Bathymetric origin shapes the physiological responses of Pterygophora californica (Laminariales, Phaeophyceae) to deep marine heatwaves
Antonella C. Almeida-Saá and Jose Miguel Sandoval-Gil contributed equally to this study.
Abstract
Kelp communities are experiencing exacerbated heat-related impacts from more intense, frequent, and deeper marine heatwaves (MHWs), imperiling the long-term survival of kelp forests in the climate change scenario. The occurrence of deep thermal anomalies is of critical importance, as elevated temperatures can impact kelp populations across their entire bathymetric range. This study evaluates the impact of MHWs on mature sporophytes of Pterygophora californica (walking kelp) from the bathymetric extremes (8–10 vs. 25–27 m) of a population situated in Baja California (Mexico). The location is near the southernmost point of the species's broad distribution (from Alaska to Mexico). The study investigated the ecophysiological responses (e.g., photobiology, nitrate uptake, oxidative stress) and growth of adult sporophytes through a two-phase experiment: warming simulating a MHW and a post-MHW phase without warming. Generally, the effects of warming differed depending on the bathymetric origin of the sporophytes. The MHW facilitated essential metabolic functions of deep-water sporophytes, including photosynthesis, and promoted their growth. In contrast, shallow-water sporophytes displayed metabolic stress, reduced growth, and oxidative damage. Upon the cessation of warming, certain responses, such as a decline in nitrate uptake and net productivity, became evident in shallow-water sporophytes, implying a delay in heat-stress response. This indicates that variation in temperatures can result in more prominent effects than warming alone. The greater heat tolerance of sporophytes in deeper waters shows convincing evidence that deep portions of P. californica populations have the potential to serve as refuges from the harmful impacts of MHWs on shallow reefs.
Abbreviations
-
- A
-
- absorptance
-
- Chl a
-
- chlorophyll a
-
- DC
-
- deep control
-
- DH
-
- Deep heat
-
- DIN
-
- dissolved inorganic nitrogen
-
- DMSO
-
- dimethylsulfoxide
-
- DP
-
- daily productivity
-
- DW
-
- dry weight
-
- Ec
-
- compensation irradiance
-
- Ek
-
- saturation irradiance
-
- ENSO
-
- El Niño-Southern Oscillation
-
- ETR
-
- electron transport rate
-
- EU
-
- experimental unit
-
- F 0
-
- minimum fluorescence
-
- F m
-
- maximum fluorescence
-
- Fv/Fm
-
- maximum quantum yield
-
- FW
-
- fresh weight
-
- FX
-
- fucoxanthin
-
- G
-
- growth
-
- gross-Pmax
-
- maximum gross photosynthesis
-
- H
-
- hole
-
- MDS
-
- non-metric multidimensional scaling
-
- MH
-
- movement of the hole
-
- MHW
-
- Marine heatwaves
-
- N
-
- nitrate
-
- net-Pmax
-
- maximum net photosynthesis
-
- NPQ
-
- non-photochemical quenching
-
- OD
-
- optical density
-
- PAM
-
- pulse amplitude modulated fluorometer
-
- P-E
-
- photosynthesis-irradiance
-
- PERMANOVA
-
- permutational multivariate analysis of variance
-
- PPFD
-
- photosynthetic photon flux density
-
- PSII
-
- photosystem II
-
- R
-
- respiration
-
- ROS
-
- reactive oxygen species
-
- SC
-
- shallow control
-
- SH
-
- shallow heat
-
- T
-
- time
-
- TBA
-
- thiobarbituric acid
-
- TCA
-
- trichloroacetic acid
-
- α
-
- photosynthetic efficiency
-
- ФPSII
-
- effective quantum yield
INTRODUCTION
Kelps form diverse and productive underwater forests with significant socio-economic and ecological benefits (Eger et al., 2023; Smale et al., 2013). However, global kelp forests face threats from both human-induced and natural factors, often acting synergistically (Krumhansl et al., 2016; Smale, 2019; Straub et al., 2019). Climate change-related ocean warming, particularly marine heatwaves (MHWs), is a key driver of these impacts (Smale, 2019; Smale et al., 2019). Therefore, understanding the effects of warming and MHWs on kelp communities is essential for developing management strategies to safeguard, recover, or facilitate artificial adaptation of these iconic macroalgae (Coleman et al., 2020; Wernberg et al., 2012).
The future of kelp forests in warmer oceans hinges on factors like genetic diversity, phenotypic plasticity, and ecological resilience (Wernberg et al., 2018). These responses, integrated with physiological and morphological variables across biological structures and life stages, play critical roles in determining their reproductive success, vegetative development, and community structure (Andrews et al., 2014; Arafeh-Dalmau et al., 2019; Liesner et al., 2020; Mabin et al., 2019a; Michaud et al., 2022). Additionally, the combination of MHWs with other stressors, such as low light (Bass et al., 2023; Xiao et al., 2015), nitrate scarcity (Fernández et al., 2020; Mabin et al., 2019b; Umanzor et al., 2021), and hyposalinity (Diehl et al., 2020), may surpass kelps' physiological tolerance thresholds, potentially overwhelming their acclimatory capacity.
Deeper, longer, and more severe MHWs are anticipated in the coming decades (Frölicher & Laufkötter, 2018; Oliver et al., 2018). This specific type of extreme MHW raises concerns due to its potential impact on kelp species with broad latitudinal and bathymetric distribution ranges. In the North American Pacific region, frequent extreme MHWs and episodic temperature anomalies, like the El Niño Southern Oscillation (ENSO), have adversely affected kelp ecosystems like those in Baja California, MX (Arafeh-Dalmau et al., 2019; Hernández-Carmona et al., 2011; Ladah & Zertuche-González, 2007). Notably, the recent extreme MHW known as “the Blob” (2013–2016) elevated sea surface temperatures by up to 3.5°C above normal, causing persistent changes in California and Baja California giant kelp forests (Bond et al., 2015; Cavanaugh et al., 2019; Hu et al., 2017). “The Blob” also triggered significant shifts in sub-canopy and invasive seaweed compositions (Arafeh-Dalmau et al., 2019; Felix-Loaiza et al., 2022; Michaud et al., 2022). This event induced strong downwelling anomalies, impacting oceanographic conditions, with potential implications for deep kelp communities (Sen Gupta et al., 2020; Zaba & Rudnick, 2016). The significance of deep kelp reefs as global climate refuges is vital but remains unexplored in the context of warming impacts (Assis et al., 2016; Davis et al., 2021; Giraldo-Ospina et al., 2020, 2023; Ladah et al., 1999).
The Baja California coastline, marking the end of the California Current System, is a crucial oceanographic transition region susceptible to climate change, carrying substantial ecological and economic implications (Smale et al., 2019; Smith et al., 2021). Anticipated warming anomalies associated with the apparent weakening of the California Current System pose significant threats to vital kelp species thriving at their southernmost distribution limit in this area. These threats include increased sea surface temperature and deepening mixing layers, as well as heightened synchronization between bottom and surface MHWs (Amaya et al., 2023; Schiel & Foster, 2015). Although studies have explored MHW impacts on the well-known giant kelp (Macrocystis pyrifera; Arafeh-Dalmau et al., 2019; Sánchez-Barredo et al., 2020; Umanzor et al., 2021), limited attention has been given to other species such as Pterygophora californica.
Pterygophora californica is long-lived stipitate kelp located from Alaska to the Baja California peninsula (Abbott & Hollenberg, 1976). This species grows under contrasting sea surface temperatures at its distribution extremes, from 2–11°C at the northernmost sites to 14–21°C at the southernmost region (according to data obtained from marineheatwaves.org). In the latter characterized by warmer waters (see Appendix S1 in the Supporting Information: Figure S1), it is likely that the persistence of the species is linked to an elevated thermo-tolerance (Hernández-Carmona et al., 2011; Matson & Edwards, 2007; Muth et al., 2019) and its resistance and recovery capabilities during ENSO events (Dayton et al., 1999; Edwards & Hernández-Carmona, 2005; Hernández-Carmona et al., 2001). However, there are no existing records on the physiological responses of P. californica to warming. Moreover, given that sporophytes can grow in a wide range of depths (from shallow subtidal areas to 35 m depth; Spalding et al., 2003), the ability of P. californica to acclimate to MHWs may be indirectly influenced by its adaptation to depth-related factors such as light and temperature. The interplay between depth adaptation and heat-stress responses in marine macrophytes requires further investigation, as this aspect has been inadequately addressed to date (Giraldo-Ospina et al., 2020; Marín-Guirao et al., 2016).
This study aimed to investigate the impact of a MHW on the physiology and growth of adult sporophytes of Pterygophora californica at different bathymetric extremes (8–10 vs. 25–27 m) within a population in Baja California, Mexico. Using a mesocosm experiment, we simulated a MHW and a subsequent warming-cessation phase. This research holds particular significance as it marks the initial exploration of MHW consequences on P. californica, providing insight into various physiological characteristics previously unknown in this species.
MATERIALS AND METHODS
Sampling site and collection of sporophytes
In spring 2021, mature sporophytes of Pterygophora californica were collected by scuba diving in Campo Kennedy, Baja California, Mexico (31°42′2.394″ N, 116°41′1.467″ W). In this location, a healthy population of the species was observed that extended from 8–10 to 25–27 m in depth. The average densities in the shallow and deep areas were 3.7 ± 1.2 and 1.7 ± 0.9 sporophytes per square meter, respectively. Twenty-four adult sporophytes (N = 24) were collected from each bathymetric limit, shallow and deep. All the sporophytes had a similar structure and size, measuring around 110 cm from the holdfast to the tip of the vegetative blade tip, with eight to 11 blades, a maximum blade length of 80–100 cm, and stipe length ranging from 40 to 60 cm. The holdfast of each sporophyte was growing naturally attached to a stone, so it was carefully preserved to avoid any damage. Additional blades were randomly collected from 12 sporophytes at each depth to evaluate their natural biological status. Black fiber meshes were applied to cover the sporophytes and blades during the collection to minimize sunlight exposure. The samples were then placed carefully in large coolers filled with seawater. Within an hour, the sporophytes were transferred to an outdoor mesocosm system situated in the Marine Botany Laboratory at the Instituto de Investigaciones Oceanológicas (Universidad Autónoma de Baja California).
Irradiance (PAR) was monitored at the bottom of both bathymetric extremes for 25 days prior to the experiment, using submersible sensors (Onset-Hobo MX2202) and excluding shaded portions by the canopy (Figure 1c). At a depth of 8–10 m, the daily light dose ranged from 13.9 to 2.2 mol photons · m−2 · d−1, with irradiance peaks between 150 and 800 μmol photons · m−2 · s−1 occurring at noon. At a depth of 25–27 m, the daily light dose ranged from 3.7 to 0.2 mol photons · m−2 · d−1, while with irradiance peaks at noon were 20 to 180 μmol photons · m−2 · s−1. The lowest recorded irradiance values were attributed to either cloudy days or reduced transparency in the water column. To obtain photon flux density measurements in μmol photons · m−2 · s−1, our submersible sensor's lux unit readings were calibrated against a cosine-corrected quantum sensor (Li-COR Biosciences, LI-192) in the laboratory. Seawater temperature throughout the year was also recorded by the same submersible sensors (Figure 1a,b). The average temperature, maximum, and minimum values at 25–27 m depth were 13.7 ± 0.01°C, 18.3°C, and 10°C, respectively. At 8–10 m, the corresponding values were 14.4 ± 0.01°C, 19.6°C, and 9.9°C.
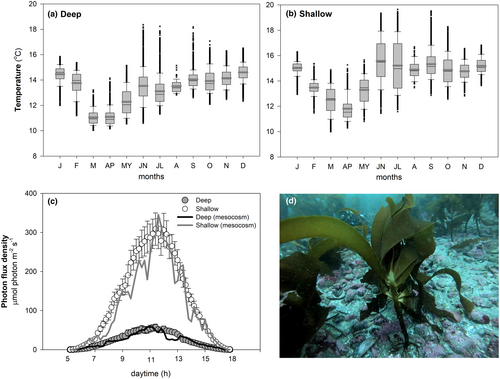
Mesocosm system and experimental design
The outdoor mesocosm system for experimentation consisted of 16 fiberglass tanks (1 m3) and closed water circulation. By using self-priming pumps with a capacity of 10,000 liters per hour, seawater was circulated and completely replaced in each tank a total of 124 times per day. The temperature of the seawater was precisely regulated through chillers (Aqua Logic MT-8, Inc., San Diego, CA) and titanium heaters. The tanks were filled with filtered (100 μm and UV) seawater with low dissolved inorganic nitrogen (DIN) concentration (1–3 μM NO3−, <1 μM NH4+) collected in an open sea area adjacent to the university. To prevent thermal gradients and improve blade movement, aeration was provided by the bottom of each tank. The temperature was set to 14 ± 0.2°C, based on average values recorded at both deep and shallow collection sites during spring (Figure 1a,b). Additionally, fiber meshes were placed on top of the tanks to simulate the mean irradiance measured in the field (Figure 1c). The average daily light dose for shallow and deep (sporophytes was adjusted at 6.9 ± 0.8 mol photons · m−2 · d−1 (~350 μmol photons · m−2 · s−1 at noon) and 1.3 ± 0.2 mol photons · m−2 · d−1 (~60 μmol photons · m−2 · s−1 at noon), respectively. To maintain the optimum nutritional condition of the sporophytes throughout the experiment, pulsed fertilization with a commercial fertilizer (Allganic Nitrogen®) was carried out every 3 days, using NaNO3 (2.9 g · m−3) to achieve a final concentration of 20 μM. Salinity and pH were monitored using a submersible multiparameter probe (YSI Pro Plus, USA), and the values of seawater ranged from 32.5 to 33.5 and 7.9 to 8.2, respectively. Filtered fresh water was added continuously to balance evaporation and maintain constant salinity.
After a 5-day acclimation period, four tanks were assigned to each experimental treatment. Each tank was designated as an experimental unit (EU). The experiment included two phases, each lasting 6 days. The first phase involved Pterygophora californica and a simulate MHW (i.e., MHW phase). The second phase consisted of sporophyte recovery to a control temperature (i.e., post-MHW phase). The MHW phase comprised of four treatments: (1) shallow sporophytes under a control temperature of 14°C (SC), (2) shallow sporophytes exposed to a high temperature of 20°C (SH), (3) deep sporophytes under a control temperature (DC), and (4) deep sporophytes exposed to high temperature (DH). The temperature was raised gradually from 14 to 20°C by 2°C each day. This simulated MHW was based on thermal anomalies recorded in the region utilizing an online MHW Tracker (Schlegel, 2020). In the post-MHW phase, the temperature in SH and DH steadily decreased by 2°C per day, reaching 14°C from 20°C. The temperature in control treatments (SC, DC) remained constant at 14°C throughout the experiment.
The biological characteristics of the sporophytes (see below) were examined at the conclusion of each experimental phase (MHW and post-MHW). At each sampling time, only one sporophyte per EU (n = 4) was used. All the physiological descriptors were measured from the first two fully mature blades beneath the vegetative (terminal) blade to minimize a possible variability between tissues in biological traits. For each physiological variable, three pseudo-replicates (blade pieces) were obtained in each sporophyte per tank. Values of pseudo-replicates were averaged per tank.
Photosynthesis and respiration (P-E curves)
Blade absorption spectra
Blade absorptance represents the efficiency of photosynthetic tissues (pigments) to harvest light for photosynthesis. Blade absorbance (OD, optical density) was measured spectrophotometrically by utilizing the opal glass technique developed by Shibata (1959) and following the guidelines outlined in Vásquez-Elizondo et al. (2017). To conduct the measurements, an intact blade piece (~2 cm2) was placed between two microscope glass slides. Additionally, another blade piece was treated with commercial bleach diluted in seawater and used as a white reference. Optical opal glasses and glass slides containing pigmented and bleached tissues were positioned near to the photomultipliers (detectors) of the spectrophotometer. Absorbance was measured in the PAR range (400–700 nm), and the resulting spectra were adjusted for residual scattering and non-photosynthetic absorption by subtracting the absorbance at 725 nm. The proportion of light captured by blade tissues was expressed as absorptance (A = 1–10−OD), assuming a negligible tissue reflectance. Values of APAR and A680 corresponded to the average absorptance in the PAR region and at the peak of chlorophyll a (chl a).
Pigment content
Chlorophyll a and Fucoxanthin were analyzed through sequential extractions following the method outlined by Seely et al. (1972) with modifications by Wheeler (1980). Initially, dimethylsulfoxide (DMSO) was used to extract fucoxanthin and chl a from 0.02 g FW of blade tissue. Subsequently, absorbance was determined at 665, 631, 582, and 480 nm. A second 24-h extraction using 100% acetone was conducted on the same tissue sample, under 4°C and darkness. The resulting solution was diluted 3:1:1 with methanol and distilled water. The pigment content was then measured by its absorbance wavelengths at 470, 581, 631, and 664 nm. The total pigment content was calculated as the sum of the pigment concentration from both sequential extractions.
Photosynthesis based on chlorophyll a-fluorescence (PSII)
Photochemical capacities were assessed by measuring changes in the fluorescence emission of the chl a of PSII using a portable Pulse Amplitude Modulated Fluorometer (Diving-PAM, Walz, Germany). Maximum Quantum Yield [(Fm − F0)/Fm or (Fv/Fm)] was measured in sporophytes dark-adapted overnight by applying a saturating pulse (0.8 s, ~5000 μmol photons · m−2 · s−1). The minimum fluorescence (F0) was measured was measured under a “measuring light” before the actinic light pulse, while Fm was the maximum fluorescence induced by the pulse. At midday, fluorescence measurements were repeated on illuminated sporophytes in the same precise blade pieces. The blades were illuminated with the actinic light of the fluorometer through the fiber optic at a saturating intensity of 300 μmol photons · m−2 · s−1. Three minutes of illumination were adequate to establish a steady state of photosynthesis after induction curve trials. Next, a saturating pulse was applied to measure F and Fm and, thus, the Effective Quantum Yield [i.e., ФPSII, (Fm′ − F)/Fm′, or (ΔF)/Fm′] and Non-Photochemical Quenching [NPQ, (Fm − Fm′)/Fm′]. In addition, the Electron Transport Rate was determined using the formula [ETR = ФPSII × E × A × 0.5]. Here, E represents the actinic light applied, A denotes tissue absorptance (refers to methods above), and 0.5 is a correction factor applied for the assumed equal distribution of photons between photosystems.
Blade growth rates
Blade growth was measured in two sporophytes per EU and treatment, using Parke's (1948) method. Growth was measured for the vegetative blade and first blade, which were approximately 20 cm lengths. A 2-mm hole was punched in the midrib of the chosen blades, 10 cm from the base of the blade (above the meristem). After exposure to the experimental treatments (6 days in each phase), the distances between the base of the meristem and the hole were re-measured, allowing for the determination of the longitudinal growth per day. Blade growth (G, cm per day) was calculated using the formula [G = MH − H/T], where MH is the hole's movement distance from the base of the blade, H (hole) is the punching's initial distance from blade's base (i.e., 10 cm), and T represents the days of the experimental phase. Blade growth was also measured in situ in six sporophytes per depth at the collection site.
Nitrate uptake kinetics and total N
Nitrate uptake rates and N content were examined to assess the effects of warming on the incorporation and assimilation of external N. Blade pieces were incubated in transparent plastic Ziploc bags containing artificial seawater with various nitrate concentrations (15KNO3 at. % = 99, Cambridge Isotope Laboratories), including 5, 10, 20, and 30 μM. Each plastic bag (2.5 L) contained four blade pieces (7 × 7 cm, ~0.15 DW). This seaweed biomass-to-volume ratio was utilized in previous trials to prevent a substantial decrease in the external nitrate concentration during the 30-min incubation period. To prevent a decrease in external nitrate concentration, it was important to avoid a concomitant decrease in nitrate uptake rates and thus an underestimation of nitrate uptake by tissues. Four bags were used for each nitrate concentration and treatment (n = 4). The bags were freely suspended within the mesocosms tanks under corresponding temperature and irradiance conditions for each treatment. All incubations were conducted at noon to avoid changes in irradiance during the day, which can affect nitrate uptake. Upon completing incubations, blade tissues were washed with deionized water to remove surface nutrients and subsequently dried at 60°C for 48 h. Samples were ground for isotope enrichment analysis. Isotopic determinations were carried out at the UC-Davis Stable Isotope Facility, using an elemental analyzer (EA) connected to a continuous flow isotope ratio mass spectrometer (IRMS). Nitrate uptake rates (V, expressed as μmol N · g−1 DW · h−1) were calculated as V = [(15Nexp − 15Nback) × Nc]/(MN × t), where the difference (15Nexp − 15Nback, at. %) is the 15N enrichment relative to natural 15N (background signal), Nc is the nitrogen content (g N·g−1 DW), MN is the molar mass of nitrogen (15 g · mol−1), and t is the duration of the incubation. Nitrogen content was also analyzed from the samples used as background (i.e., those not exposed to the 15NO3− tracer) for isotope analyses.
Total soluble carbohydrates
Total intracellular carbohydrate content was analyzed to assess the internal C-reserves in the blades. Carbohydrates were measured using the phenol-sulfuric acid colorimetric method (Dubois et al., 1956). Approximately, 0.02 g FW of tissue was dried, ground, and then digested in 0.2 M HCl at 60°C for 3 h. After centrifugation (5 min, 1000 g), the supernatant was mixed with 3% phenol and sulfuric acid (95%). Absorbances were read at 490 nm, using glucose as a standard.
Lipid peroxidation
Lipid peroxidation (i.e., a variable that represents the oxidative damage of reactive oxygen species over biological membranes) was determined using the thiobarbituric acid reactive substances (TBARS) assay as described by Hodges et al. (1999) and Correia et al. (2006). Blade fresh tissue (~0.5 g) was homogenized with trichloroacetic acid (TCA, 20%). Next, the homogenates were centrifuged (10 min, 3000 g, 4°C), and the resulting supernatants were mixed with a solution of TCA (20%) and thiobarbituric acid (TBA, 0.5%). The solutions were heated at 90°C for 30 min, followed by another round of centrifugation (10 min, 10,000 g). The supernatants were extracted, and their absorbances (440, 532, and 600 nm) were measured.
Total phenolic content and antioxidant capacity
The antioxidant capacity and total phenols were measured as a proxy of the general antioxidant response under a potential oxidative stress caused by warming. Approximately 0.02 g DW were ground and mixed with 80% methanol. The mixture was left in darkness for 24 h, and then centrifuged (10 rpm for 10 min). The resulting supernatant was used to measure phenolic compound content through a modified Folin–Ciocalteu assay, with gallic acid as a reference (Singleton & Rossi, 1965). To briefly summarize, an aliquot of the methanolic extract was diluted in 1 mL of distilled water. Then, 0.1 mL of Folin–Ciocalteu reagent and 0.3 mL of saturated NaCO3 were added, homogenized, and heated at 40°C for 3 min. Subsequently, absorbance was read at 765 nm. The radical scavenging activity of the same methanolic extracts was determined over the stable free radical, DPPH, using ascorbic acid as the standard (Sabeena Farvin & Jacobsen, 2013). The reactive solution was prepared by diluting 0.1 mL of diluted extract in aqueous methanol (80% concentration) in a 1:4 ratio and mixing it with 1 mL of 30 μM DPPH freshly prepared in aqueous methanol (90% concentration). After precisely 30 min, the DPPH was added, and the absorbance was measured at 517 nm. A blank control was also prepared with the same proportions of DPPH and aqueous methanol, but without the algal extract. The absorbance of the blank solution was used as a reference.
Statistical analysis
A t-test was conducted to compare the averages of the different biological variables measured in Pterygophora terygophora sporophytes growing at both depths in the natural population (Table 1). For each experimental phase (MHW and post-MHW), a two-way ANOVA (posthoc Student–Newman–Keuls) was applied with temperature (two levels; control and high) and depth (two levels: shallow and deep) as fixed and independent factors. Significant differences were identified at p < 0.05. Data were tested for the assumptions of normality and homoscedasticity through the Shapiro–Wilk and Levene tests, respectively. A permutation ANOVA test (10,000 permutations) was performed when the assumptions of normality and homoscedasticity were not met even after transformations. Differences in nitrate uptake rates were statistically analyzed using a three-way ANOVA for each experimental phase, with temperature, depth, and nitrate concentration as fixed factors. Rstudio software and Sigmaplot 14.5 were applied for statistical analyses. Supplementary material (Appendix S1: Tables S1–S4) includes the provided statistical outcomes.
| Biological variables | Pterygophora californica (Deep) | Pterygophora californica (Shallow) | p |
|---|---|---|---|
| Mean ± SE | Mean ± SE | ||
|
Net-Pmax μmol O2 · g−1 DW · h−1 |
232 ± 1 | 257 ± 10 | 0.04 |
|
Gross-Pmax μmol O2 · g−1 DW · h−1 |
266 ± 5 | 278 ± 10 | 0.339 |
|
Respiration μmol O2 · g−1 DW · h−1 |
14 ± 1 | 22 ± 1 | <0.001 |
| α | 1.5 ± 0.1 | 1.3 ± 0.1 | 0.099 |
|
Ec μmol photons · m−2 · s−1 |
19 ± 1 | <0.001 | |
| Ek | 174 ± 9 | 195 ± 6 | 0.092 |
|
Total soluble carbohydrates % DW |
3.27 ± 0.06 | 2.98 ± 0.04 | 0.003 |
| A PAR | 0.73 ± 0.003 | 0.71 ± 0.003 | <0.001 |
| A 680 | 0.94 ± 0.002 | 0.93 ± 0.002 | 0.224 |
|
Chl a μg · g−1 FW |
470 ± 41 | 683 ± 32 | 0.001 |
|
Fucoxanthin μg · g−1 FW |
1211 ± 97 | 1151 ± 52 | 0.588 |
| Fv/Fm | 0.77 ± 0.01 | 0.70 ± 0.01 | 0.001 |
| ФPSII | 0.33 ± 0.01 | 0.37 ± 0.01 | 0.038 |
| NPQ | 0.41 ± 0.04 | 0.53 ± 0.05 | 0.094 |
|
ETR μmol e− · m−2 · s−1 |
25 ± 0.4 | 27 ± 1 | 0.116 |
| δ15N | 6.74 ± 0.26 | 6.29 ± 0.29 | 0.271 |
|
N % DW |
1.60 ± 0.1 | 1.62 ± 0.07 | 0.907 |
|
Lipid peroxidation nmol equation AA · g−1 DW |
38.16 ± 3.03 | 0.009 | |
|
Total phenols mg equation AA · g−1 DW |
2.69 ± 0.21 | 3.13 ± 0.16 | 0.119 |
|
Antioxidant capacity mg equation AA · g−1 DW |
0.71 ± 0.1 | 1.58 ± 0.04 | <0.001 |
|
Growth (first blade) cm · d−1 |
0.35 ± 0.05 | 0.98 ± 0.05 | <0.001 |
- Note: Measurements were taken immediately after collection (i.e., before the experiment). Bold values denote significant values (p < 0.05, t-test).
Multivariate analyses were conducted using normalized data from all response variables, and a ranked triangular similarity matrix was created based on Euclidean distances. A two-way PERMANOVA crossed design (9999 permutations) was completed to assess the interactive effects of the factors Depth and Temperature. A Monte Carlo p-values, P(MC), was used to verify significant differences in pair-wise posterior comparisons, as the number of possible permutations was limited. A non-metric multidimensional scaling (MDS) ordination was utilized to visualize multivariate patterns (Appendix S1: Figure S2). The statistical procedures were carried out with the PRIMER 6 & PERMANOVA+ v.1.0.2 software package (Anderson et al., 2008).
RESULTS
Comparison of deep and shallow Pterygophora californica from the natural population
The following result corresponds to the biological descriptors measured in sporophytes just after collection, before the beginning of the experiment. Values of net-Pmax, respiration, and Ec were significantly higher (10%, 60%, and 140%, respectively) in shallow-water sporophytes compared to deep-water sporophytes (Table 1). Similarly, values of lipid peroxidation, antioxidant capacity, chl a content, and ФPSII were higher in shallow-water individuals. Blade growth was three-fold higher in shallow Pterygophora californica (Table 1). Conversely, deep-water sporophytes showed higher Fv/Fm, soluble carbohydrates concentration, and blade absortance, APAR (Table 1). Values of gross-Pmax, Ek, α, A680, fucoxanthin, NPQ, ETR, total phenols, δ15N, and total N were similar in sporophytes collected from depths (Table 1).
Biological responses in the MHW and post-MHW phases
The two-way PERMANOVA (Table 2) revealed significant differences in the analyzed biological characteristics between sporophytes from both depths (p = 0.001). Furthermore, this multivariate analysis demonstrated the significant impact of the MHW (p = 0.001) on the physiology of Pterygophora californica during both experimental phases (MHW and post-MHW), and these varied significantly between deep-water and shallow-water individuals (i.e., significant interaction depth x temperature, p < 0.001). The non-metric multidimensional scaling analysis (MDS, Figure S2) supports the PERMANOVA findings. The MDS also demonstrated greater 2D distance differences between control and heated deep-water sporophytes (DC vs. DH).
| Experimental phase | Main test | df | SS | Pseudo-F | p (Perm) |
|---|---|---|---|---|---|
| MHW | Depth | 1 | 119.65 | 15.837 | 0.001 |
| Temperature | 1 | 39.575 | 5.238 | 0.001 | |
| D × T | 1 | 35.109 | 4.647 | 0.001 | |
| Residuals | 12 | 7.555 | |||
| Post-MHW | Depth | 1 | 104.72 | 18.252 | 0.001 |
| Temperature | 1 | 51.677 | 9.007 | 0.001 | |
| D × T | 1 | 59.749 | 10.414 | 0.001 | |
| Residuals | 12 | 68.851 |
- Note: Bold numbers indicate significant differences.
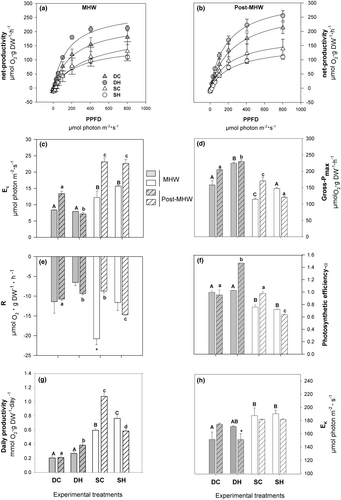
The P-E curves (Figure 2a,b) were fitted to tangential hyperbolic adjustments and showed a strong correlation (R2 > 0.97, p < 0.05). The values of Ec (Figure 2c) decreased by 50% in DH relative to control sporophytes during the post-MHW phase (Table S2). Gross-Pmax (Figure 2d) significantly increased by ~25%–50% in DH and SH sporophytes exposed to high temperature (Table S1). In the post-MHW phase, DH sporophytes exhibited a similar trend, but their values decreased by 20% compared to their control in SH (Table S2). Warming exposure induced a 40%–60% drop in respiration (R, Figure 2e) in both SH and DH sporophytes, but significant differences were found only for DH (Table S1). During the post-MHW phase, the interaction between factors (D × T) was found to be statistically significant (Table S2). The decrease of R by 40% in SH (Table S2) and the increase of R by 10% in DH (Table S2) further supported these results. Photosynthetic efficiency (α, Figure 2f) did not vary in either SH or DH sporophytes. However, the D × T interaction was significant during the post-MHW phase (Table S2) due to a 60% increase in values observed in DH, while decreasing by 40% in SH. In shallow sporophytes, daily net productivity (Figure 2g) increased by ~15% when exposed to warming (Table S1), while decreasing by 40% during the post-MHW phase. Deep sporophytes (DH) exhibited a notable rise in their daily productivity (by ~50%) during the post-MHW phase (Figure 2i; Table S2). During the post-MHW phase, saturation irradiance (Ek, Figure 2h) uniquely decreased in DH sporophytes compared to the control (DC; Table S2).
Blade absorptance in the PAR range (APAR, Figure 3c) increased in sporophytes that were exposed to high temperatures (SH) in the post-MHW phase (Table S2). Nonetheless, temperature did not significantly impact A680 in DH and SH sporophytes during any phase. The contents of chl a and Fx did not undergo changes in deep and shallow sporophytes upon warming (Figure 3e,f; Table S1). However, both pigments exhibited a decrease in DH sporophytes during the post-MHW phase (Figure 3e,f; Table S2).
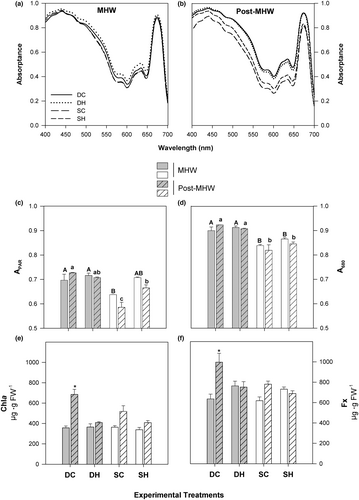
The Fv/Fm values (Figure 4a) were similar across treatments in both experimental phases. During the exposure phase, there was an increase in ФPSII in DH and SH sporophytes, but the difference was statistically significant only for DH (Figure 4b; Table S1). On the contrary, DH sporophytes displayed lower ФPSII compared to their respective controls in the post-MHW phase (Figure 4c; Table S2). Non-photochemical quenching (Figure 4c) exhibited no significant variation in Pterygophora californica between treatments across both experimental phases, with the exception of a decline in SH sporophytes observed in the post-MHW phase (Table S2). Exposure to high temperature elevated the ETR of SH sporophytes (Figure 4d; Table S1).

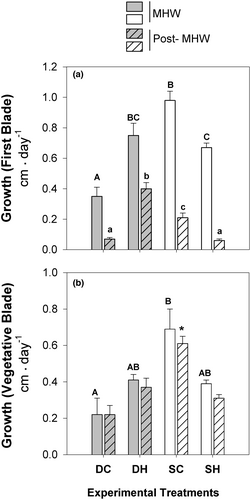
The statistical significance of the interaction between the factors (D × T, depth) was observed for blade growth in both phases (Figure 5a,b; Tables S1 and S2). The growth of the first blade (Figure 5a) and the vegetative blade increased by 140%–471% in DH sporophytes and decreased by 32%–72% in SH sporophytes. Although statistically significant changes were observed in the growth of the first blade (Tables S1 and S2), only significant differences were found between SC and SH during the post-MHW phase (Table S2).
Nitrate uptake rates (Figure 6a,b) of both shallow and deep sporophytes did not reach saturation at the tested nitrate concentrations (up to 30 μM). Linear regression adjustments indicated a good fit for all the treatments (R2 > 0.92; p < 0.05). There was a significant decrease in nitrate uptake rates for both deep and shallow sporophytes in the post-MHW phase compared to control values (Table S4), with differences being higher for the former.
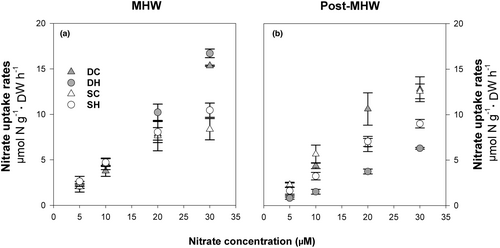
Nitrogen content (Table 3) was significantly greater in sporophytes from deeper waters than those from shallower water in both experimental stages, regardless of temperature treatment (Table S1 and S2). Total soluble carbohydrates (Table 3) decreased by ~30% in deep sporophytes exposed to warm temperatures compared to DC individuals (Table S1). In the post-MHW phase, total carbohydrates decreased in both deep and shallow sporophytes compared to their respective controls (Table S2).
| Exp. phase | DC | DH | SC | SH | |
|---|---|---|---|---|---|
| % N | MHW | 1.37 ± 0.13a | 1.49 ± 0.09a | 1.23 ± 0.05b | 1.21 ± 0.05b |
| Post-MHW | 1.44 ± 0.07a | 1.56 ± 0.06a | 1.22 ± 0.05b | 1.37 ± 0.06b | |
| TSC | MHW | 3.20 ± 0.06a | 2.45 ± 0.05b | 2.99 ± 0.03ac | 2.89 ± 0.09c |
| Post-MHW | 2.97 ± 0.16a | 2.63 ± 0.1b | 2.89 ± 0.12a | 2.61 ± 0.07b |
- Note: Values show means and standard errors (n = 4). Different letters on the bars indicate statistical differences among treatments (two-way ANOVA, post hoc SNK; Tables S1 and S2). See Figure 1 for further details on the meaning of treatment abbreviations.
- Abbreviations: DC, deep control; DH, deep heat; SC, shallow control; SH, shallow heat.
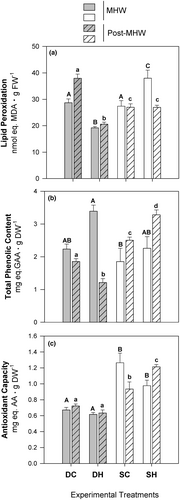
During the exposure phase, there was a significant interaction of factors for lipid peroxidation (D × T; Figure 7a; Table S1). Specifically, this variable decreased by ~30% in DH sporophytes but increased by ~30% in SH (Table S1). In the post-MHW phase, lower values of lipid peroxidation continued to persist in DH sporophytes (Table S2), whereas SH and SC sporophytes displayed comparable lipid peroxidation. The total amount of phenolic compounds (Figure 7b) rose by ~35% in DH sporophytes following exposure to warming conditions (Table S1). However, DH sporophytes experienced a decline in total phenols while SH sporophytes showed an increase after the occurrence of MHW (i.e., the interaction of factors D × T was significative; Table S2). Only SH sporophytes demonstrated an increase in antioxidant capacity during the post-MHW phase (Figure 7c; Table S2).
DISCUSSION
Ecophysiology of Pterygophora californica in its bathymetric extremes
Pterygophora californica undergoes significant variations in lighting throughout its bathymetric distribution. In the collection site during spring, the shallow portion of the population (8–10 m depth) receives a higher light dose of approximately 7 mol photons · m−2 · d−1. Maximum irradiance at midday reached around 350 μmol photons · m−2 · s−1. In contrast, the deeper sporophytes (25–27 m) are exposed to lower light levels, receiving approximately 1 mol photons · m−2 · d−1 with irradiance peaks of around 60 μmol photons · m−2 · s−1. These differences in light exposure significantly impact the photo-acclimation and photo-adaptation of P. californica (Table 1). Shallow-water sporophytes showed higher maximum photosynthetic rates (gross-Pmax), electron transport rate (ETR), and saturation irradiance (Ek). Blades of these sporophytes also contain more chl a, indicating more photosystem reaction centers (Beer et al., 2014; Davison et al., 1991). These photobiological properties enable more efficient use of light above saturating irradiances and are typically associated with seaweeds acclimated to high light conditions (Hurd et al., 2014). As the light intensity increases, the photosynthetic apparatus at the thylakoid level becomes more excited and undergoes greater reduction (Beer et al., 2014; Lobban & Harrison, 1994). The increased values of NPQ observed in shallow sporophytes were consistent with this state, potentially functioning as a mechanism to dissipate excitation energy as heat through the xanthophylls cycle (XC; Beer et al., 2014; García-Mendoza & Colombo-Pallotta, 2007).
Shallow-water sporophytes exhibit higher respiration rates than those growing in deeper waters, likely due to a stimulated carbon metabolism or other energy-demanding processes such as growth (Eggert, 2012; Markager & Sand-Jensen, 1994). Stimulated respiration and photosynthesis inevitably produce more reactive oxygen species (ROS) via redox reactions in thylakoids and mitochondria (Beer et al., 2014). This is likely responsible for the higher lipid peroxidation levels (as a sign of oxidative damage) in these individuals compared to deep-water sporophytes (Umanzor et al., 2020).
Deep-water Pterygophora californica demonstrated photoacclimation to low-light conditions. These sporophytes showed greater photosynthetic efficiency (α, and, thus, greater Fv/Fm) and blade absorptance values, indicating their enhanced capacity to harvest light in the PAR range. Therefore, photosynthesis becomes more efficient at sub-saturating irradiances in low-light regimes (Hanelt & Figueroa, 2012; Hurd et al., 2014; Tait & Schiel, 2013). As a result of a higher α, the compensation irradiance (Ec) is reduced, requiring less light for photosynthesis to balance respiration and achieve positive net metabolic productivity. The sporophytes' low respiratory activity optimizes carbon balance under light limitation (Bernardeau-Esteller et al., 2011, 2015; Sandoval-Gil et al., 2014; Umanzor et al., 2020).
Several biological differences between the natural deep-water and shallow-water Pterygophora californica were also observed when comparing control individuals from both depths, referred to as DC and SC, respectively. These differences included physiological descriptors such as respiration, α, Ec, NPQ, antioxidant capacity, blade absorptance, and growth rate. This suggests that the collection and transplantation of these sporophytes had no significant impact on their physiological status and that the mesocosm system maintained the biological differences attributed to adaptation to depth. It additionally confirmed that the effects and responses observed in P. californica were due to the implemented experimental treatments rather than any confounding effects associated with manipulating sporophytes. Furthermore, and regarding thermal conditions, deep and shallow sporophytes encountered analogous thermal regimes in the studied population (see Figure 1a,b), despite growing in the bathymetric extremes. Thus, rather than thermo-adaptative mechanisms, photo-acclimation to light availability and other adaptive processes related to environmental conditions not evaluated in this study (such as nutrients and hydrodynamic) may significantly impact the distinct thermo-tolerance of P. californica at different depths (see below), rather than thermo-adaptive mechanisms.
Deep and shallow Pterygophora californica under MWHs
Our study demonstrated that exposure of sporophytes of Pterygophora californica to heatwave-like warming had varying impacts on their physiology. These effects were dependent on the bathymetric origin of the tested sporophytes.
Blade growth of deep-water sporophytes increased during both the exposure to warming and post-MHW phase. Overall, this can be explained by the high thermo-tolerance of the photobiological processes. Specifically, the warming applied during the MHW phase did not affect most of the photobiological variables studied in the deep-water sporophytes, except for a rise in gross-Pmax and ΦPSII. However, in the post-MHW phase, these sporophytes displayed enhanced photosynthetic capability and daily productivity due to increased values of gross-Pmax, α, and Ec. This stimulation of photosynthesis may result from positive warming effects that are delayed and impact various related processes, including the activity of enzymes involved in carbon assimilation and the mobility of proteins in the thylakoid membranes (Andersen et al., 2013; Shindo et al., 2022; Vivanco-Bercovich et al., 2022; Wernberg et al., 2016). The unaltered values of Fv/Fm, ETR, and NPQ recorded during both the experimental periods (MHW and post-MHW) point toward the functional and structural integrity of the photosynthetic apparatus (Murchie & Lawson, 2013; Sánchez-Barredo et al., 2020; Umanzor et al., 2021), thereby sustaining the positive photosynthetic response of deep-water sporophytes. The lower pigment concentration (chl a and Fx) and the similar blade absorptance (APAR, A680) measured in these sporophytes appear to contradict this trend. Many studies (e.g., Sandoval-Gil et al., 2023) have documented a similar lack of correlation between light-harvesting and photosynthetic abilities. Processes not measured in this study are likely to be responsible for the increase in photosynthesis, rather than light capture capacities.
During the period of warming, shallow-water sporophytes of Pterygophora californica exhibited a slight increase in gross-Pmax and a decrease in respiratory activity and showed a higher daily productivity. An increase in gross-Pmax was also observed in deep-water individuals. However, the results observed during the post-MHW period differed significantly between deep-water and shallow-water P. californica, since gross-Pmax and α of shallow-water sporophytes drastically decreased. This delayed stress induced by heat is likely a result of multiple alterations affecting both the light-dependent reactions of photosynthesis and subsequent photo-assimilation processes, such as carbon fixation/assimilation. These changes may encompass modifications in the structure and functionality of the PSII and thylakoid membranes, dissociation of the water-splitting complex, or the deceleration of electron transport between photosystems (Heinrich et al., 2012; Sandoval-Gil et al., 2014; Shindo et al., 2022). Despite a decline in gross-Pmax and α, Fv/Fm, ΦPSII, and ETR values remained similar to control levels. Discrepancies in photobiological parameters obtained from P-E curves and chl a-fluorescence can be attributed to the activation of mechanisms providing alternative electron sinks beyond photosynthesis, including the water–water cycle, photorespiration, and cyclic electron transport of PSI (Niyogi, 2000; Voss et al., 2013), as documented in other marine macrophytes (Marín-Guirao et al., 2016). Furthermore, the induction of these electron-draining processes likely contributed to reactive oxygen species (ROS) production in shallow P. californica, triggering antioxidant and free-radical scavenging responses, leading to a higher phenolic content and antioxidant capacity. Although warming enhanced certain photosynthetic capabilities and daily net productivity during the MHW phase, shallow-water P. californica exhibited reduced blade growth rates and increased lipid peroxidation (i.e., oxidative damage), indicating unassessed heat-related metabolic stress in our study.
The reduced photosynthetic capacity of shallow Pterygophora californica in the post-MHW phase led to a severe decline in daily net productivity and internal carbon reserves. Respiration also increased in these sporophytes, further contributing to these metabolic carbon imbalances. Increased respiration to sustain high energy-demanding stress responses has been described in seaweeds under warming and other stressors (Anderson, 2006; Andersen et al., 2013; Britton et al., 2020). However, the opposite was observed for deep-water sporophytes, in which stimulated photosynthesis and reduced respiration likely fueled blade growth. Down-regulation of respiration not only reduces internal carbon losses but also allows optimization of net productivity at low (sub-saturating) irradiances near the compensation point, Ec (Blain & Shears, 2020; Davison et al., 1991; Fairhead & Chesire, 2004; Hurd et al., 2014; Rodrigues et al., 2000).
Nitrate uptake rate was another physiological trait affected in the post-MHW phase instead of during the warming period. Both deep-water and shallow-water Pterygophora californica sporophytes significantly declined their nitrate acquisition capacities. Evidence of the effects of warming on the N-uptake kinetics of seaweeds is limited (e.g., Fernández et al., 2023; Gerard, 1997; Sánchez-Barredo et al., 2020). Warming can directly affect the transmembrane transportation of nitrate and indirectly modify processes that determine its incorporation (e.g., Hurd et al., 2014; Sánchez-Barredo et al., 2020; Umanzor et al., 2023). In our study, for example, exposure to warming could have resulted in metabolic energy depletion of shallow-water sporophytes, which can potentially hinder the activity of N-assimilatory enzymes. A decrease in nitrate uptake rates was observed for P. californica for common external nitrate concentrations in the water column of the region (Camacho-Ibar et al., 2003; Hernández-Ayón et al., 2004) and was more pronounced at levels linked to upwelling events (~20 μM; Camacho-Ibar et al., 2003). This hinders P. californica's ability to utilize this primary source of DIN that is necessary for the growth and survival of this and other foundation kelp species (Fernández et al., 2020; Hurd et al., 2014; Sandoval-Gil et al., 2023; Zimmerman & Kremer, 1986). Despite the decreased uptake of nitrate, the total N content in blade tissues did not decay, indicating that internal N-storage may have been sustained by N mobilization and reabsorption from other vegetative compartments (Forbord et al., 2021; Gerard, 1982; Young et al., 2007). This decoupling between nitrate uptake and total N content in blade tissues has also been described for juvenile sporophytes of giant kelp exposed to warming and light limitation (Sandoval-Gil et al., 2023; Umanzor et al., 2021).
Overall, our study reveals that MHWs can impact the physiology and growth of the kelp Pterygophora californica in the North American Pacific, particularly near the southern limit of its distribution. The effects of warming vary based on the bathymetric origin of sporophytes. While MHWs may benefit deeper sporophytes (25–27 m), sporophytes in shallow waters (8–10 m) exhibit signs of metabolic stress, diminished growth, and oxidative damage. Like other kelp species, P. californica is expected to face more intense and deep MHWs. Shallow-water sporophytes may be significantly affected, while deeper ones may benefit. However, species responses could differ due to local adaptation and thermal niches (Andersen et al., 2013; Delebecq et al., 2013; Eggert, 2012; King et al., 2018). The potential within-species variability and unexplored factors affecting P. californica's thermo-tolerance, such as early-life stage resistance and seaweed-microbiome interaction, require further investigation.
Our findings support the concept that deeper kelp populations can serve as refuges from MHWs (Giraldo-Ospina et al., 2020). This outcome presents an opportunity for future restoration strategies, such as transplanting deep-water sporophytes to revitalize damaged shallow Pterygophora californica beds or exploring their potential as a source of reproductive propagules for natural repopulation, as seen in other kelp species (e.g., Ecklonia radiata; Giraldo-Ospina et al., 2023). Notably, most effects and responses were observed after MHW cessation, aligning with studies on other macrophytes (Marín-Guirao et al., 2016; Umanzor et al., 2021; Vivanco-Bercovich et al., 2022) and indicating a delayed response to heat stress. Our results emphasize the need for extended experimental periods to comprehend seaweed's physiological tolerance and resilience capacities within the context of MHWs.
AUTHOR CONTRIBUTIONS
Antonella C. Almeida-Saá: Conceptualization (lead); data curation (lead); formal analysis (lead); investigation (lead); methodology (lead); visualization (lead); writing – original draft (lead); writing – review and editing (lead). Schery Umanzor: Investigation (equal); writing – original draft (supporting); writing – review and editing (supporting). Jose Antonio Zertuche-González: Investigation (equal); writing – review and editing (supporting). Ricardo Cruz-López: Investigation (equal); writing – review and editing (supporting). Raquel Muñiz-Salazar: Investigation (equal); writing – review and editing (supporting). Alejandra Ferreira-Arrieta: Methodology (equal); supervision (equal). Paula Bonet Melià: Methodology (equal). Jessica Anayansi García-Pantoja: Methodology (equal). Laura K. Rangel-Mendoza: Methodology (equal). Manuel Vivanco-Bercovich: Methodology (equal). Leonardo Ruiz-Montoya: Methodology (equal). Jose Manuel Guzmán-Calderón: Methodology (equal). Jose Miguel Sandoval-Gil: Conceptualization (lead); data curation (lead); formal analysis (lead); funding acquisition (lead); investigation (lead); methodology (lead); project administration (lead); supervision (lead); validation (lead); visualization (lead); writing – original draft (lead); writing – review and editing (lead).
ACKNOWLEDGMENTS
This research was supported by a CONACYT (Consejo Nacional de Ciencia y Tecnología) Project CB-A1-S-8382 and an internal project (403/1/C/2/22) at UABC, under the leadership of JMS-G. The assistance of all the members of the Marine Botany research group (IIO-UABC) was invaluable. CONACYT Doctoral scholarships were granted to ACA-S, MV-B, and PB-M. LR-M was supported by a CONACYT postdoctoral fellowship. The picture for Figure 1d was kindly provided by our friend Ángela San Martín de Haro.




