Insights into gut fungi in pigs: A comprehensive review
Abstract
Fungi in the gut microbiota of mammals play a crucial role in host physiological regulation, including intestinal homeostasis and host immune regulation. However, our understanding of gut fungi in mammals remains limited, especially in economically valuable animals, such as pigs. Therefore, this review first describes the classification and characterisation of fungi, provides insights into the methods used to study gut fungi, and summarises the recent progress on pig gut fungi. Additionally, it discusses the challenges in the study of pig gut fungi and highlights potential perspectives. The aim of this review is to serve as a valuable reference for advancing our knowledge of gut fungi in animals.
1 INTRODUCTION
Gut microbiota is a complex microecosystem containing a large number of bacteria, fungi, archaea, viruses, and protists. It interacts with the host and plays an important role in host immunity, metabolism, intestinal endocrine function, nerve signal regulation, and so on (Fan & Pedersen, 2021; Lynch & Pedersen, 2016). With the advances in high-throughput sequencing technologies, a huge number of studies have focused on the gut microbiome. However, most of these studies have concentrated on bacteria. Fungi, another important microbial component, have been neglected for a long time (Huffnagle & Noverr, 2013; Qin et al., 2010). Fungi are far less abundant than bacteria in the gastrointestinal tract, which account for less than 1% of the total abundance of microorganisms in the human gastrointestinal tract (Nash et al., 2017; Qin et al., 2010; Sender et al., 2016). However, its cell volume is more than 100 times, and its sizes are ten times longer than bacteria (Richard & Sokol, 2019; Wang et al., 2022). In general, fungi serve as a valuable source of food and medicine, exhibit significant economic importance, and hold a vital role in balancing the ecosystem. In addition, fungi also play a crucial role in maintaining gut homeostasis and host health (Gutierrez & Arrieta, 2021; Huseyin et al., 2017a; Pérez, 2021). However, certain fungi have shown negative effects on host health, such as pathopoiesis, toxin production, allergic reactions, and ecological disruption (Iliev & Leonardi, 2017; Vignal et al., 2018; Wheeler et al., 2016; Zhang et al., 2022). At present, most studies on fungi primarily focus on their effects on human health and diseases. An imbalance of fungal compositions would lead to various human diseases, such as intestinal disorders (Lin et al., 2022; Richard & Sokol, 2019), infectious diseases (Zuo et al., 2020), neurological diseases (Hao et al., 2023; Nagpal et al., 2020), immune system diseases (Lee et al., 2022; Wang et al., 2023a), and metabolic disorders (Sun et al., 2021; Zhou et al., 2023c). However, the studies on gut fungi in animals have been rarely reported, especially in livestock.
Pigs are important livestock. Their health significantly affects economic benefit of pig production. The close relationships between gut fungi and pig health or phenotypes of economic traits have been observed. For example, a recent study found a significant reduction in the abundance of the fungus Candida tropicalis in feces of weaned piglets with diarrhea. Decreased abundance of C. tropicalis reduced its consumption of choline phosphate in the colon. Choline phosphate can promote cAMP production and water secretion by activating adenylyl cyclase, thus promoting diarrhea (Zhou et al., 2023b). Therefore, the utilisation of intestinal microbe agents has garnered considerable attention in recent decades. In addition, previous studies have reported that fungi added to pig diets can improve feed efficiency, enhance production performance, reduce pathogenic bacteria, promote animal health, and reduce the negative impact on environmental pollution (Bass et al., 2019; Elghandour et al., 2020; Liu et al., 2020; Luo et al., 2021a; Pang et al., 2022). These findings suggest that intestinal fungi play a crucial role in maintaining intestinal function and contribute to disease pathogenesis in pigs. However, the corresponding mechanisms have yet been unclear. A comprehensive understanding of the composition and functional capacities of fungi in the pig gut microbiota would greatly contribute to increasing feed efficiency, preventing diseases, and improving production performance. Furthermore, exploring how fungi in animals impact host physiology and diseases could open up new avenues for enhancing economically important traits through regulating gut fungi. Therefore, this review summarises the research progress on gut fungi in pigs in recent years and provides some valuable perspectives for future studies on animal fungi.
2 CLASSIFICATION AND CHARACTERISATION OF FUNGI
Fungi are a diverse and widely distributed group of eukaryotes (Naranjo-Ortiz & Gabaldón, 2019). The estimated number of fungal species ranges from 2.2 to 3.8 million (Hawksworth & Lücking, 2017). Until now, approximately 150,000 species of fungi have been described worldwide (Cheek et al., 2020). In previous studies, the classification of fungi primarily relied on their morphological characteristics. However, with the development of high-throughput sequencing technology, an increasing number of fungal genomes have been sequenced. In recent years, the taxonomic system of fungi has been updated using phylogenetic tree analyses based on whole-genome sequences. As a result, the number of phyla within the fungi kingdom has significantly increased from four to nine (Hibbett et al., 2007; Whittaker, 1969). Nevertheless, different classification systems resulted in different numbers of phyla (Tedersoo et al., 2018). The most prevalent fungi found in the intestinal tract are Ascomycota and Basidiomycota in all of humans, mice, pigs, and chickens (Gupta et al., 2023; Hu et al., 2023; Richard & Sokol, 2019; Robinson et al., 2022). Among them, Ascomycota is the largest group of fungi, including single-celled yeasts (Candida, Saccharomyces, etc.) and various filamentous molds (Aspergillus, Penicillium, etc.) (Radford & Parish, 1997). Basidiomycota is closely related to Ascomycota and mainly consists of fungi with large sizes, and Malassezia is a common fungus in the gut (Nash et al., 2017). In the gastrointestinal tract of most healthy people, there are ten genera which are considered as core fungal genera, including Candida (especially Candida albicans), Saccharomyces (particularly Saccharomyces cerevisiae), Penicillium, Aspergillus, Cryptococcus, Malassezia (particularly Malassezia restricta), Cladosporium, Galactomyces, Debaryomyces, and Trichosporon, following their abundances (Hallen-Adams & Suhr, 2017; Richard & Sokol, 2019; Suhr & Hallen-Adams, 2015).
Fungal life cycles are complex and their morphological transformations can have an impact on adhesion, metabolic secretion, toxin secretion, and intestinal colonisation (Ost & Round, 2023). Compared to bacteria, fungi show a lower amount and abundance in the gut. However, they play important roles in host physiology. Gut fungi take part in the metabolism of nutrients and the production of enzymes (Hoffmann et al., 2013). It also plays a significant role in host immunity as a form of antigen exposure (Underhill & Iliev, 2014). Candida and yeast are the most common fungi in the gut. Among species in Candida, C. albicans belongs to an opportunistic pathogen. Overgrowth of this fungus is often associated with a variety of diseases, especially in immunocompromised individuals. For example, a recent study found that C. albicans was significantly more abundant in the colonic mucosa of patients with ulcerative colitis. Certain strains can promote intestinal inflammation by secreting the peptide toxin candidalysin. This toxin damaged immune cells of macrophages and prompted high level of the pro-inflammatory cytokine interleukin (IL)-1β (Li et al., 2022b). However, another study indicated that the intestinal commensal fungus C. albicans contributed to aberrant inflammatory CD4 T cell responses in patients with Crohn's disease (Martini et al., 2023). This different result should be owning to the different strains of C. albicans. Furthermore, other species of Candida may also have potential disease-resistant effects. A promising finding reported the increased abundance of Candida in the gut microbiota of patients with inflammatory bowel disease (Huo et al., 2022). Among them, Candida metapsilosis M2006B has been identified as an agonist that can metabolise farnitol X receptor to relieve colitis in mice (Huo et al., 2022). To our knowledge, current studies have indicated that yeast shows numerous benefits in the gut (Jiang et al., 2017). For instance, yeast in the gut plays a role in regulating host immune homeostasis (Doron et al., 2021). Interestingly, yeast can also promote the gut barrier function and enhance the mouse social behaviours. A novel finding has suggested that yeast residing in the gut mucosa can protect the gut barrier by inducing Th cells to secrete IL-22 that has a role in anti-infection. It can also affect host behaviours through the IL-17 signalling pathway in neuronal cells (Leonardi et al., 2022).
3 THE METHODS APPLYING FOR THE STUDY OF GUT FUNGI
The composition of gut fungi, the abundance of each fungal taxon, and their potential functional capacities are common subjects that have garnered the attention of researchers. Previous studies of fungi mainly focused on the morphological observations and biochemical characterisation. To facilitate the comparison between different studies, a consistent methodology for studying gut fungi needs to be established. The current technologies used for characterising fungi mainly include culture-dependent methods and culture-independent methods.
3.1 Culture-dependent methods
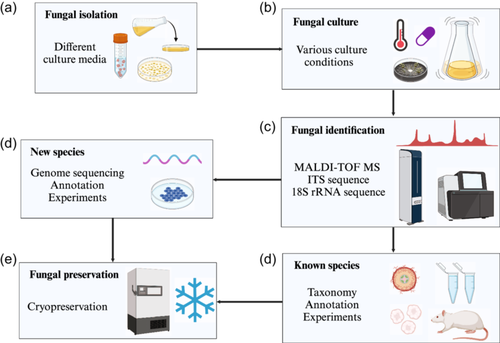
Traditionally, culture-dependent methods have commonly been used to isolate gut fungi from fecal samples. The process of culturing gut fungi primarily includes isolation, cultivation, and identification, which is similar to bacterial culture (Figure 1). However, because of the low abundance of intestinal fungi, antibiotics have always been added to the culture medium to inhibit bacterial growth in fungal isolation to improve the fungal strain isolation. For example, vancomycin is used to remove Gram-positive bacteria, while gentamicin is employed to eliminate Gram-negative bacteria. Fungal isolation highly depends on the culture medium. Different media have selectively been used to culture various fungal species. For example, Sabouraud dextrose agar is a generally used medium for fungi (Das et al., 2010; Odds, 1991; Wiwanitkit, 2010), while CHROMagarTM Candida medium has always been used for Candida isolation (Malcok et al., 2009; Tang et al., 2019). Other media commonly used for fungal isolation include malt agar, potato dextrose agar, Czapek-Dox agar, Colombia agar, Dixon agar, modified Leeming Notman agar, Banana agar, glycine-vancomycinpolymyxin B agar and yeast peptone dextrose medium, and so on (Khelaifia et al., 2023; Tidjani Alou et al., 2020) (Table 1). Additionally, temperature, culture time, pH, and oxygen content are also important environmental factors influencing the isolation and culture of fungi. Most fungi are mesophilic and the cultivation temperature varies from 25°C for filamentous fungi to 37°C for yeast (Barzee et al., 2021; Tidjani Alou et al., 2020). The culture time depends on the kinds of fungi. Fungi can grow within a relatively wide pH range, but different pH may cause different morphologies of cultured fungi (Barzee et al., 2021). As for the effect of oxygen on the culture of gut fungi, most intestinal fungi can be cultured in anaerobic or strictly anaerobic environments due to low oxygen levels in the gut. Generally, different fungi can promote or antagonise each other under specific culture conditions. Consequently, the culturability of uncultured fungi can be enhanced by improving the nutrient components of the medium, optimising the culture conditions, and manipulating the temperature, time, and pH. Classical identification methods for gut fungi primarily rely on the observation of their morphological characteristics including macroscopic and microscopic morphology, colony colour, texture, shape, and cellular morphology. With the development of technologies, MALDI-TOF MS, ITS sequencing, and 18S rRNA gene sequencing have become the main technologies used for fungal identification in fungal culture (Becker et al., 2014; Hoggard et al., 2018; Robert et al., 2020).
| Medium | Abbreviation | Purpose/description | Application fungi | Reference |
|---|---|---|---|---|
| Potato dextrose agar | PDA | Suitable for cultivation of various fungi, commonly used for preliminary screening of intestinal fungi | Various fungi, Aspergillus spp., etc. | Huseyin et al. (2017b); Raimondi et al. (2019); Yan et al. (2024) |
| Sabouraud dextrose broth | SDB | General-purpose medium suitable for fungal cultivation, rich in nutrients | Various fungi, Candida spp., Aspergillus spp., etc. | Malcok et al. (2009); Summers et al. (2021) |
| Sabouraud dextrose agar | SDA | General-purpose medium suitable for fungal cultivation, rich in nutrients | Various fungi, filamentous fungi, yeast, Candida spp., Aspergillus spp., etc. | Borges et al. (2018); Huseyin et al. (2017b); Malcok et al. (2009); Rhimi et al. (2022); Summers et al. (2019); Summers et al. (2021) |
| Dixon agar | DIX | Mainly used for the cultivation and research of specific fungi | Lipophilic fungal species, Aspergillus niger, Pseudomonas aeruginosa, Candida, etc. | Huseyin et al. (2017b); Sun et al. (2021); Yan et al. (2024) |
| Yeast peptone dextrose | YPD | A universal medium suitable for the cultivation and propagation of various yeast strains | Saccharomyces cerevisiae, Candida albicans, Cryptococcus neoformans, etc. | Davies et al. (2021); Hu et al. (2023); Summers et al. (2021); Yan et al. (2024) |
| Czapek-Dox agar | CZAPEK | Used for isolation and cultivation of Aspergillus and Penicillium strains | Penicillium, Aspergillus spp., etc. | Gouba et al. (2013); Gouba et al. (2014); Huseyin et al. (2017b); Yan et al. (2024) |
| Banana blossom agar | BABA | Suitable for the purification and cultivation of fungi | Cryptococcus neoformans/Cryptococcus gattii etc. | Khayhan et al. (2018) |
| Malt agar | MA | A commonly used fungal culture medium suitable for the growth and propagation of various fungi | Various fungi, yeasts, Aspergillus, Trichoderma, etc. | Scanlan & Marchesi (2008) |
| Rumen-fluid (RF) cellobiose agar | – | Suitable for the purification and cultivation of fungi | Suitable for the isolation of cellulose-degrading fungi from rumen fluid, etc. | Hanafy et al. (2020) |
| Modified Leeming and Notman agar | Mlna | Used for fungal culture and identification, but cannot differentiate between different types of fungi | Lipophilic fungi belonging to the genus Malassezia | Leong et al. (2019); Suzuki et al. (2022) |
| Glycine-vancomycinpolymyxin B agar | – | Selectively promote the growth of Candida species | Candida spp., etc. | Gouba & Drancourt (2015) |
| CHROMagar Candia | – | Identifies different species of Candida fungi based on colour reactions of colonies | Candida spp. | Malcok et al. (2009) |
In previous studies, the assessments of fungal diversity have mainly relied on culture-based methods. Through culture, we can discover some strains that cannot be identified by high-throughput sequencing, exam the morphological characteristics, and determine their culture conditions directly. The fungal strains isolated and identified by cultural methods can enrich the resource library of fungal strains. Simultaneously, we can also perform various in vivo and in vitro experiments on isolated strains to further explore their metabolites and physiological function properties. Furthermore, some strains that have been shown to be beneficial to the host also provide an excellent source of strains for disease prevention as well as for the development of probiotics. Thus, the cultivated fungal strains show great significance for the studies of gut fungi. A recent study isolated 12,453 fungal strains from fresh fecal samples of 135 healthy volunteers using multiple fungus-specific media. And then, A cultivated gut fungi catalog was established, including 760 fungal genomes. The catalog covered 206 species in 48 families, 69 of which were not found in existing databases and defined as unknown species. This catalog has significantly increased the genomic resources of gut fungal species (Yan et al., 2024). Nevertheless, the gut microbiota is a complex and dynamic microecosystem. Many fungi cannot be cultured due to low abundance and limited culture conditions (O'Brien et al., 2005). Previous studies have shown that the number of culturable fungal species in feces range from 102 to 107 cfu/g (Krawczyk et al., 2021). External culture is not only time-consuming and labour-intensive, but also unable to truly reflect the dynamics of intestinal microbiota. In the studies of human gut fungi, the number of fungal species cultivated was far less than that of sequenced species. For example, amplicon-based ITS1 sequencing identified over 90 fungal taxa, whereas only 34 fungal species were isolated using the culture-based approach (Gouba et al., 2014). Up to now, there are few studies on the culture of human intestinal fungi, and even fewer in other mammals (Borges et al., 2018; Huseyin et al., 2017b; Yan et al., 2024). In pigs, a previous study investigated the dynamics of the gut mycobiome in piglets at pre- and post-weaning using culture-based method, and found a significant increase in fungal burden following weaning that does not differ from adult levels (Summers et al., 2019). However, to our knowledge, there are few studies about the comparison of fungal identification between sequencing- and culture-based approaches.
3.1.1 Culture-independent methods
Due to the low efficiency and unculturability for large numbers of microbes using traditional culture methods, researchers require more efficient and rapid methods to detect the composition of gut microbes. With the rapid development of biotechnology, high-throughput sequencing has become the most commonly used method for gut microbiota analysis including bacteria and fungi. Amplicon sequencing breaks through the shortcomings of unculturable microbes. Meanwhile, this method can obtain the information, such as the evolutionary relationship of microbial community structure and the correlation between microorganisms and environments. For fungal analysis, amplicon sequencing has always been performed using fungal-specific primers to amplify the ITS or 18S region of the rRNA gene (Berbee & Taylor, 1992; Schoch et al., 2012; Tang et al., 2015). Due to the longer length and higher resolution of the ITS2 region compared to ITS1, most studies have amplified and sequenced the ITS2 region, while 18S rRNA gene sequencing is commonly used for eukaryotic analysis. The process for data analysis of amplicon sequencing is shown in Figure 2a. Of note, in order to facilitate the analysis, a series of amplicon analysis pipelines have been developed, such as AMPtk (Palmer et al., 2018), Lotus2 (Özkurt et al., 2022), PipeCraft (Anslan et al., 2017), Pipits (Gweon et al., 2015), etc. However, the amplified and sequenced regions of marker genes show significant influence in the results of gut fungal compositions. For example, Arfken et al. (2023) compared the effects of amplicon sequencing of ITS1, ITS2, and 18S rRNA gene on the taxonomic annotation and the abundance of swine gut mycobiome, and found that ITS markers were slightly superior to 18S in the identification of species, but Lichtheimia corymbifera was not amplified by ITS1 and ITS2 primers. 18S marker-based abundance estimates of taxa were more accurate than ITS-based approach. At the species level, Kazachstania slooffiae showed the most stable copy numbers, while L. corymbifera displayed significant variability across gene regions. Amplicon analysis can quickly obtain fungal taxonomy information (D'Andreano et al., 2021), but this method has been criticised for having a low phylogenetic resolution that cannot achieve the species level (Ranjan et al., 2016). Some studies have demonstrated that the copy numbers of the ribosomal DNA in fungi vary greatly at both the species and strain level, ranging from tens to thousands (Lofgren et al., 2019; Sharma et al., 2022). The variable copy numbers of ITS region were also observed by another study, which reported that the copy numbers of the ITS region in 32 S. cerevisiae strains varied from 15 to 137, and ranged from 11 to 74 in 182 C. albicans strains (Xie et al., 2023). This variation presents a challenge for the quantitative taxonomic profiling of fungal communities, so more studies would need to address the limitations of this method.

- i.
Reference genome-based approach
The reference genome-based approach shows high accuracy for fungal identification. At present, the NCBI database contains a large number of 16,258 fungal genomes at various assembly levels (before 7 September 2023). Representative software tools for this analysis mainly included Kraken2 (Wood et al., 2019), bowtie2 (Langmead & Salzberg, 2012), and CCMetagen (Marcelino et al., 2020). In this approach, clean reads of metagenomic sequencing are mapped directly to the fungal genome to obtain taxonomic information. And then, various subsequent analyses, such as functional capacity analysis, are performed. However, this method has been limited by the incomplete and scattered data of fungal genome databases. Specifically, it can only describe the fungal community with genome information, and the fungal species without genome information cannot be analysed. At the same time, fungal genomes are easy to be contaminated by bacterial genomes, resulting in a high false discovery rate.
- ii.
Marker genes-based approach
Genome and protein sequences of fungi are prone to be contaminated by bacterial sequences, leading to incorrect identification. Fungal genomes take up a lot of memory and require a lot of time and resources to operate. However, these problemes can be effectively avoided by using marker genes. As an alternative to ribosomal DNAs, a set of single-copy marker genes can be candidates for microbiome classification annotation such as bacteria (Lind & Pollard, 2021). Currently, many software tools have been developed for marker gene analysis, such as EukDetect (Lind & Pollard, 2021), TaxaTarget (Commichaux et al., 2022), and CORRAL (Bazant et al., 2023). Identification of fungi based on marker genes shows a high specificity to eukaryotes with a strong phylogenetic signal (Simão et al., 2015). The disadvantage is that some marker genes are universal, but vary across species. Furthermore, the fungi unknown at present cannot be identified, and the marker genes only occupy a small fraction of fungal genomes, which may result in low sensitivity in identifying fungi from metagenomic sequencing data.
- iii.
Database independent approach
At present, only a limited number of fungal genomes are available in the database. For many undiscovered fungi, applications of various machine learning approaches independently from databases are a practical method. This method has always used K-Mer to recognise fungal contigs or bins from assembly. And then, the identified contigs or bins are used for further analyses (Kayani et al., 2021). Currently, the pipelines or software used in this kind of analyses mainly include Tiara (Karlicki et al., 2022), EukRep (West et al., 2018), and Whokaryote (Pronk & Medema, 2022). These tools used K-Mer frequencies and linear vectors for DNA sequence classification, which do not require a database and can provide access to a larger library of genetic resources. To assess the quality of eukaryotic Metagenome-Assembled Genomes (MAGs), EUKCC is frequently used to evaluate the quality of eukaryotic MAGs (Saary et al., 2020). EukRep identifies assembled contigs from different environments, and can accurately predict 97.5% of eukaryotic sequences (West et al., 2018). In fact, EukRep has been utilised as a tool for fungal metagenomic analysis in the construction of the first pan-cancer fungal atlas (Narunsky-Haziza et al., 2022) and for investigating the diversity and functional capacity of skin fungi (Saheb Kashaf et al., 2022). Nevertheless, to most researchers, the process of this approach is relatively complex.
| Sofware/pipelines | Description | Advantages | Link |
|---|---|---|---|
| Kraken2 | Bases on k-mer approach provides a fast taxonomic classification of metagenomic sequence data, allowing greater amounts of reference genomic data to be used | High accuracy and fast | https://github.com/DerrickWood/kraken2 |
| Bowtie2 | Uses the FM index to index the reference genome | High speed, sensitivity and accuracy | https://github.com/BenLangmead/bowtie2 |
| CCMetagen | Uses the entire NCBI nucleotide collection as a reference to detect species with incomplete genome data from all biological kingdoms | Efficiently, user-friendly, and the results can be easily integrated into microbial community analysis software for streamlined and automated microbiome studies | https://github.com/vrmarcelino/CCMetagen |
| EukDetect | Aligns metagenomic sequencing reads to a database of universally conserved eukaryotic marker genes | Avoids the bacterial contamination in eukaryotic genomes | https://github.com/allind/EukDetect |
| TaxaTarget | Uses a database of eukaryotic marker genes and a supervised learning approach for training | Efficient, with higher sensitivity, and often higher precision than similar tools using marker sequence databases | https://github.com/SethCommichaux/taxaTarget |
| CORRAL | Bases on alignments to eukaryote-specific marker genes and Markov clustering | Sensitive and accurate, capable of inferring the presence of eukaryotes not included in the marker gene reference, such as novel strains | https://github.com/wbazant/CORRAL |
| Tiara | Uses k-mer counts as features for deep learning models | Performs similarly to EukRep for prokaryotes classification and outperformed it for eukaryotes classification with lower calculation time | https://ibe-uw.github.io/tiara |
| EukRep | k-mer-based strategy for eukaryotic sequence identification | Assignment to draft genomes and improvement of the quality of gene predictions | https://github.com/patrickwest/EukRep |
| Whokaryote | A random forest classifier that uses intergenic distance, gene density and gene length as the most important features | Use k-mer frequencies as features, perform almost as well as the classifiers EukRep and Tiara | https://github.com/LottePronk/whokaryote |
| Mciop | Uses fast-mapping of reads to build a comprehensive reference database of full genomes from viruses and eukaryotes to achieve maximum read usage and enable the analysis of the virome and eukaryome in each sample | Proves more effective than existing methods at abundance profiling of viruses and eukaryotes in metagenomic samples | https://github.com/smangul1/MiCoP |
| HumanMycobiome | A bioinformatics tool for the taxonomic profiling of the mycobiome directly from raw data of next-generation sequencing | Characterisation of the fungal component of microbiomes, customising the database | https://sourceforge.net/projects/hmscan/ |
| FunOmic | Two built-in fungal databases, FunOMIC-T and FunOMIC-P, integrated into an automated pipeline for taxonomic and functional profiling | Can effectively detect fungal species from shotgun metagenomic sequencing data | https://manichanh.vhir.org/funomic/ |
| FindFungi | Identifies fungal sequences in public metagenome datasets | Can be applied to any shotgun metagenomics dataset | https://github.com/GiantSpaceRobot/FindFungi |
There are several pipelines integrating multiple approaches and optimising the process of fungus identification, such as Mciop (LaPierre et al., 2019), HumanMycobiome (Soverini et al., 2019), FunOmic (Xie & Manichanh, 2022), FindFungi (Donovan et al., 2018), etc. These pipelines are friendly to beginners, but lack flexibility. For example, FunOmic has constructed a fungal marker gene (FunOmic-T) and protein (FunOmic-P) database (Xie & Manichanh, 2022). The users can input human metagenomic sequencing data directly. And then, the fungal taxonomic and functional profiling would be obtained. However, this pipeline is computational resource-consuming and fails to identify novel fungi. Among these pipelines from the literature, FindFungi has been the most widely used. It has placed 942 fungal genomes into Kraken2 for fungal identification from public metagenome datasets (Donovan et al., 2018). Generally, there is no standard process for all these current methods (Suhr & Hallen-Adams, 2015; Wickes & Wiederhold, 2018). Different pipelines can result in various outcomes, which may be the main issue in the identification of fungi (Nilsson et al., 2019). All described above highlight the importance of carefully selecting appropriate bioinformation methods based on the available data to achieve the most accurate and reliable results (Thielemann et al., 2022).
4 THE GUT FUNGI IN PIGS
4.1 The origin of pig gut fungi
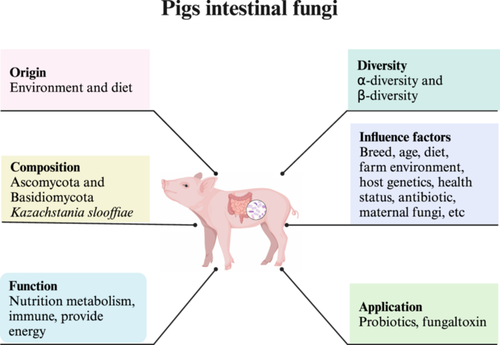
Fungi are widely found in a variety of environments. According to the existing reports, it has generally been considered that fungi in the gastrointestinal tract mainly originate from food and the environments. A study on the origin of chicken gut fungi suggested that the majority of gut fungi were derived from food and the environments by using the Source Tracker software (Robinson et al., 2022). Similarly, another study showed that the gut mycobiome of healthy mice was shaped by the environment (Mims et al., 2021). However, it remains unclear which fungi merely pass through the gut from food and the environments, and which fungi actually can establish a permanent colonisation in the gut. The sources of fungi in the gut of pigs should be similar to that in the chickens, which primarily originated from diets and the environments (Figure 3). Fungi were detected in the feces of piglets on the first day after birth (Arfken et al., 2020; Summers et al., 2019). However, whether fungi presented in the gut of newborn piglets originated from the sow's feces, breast milk, or the vaginal tract has not been reported. The maternal mycobiome plays an essential role in shaping the gut mycobiome of piglets in early life (Prisnee et al., 2022). Previous studies have shown that the gut mycobiomes of piglets was closely similar to that in feces of their maternal sows. Additionally, another study revealed that K. slooffiae in the pig gut has been maintained in the same status as their mothers until the end of the production cycle (Prisnee et al., 2023). In summary, all of these findings suggest that maternal fungal colonisation is the driving factor in piglet mycobiome development (Prisnee et al., 2022; Prisnee et al., 2023). As we have well known, pigs have the habit of digging their noses through the earth, which may lead to some fungi in the gut being original from the soil. In a previous study, the Source Tracker software was used to track whether the fungi found in pig colonic content were derived from their diets. The results found that the fungal composition in diets was completely different from that in colonic content samples (Luo et al., 2021b). This observation suggested that fungi in diets was not easy to colonise in the gut. Whether other sources, such as sow's feces, breast milk, or vaginal tract contributed to the gut fungi in newborn piglets remains unknown at present.
The compositions of gut fungi vary greatly among individuals, which has inhibited to deeply investigate their origin (Nash et al., 2017). Moreover, the temporary presence of many fungi in the gastrointestinal tract has suggested a limited capacity for colonisation. The colonisation of fungi in the intestine is influenced by various factors, including fungal abundance, intrinsic characteristics of fungi, and the unique gastrointestinal microenvironments. Some members of fungi are able to grow under various environments. Factors, such as temperature, humidity, pH, nutrients, and competition with other microbes play crucial roles in fungal growth. Different pH values, nutrient levels, oxygen concentrations, and the interactions between microbes determined the colonisation and composition of fungi in the pig gastrointestinal tract. Understanding the factors influencing fungal colonisation can provide valuable insights into the origin of pig gut fungi and the interactions between fungi and other microbes in the gut microecosystem.
4.2 The diversity of pig gut fungal compositions
Fungi have been detected in the feces of healthy piglets at birth. From birth to 2 weeks after weaning, bacterial diversity gradually increases, while fungal diversity is variable (Arfken et al., 2020) (Figure 3). A recent study in weaned and finishing pigs found that the α-diversity of fungi in the feces of pigs was increased following the age from amplicon and metagenomic sequencing analysis (Hu et al., 2023). However, in healthy people, this tendency was not observed and the α-diversity of gut fungi remains low in the adults. Increased diversity and abundance of fungi often mean the emergence of diseases (Ott et al., 2008; Raimondi et al., 2019). Therefore, low fungal diversity and abundance in piglets may be considered a sign of good health. For instance, a recent study showed that the observed species and Chao1 indices were significantly higher in diarrheal piglets than in healthy control piglets (Zhou et al., 2023a). In human studies, the composition of gut fungi varies widely between individuals, suggesting that the composition of gut fungi may be more personalised (Shuai et al., 2022). Nonetheless, a recent study has found that the β-diversity of gut fungi in different breeds of Chinese indigenous pigs was highly similar (Wang et al., 2023b). This may be due to the fact that these experimental animals were raised in the same environments and fed the same diets.
Apart from the observations mentioned above, another previous research demonstrated that the α-diversity of gut mycobiome and ASV counts were highest in the stomach tissue (Arfken et al., 2019). The high diversity of fungal composition in the stomach may be due to the stronger ability of fungi to survive in an environment with low pH, or it may be that the stomach has less competition from bacteria compared to other parts of the gut locations, but certainly it is also possible that there is a bias in the detection. The reason for this phenomenon needs to be further clarified. Another study found that the α-diversity of cecal fungi in weaned piglets was significantly higher than that in the colon (Li et al., 2020b). In addition, even with the same diet and feeding environment, pigs with similar genetic backgrounds and body weights may have completely different fungi diversity along the gastrointestinal tract (Li et al., 2022a). These different results mentioned above would need to be confirmed in future studies.
4.3 The composition of pig gut fungi
Similar to the composition of fungal phyla in the gut of humans and mice, Ascomycota and Basidiomycota are the dominant phyla of fungi in the pig gut microbiota (Arfken et al., 2019; Hu et al., 2023; Li et al., 2018) (Figure 3). At the weaning stage, the fungal composition in the gut varies greatly among pigs. However, from 24 to 35 days after weaning, the composition of piglet gut fungi became more and more similar to that of adult pigs (Arfken et al., 2019; Kong et al., 2021). K. slooffiae was a commensal fungus with a high prevalence and abundance in pigs (Figure 3). Its abundance achieved the highest level at the weaning stage. After that, it decreased to a stable level until adulthood (Davies et al., 2021). A recent study presented that K. slooffiae was the most abundant fungus in the gut of weaned piglets from seven different breeds (Hu et al., 2023). K. slooffiae could be isolated and cultured from the gastrointestinal contents and feces of weaned piglets (Summers et al., 2021). As a core fungus in the gut, K. slooffiae has been detected in all gut locations of weaned piglets (Arfken et al., 2019; Li et al., 2020b; Ramayo-Caldas et al., 2020; Summers et al., 2019). However, the abundance of this fungus is very low or even undetectable in the gut of humans and mice (Auchtung et al., 2018; Gupta et al., 2023; Li et al., 2018), indicating that there may be a unique fungal ecosystem in the gastrointestinal tract of pigs. In addition, previous studies have shown that the compositions of fungi in different gut locations of weaned piglets are significantly different (Li et al., 2020b). Recent studies found that the dominant fungi in the cecum were Saccharomycopsis, Wallemia, Bifiguratus, Mrakia, and Kazachstania, while the predominant genera in colon were identified as Kazachstania, Saccharomycopsis, Aspergillus, Scheffersomyces, and Issatchenkia (Hu et al., 2023; Li et al., 2020b).
In finishing pigs, the most abundant fungi in the gut are different in different pig breeds. In Chenghua, Yorkshire, and Tibetan pigs, Loreleia, Russula, and Candida were the core fungi whose abundances were listed in the top three. These fungi were related to the production of short-chain fatty acids (SCFAs) (Li et al., 2020a). In another study, K. slooffiae had the most abundance in the gut of Duchangda, Shaziling and Ningxiang finishing pigs, while Aspergillus was the most abundant fungal genus in Laiwu and Congjiang finishing pigs (Hu et al., 2023). Most of Aspergillus spp. are harmful, and its metabolites have also been reported to show disadvantage to the host (Kanora & Maes, 2018). High abundance of these fungal species may suggest that pigs are at a higher risk of pathological state. These differential core fungi among pig breeds should be caused by different host genetic background, diets, pig farms, and environments. Similar to that in the gut of humans, the fungal community in the gastrointestinal tract of pigs is highly variable. Compared with bacteria, the development of the mycobiome is more variable and more likely transient and environmentally driven (Tiew et al., 2020).
4.4 The factors influencing the composition of pig gut fungi
Gut microbiota is a dynamic microecosystem influenced by multiple factors, mainly including host factors and external factors. Host factors include genetics, age, sex, and health states, while external factors are mainly comprised of diets, housing, and environmental factors. Environmental factors account for a large proportion of the variations of the gut microbial composition, while host factors are always underestimated because of the interference from other factors (Rothschild et al., 2018). Early studies have shown that species, diet, host genetics, environments, age, sex, lifestyle, bacteria, antibiotics and other factors can impact the composition of the gut fungi in mammals (Lai et al., 2019). However, few studies have definitively established the extent to which these factors influence the variation of gut fungi. This is likely due to the low abundance of fungi in the gut, as well as the fact that many studies have shown the instability of fungal colonisation in the gut. Furthermore, the core fungi present in the gut are still unknown and require further elucidation through relevant studies. Existing studies have shown that various factors, such as pig breed (Hu et al., 2023), age (Hu et al., 2023), diets (Luo et al., 2021b), farm environments (Ramayo-Caldas et al., 2020), host genetics (Ramayo-Caldas et al., 2020), health status (Kong et al., 2021), antibiotics, and maternal fungi (Prisnee et al., 2022), have significant impacts on the composition of gut fungi (Figure 3). Fungi are not relatively stable between and within individuals. Li et al. (2020a) reported for the first time that the fungal composition of feces in different pig breeds varied at both phylum and genus level. A more recent study reported that the fungal composition of colonic contents varied in different breeds (Wang et al., 2023b). Summers et al. (2019) characterised the changes in the gut fungal community of piglets throughout the whole-weaning period (seven time points) using 20 piglets from three litters by ITS sequencing and culture-dependent method. Their results confirmed that both the dominant fungal species and their abundances in piglets were changed during the whole-weaning period. The changed structure of the gut mycobiome might be caused by dietary changes, immune system maturation, and weanling stress. Especially, a previous study showed that dietary carbohydrates caused the differences in the composition of colon fungi in piglets (Luo et al., 2021b). The composition and diversity of fungi were also distinct in different parts of gastrointestinal tract (Li et al., 2020b; Li et al., 2022a). Furthermore, the utilisation of antibiotics can cause the dysbacteriosis of gut microbiota, which further influences the composition of gut fungi because of the complex interaction between gut bacteria and fungi (Hedin et al., 2022; Spatz et al., 2023). Previous studies have demonstrated that antibiotics alter the bacterial composition of piglets at birth and have some impact on fungi as well (Prisnee et al., 2022). At the same time, researchers also found that the colonisation of fungi in the maternal body was a driving factor for fungal development in piglets (Prisnee et al., 2022). For other factors, farm and pig ages explained 44% of the fungal variation (Ramayo-Caldas et al., 2020). The extent of these factors on gut fungal composition was not consistent in different studies. This instability in the compositions hampers the progress of the studies about gut fungi. Understanding the effects of different influencing factors on intestinal fungi is of great theoretical and potential practical significance for implementing interventions that target gut fungi to improve the growth and health of pigs.
4.5 The function capacities of pig intestinal fungi
Fungal colonisation in the gastrointestinal tract not only affects the physiological functions of the intestinal tract, but also influences the physiological functions of other organs of the host, and has important correlations with many diseases. In the gastrointestinal tract, fungi may regulate host energy metabolism by interacting with bacteria, or competing with each other (Seelbinder et al., 2023). The effect of gut fungi on immune function is similar to that of bacteria. Some fungi affect host health by producing metabolites to mediate immune response (Belvoncikova et al., 2022; Wu et al., 2021). Some fungi colonising in the gut are treated as immunologically active components can influence host immunophysiology through both commensal and synergistic effects with intestinal bacteria (Zeng et al., 2023).
To date, there have been limited reports on the roles of gut fungi in pigs. A study in pigs showed that fungi could degrade dietary carbohydrates in the colon, but the mechanism was unknown (Luo et al., 2021b) (Figure 3). The other two studies have indicated that fungi in the gut of weaned piglets and fishing pigs were related to the production of SCFAs. SCFAs may indicate the interaction between fungi and bacteria (Li et al., 2020a; 2020b). K. slooffiae shows probiotic characterisation and is a potential probiotic (Arfken et al., 2019). Previous studies discovered that K. slooffiae could rapidly colonise in all gut locations of weaned piglets and was positively correlated with SCFAs, especially butyric acid, acetic acid, and propionic acid (Urubschurov et al., 2017, 2018). Several reports have suggested that K. slooffiae showed positive interactions with gut bacteria, such as Prevotella and Lactobacillus (Arfken et al., 2019, 2020). Moreover, K. slooffiae may also be a novel protein source, which provides amino acids that benefit the growth of both microorganisms and piglets as an energy source (Summers et al., 2021; Urubschurov et al., 2017). A recent study found that K. slooffiae can promote glycolysis of pig intestinal epithelium through de-succinylation modification of lysine (Hu et al., 2023). Gut fungi were also involved in the health of pigs. A recent study revealed that decreased abundance of C. tropicalis leaded to reduced phosphocholine consumption in diarrheal piglets. Phosphocholine accumulation in the colon subsequently drove water efflux through decreasing fluid absorption mediated by adenylyl cyclase activation, and finally resulted in diarrhea (Zhou et al., 2023a). As mentioned above, nutrient metabolism and immunity are two most key functions of fungi in the pig gut. Further explorations about the roles of porcine intestinal fungi in vivo can provide new knowledge and strategies for improving energy metabolism and growth of pigs by regulating the composition of gut fungi. As an example, β-glucan is a major component of fungal cell walls. It plays crucial roles in keeping the intestinal microecological balance and the interactions between fungi and hosts. Although there are few studies about the fungal β-glucan in the pig gut, and its roles in pig physiology are largely unknown, the extensive roles of β-glucan in immune responses of human gut suggest great research values in pigs.
4.6 The application of pig intestinal fungi
In humans, the main methods for effectively regulating intestinal fungi include fecal microbiota transplantation, antifungal medications, antibiotics, dietary interventions, and probiotics (Zhang et al., 2022). Recent years, the utilisation of fungi in pig production has been increasing quickly. Probiotics are a group of microorganisms that can provide beneficial effects on the host (Hill et al., 2014). Most of the probiotics currently used belong to bacteria, such as some species of Lactobacillus and Bifidobacterium. Although it has been used less frequently than bacterial probiotics, fungal probiotics have been studied in more comprehensive areas. S. cerevisiae, Candida yeast, Brady yeast, and Pichia are among the fungal probiotics that have been extensively studied. In livestock production, these fungal probiotics have played important roles in immune regulation, metabolic activity, regulation of intestinal microbiome, and removal of oxygen (Elghandour et al., 2020). The European Commission approved S. cerevisiae as a feed additive for pigs as an animal nutrition additive, and classified it into the additive category of “Animal Technical Additives” and the functional group as “Intestinal Microbiota Stabiliser”. Adding S. cerevisiae to pig diets can enhance mucosal immunity by increasing the activity of immunoglobulin M and immunoglobulin A against pathogenic bacteria, promoting intestinal development and function, improving the excretion of mycotoxins, regulating gut microbiota, and reducing diarrhea after weaning (Elghandour et al., 2020; Hill et al., 2014; Jiang et al., 2015; Kogan & Kocher, 2007; Shen et al., 2009; Xu et al., 2018) (Figure 3). These findings have demonstrated the potential of S. cerevisiae as a beneficial addition to pig diets, with impacts on multiple aspects of pig health and performance. Interestingly, the studies also have found that the supplementation of S. cerevisiae YST2 can significantly decrease methane production in pig intestine (Gong et al., 2018). This would benefit the protection of the environment by reducing methane emissions. Supplementation of active S. cerevisiae Actisaf Sc 47 to newborn piglets from birth to weaning improved average daily feed intake and average daily weight gain of piglets, and altered the microbial composition in the hindgut of piglets by positively interacting with beneficial bacteria from Actinobacteria and Firmicutes (Kiros et al., 2018). Compared to bacteria, one advantage of using yeast as probiotics is that yeast is not affected by antibiotics (Hatoum et al., 2012). Therefore, probiotic fungi not only become an alternative to antibiotics, promote the growth of animals, but also prevent and treat some diseases.
Preventing the proliferation of harmful fungi in pigs is another crucial aspect in the application of fungi. Pigs that consume diets contaminated by molds, such as Aspergillus, Penicillium, and Fusarium, etc., have been reported to exhibit various toxic symptoms caused by secondary metabolites of mycotoxins, including aflatoxin, citrinin, F-2 toxin, and fumonisin, which are produced by the growth of these molds (Bryden, 2012; Holanda & Kim, 2021) (Figure 3). The presence of toxins in feed can increase the translocation of bacteria; and impair the immune response making animals more susceptible to infectious diseases (Pierron et al., 2016). Furthermore, these toxins can also easily cause human food poisoning through the food chain. In general, harmful fungal metabolites in the gut at low concentrations are not sufficient to cause obvious diseases, but may lead to economic losses through adverse effects on pig growth, production performance, and immunity. Therefore, we need to continuously strengthen the studies on harmful fungi, and clarify the deleterious effect of these fungi and their metabolites on the host. This would help us to better prevent their harmful effects on pigs.
5 CURRENT CHALLENGES
Gut fungi have received less attention compared to other gut microorganisms, particularly bacteria. Further investigation and understanding of gut fungi are necessary. However, numerous challenges hinder the progress in the study of pig gut fungi, primarily related to the changes in methods and applications. Addressing these obstacles will be crucial for advancing our understanding of the roles of pig gut fungi in host physiology and developing effective strategies to promote gut health.
The main challenge in the study of pig gut fungi is their relatively low abundances. Culturing fungi in laboratory conditions has been proven difficult, and more suitable cultivation methods should need to be developed. As for the bioinformatic analysis of metagenomic sequencing data of gut fungi, many pipelines and software that have always been designed for the studies of bacteria are not suitable for fungi. Consequently, only a small number of fungi have been identified using this approach. In terms of amplicon analysis, the 18S rRNA gene sequencing has shown low accuracy for the identification and annotation of gut fungi and the ITS discrimination is insufficient. Mycobiome composition shows highly heterogeneous in different individuals. Some fungi can change their morphology under different conditions, such as transitioning between mycelium and yeast. Unfortunately, such variations are often not recognised as the same fungus in ITS sequencing analysis. At the same time, cell walls of fungi are thick, which makes it difficult to extract fungal DNA. Additionally, the reference genome database has yet been absent because of the relatively limited number of fungal genomes available. This deficiency significantly restricted the mining of metagenomic sequencing data of gut fungi especially under the condition of low abundance in the gut. Therefore, how to obtain the composition and functional capacity information of gut fungi from metagenomic sequencing data more effectively urgently needs to be solved in future studies.
In addition, there are few reports on the applications of porcine gut fungi because of the limited number of studies on porcine gut fungi. Furthermore, compared to that in the gut bacteria, the progress on gut fungi is far slowly and less in-depth. The effects of porcine gut fungi on host physiology and immunity are unclear. The interactions of fungi with bacteria in the gut have been largely unknown, and the cultivation of fungi is not quite enough. Furthermore, the origin and function capacity of core fungi in pig intestines are unknown. The significance of fungi in gut habitats is often overlooked, even though they play various roles in different environments. In existing studies, diseases are significantly correlated with fungi, but their causality has yet been unclear and is worth exploring further. Additionally, besides the fungi themselves, the metabolites or signalling molecules produced by intestinal fungi are also relevant to the host. However, there few studies that have concerned this.
6 PERSPECTIVES
The study of gut fungi has been rapidly developing, but many fundamental and essential biological questions about gut fungi remain unanswered. For example, the composition of gut fungi in pigs is still unclear and it is largely unknown which fungi are stably colonised in the gastrointestinal tract of pigs. Further exploration is necessary to distinguish whether intestinal fungi are colonised or derived from the diets and environments. For the culture of gut fungi, how to optimise the culture conditions to isolate and culture more valuable fungi requests to be explored. At the same time, more bioinformatic tools need to be developed to dig up more fungi from high throughput sequencing data. Fungi not only affect the growth of their hosts, but are also closely related to many diseases. Unlike bacteria, fungi are generally more susceptible to environmental influences. However, analysing feces and intestinal contents alone does not provide a comprehensive understanding of the real composition of gut fungi in the host. Instead, studying the fungal composition in the intestinal mucosa may yield more meaningful results. To comprehensively understand the composition of gut fungi, it is crucial to employ more rigorous experimental designs, utilise large sample sizes, and conduct longitudinal studies. Additionally, high-throughput sequencing of gut microbiota from different segments of the intestine is necessary.
Following the progress on the studies of gut fungi, there is a requirement to explore the application of gut fungi in improving pig production performance. Firstly, the relationship between fungal composition in the gut and pig phenotype needed to be elucidated. For example, analyzing the association between gut fungi and health status of pigs can provide a better understanding of the complexity of fungi and their role in diseases. Feed cost accounts for more than 60% of the total cost in pig production (Banerjee et al., 2020). Therefore, it is crucial to investigate whether some fungi in the gastrointestinal tract affect the nutrient absorption and energy conversion in pigs. Future studies should focus on exploring the functions of gut fungi in pigs, such as the functional capacities in cellulose degradation, protein digestion, and enzyme production, to optimise pig feed formulations and enhance nutrient utilisation efficiency. Additionally, the integrations of culturomics, metabolomics, genomics, and macro-transcriptomics would promote the characterisation of functional capacities of gut fungi. Furthermore, studying the role of fungi in the pig gastrointestinal tract within specific intestinal axes, such as the brain-gut axis and intestine-lung axis, can provide valuable insights into their impact on pig health and production performances. In addition, whether the metabolites derived from fungi should affect host behaviours, such as aggressiveness, infanticide, anxiety, pica, and so on is also worth to be investigated in depth. Understanding the mechanism behind the relationship between gut fungi and host physiology and discovering the causal relationship are of great significance for both pig production and human health. Moreover, it is worth studying diseases caused by fungi in the intestine from an immunological perspective (Ost & Round, 2023; Zhang et al., 2022) because this can help to prevent diseases in pigs proactively and promote the development of probiotic preparations. Pigs serve as a good study model for the study of human diseases (Lunney et al., 2021). Understanding the dynamics, succession, origin of the intestinal fungal community in animals, as well as its relationships with host physiological phenotype, immunity, and metabolism will help us to develop new methods for manipulating the intestinal fungal community to improve host health and production performance in the future.
7 CONCLUSION
In conclusion, fungi in the pig gut are crucial components of gut microecosystem and are involved in pig health and production traits. Although many challenges have still existed in the studies of pig gut fungi, more and more findings have uncovered its interactions with gut microbiota, and identified its correlations with host health, diseases, and economic traits, such as feed efficiency in pigs. By using multi-omics approaches, there are significant potentials to identify key fungi involved in pig health and production traits, and to facilitate to elucidate their mechanisms. Therefore, systematically investigating the composition, and the functional capacities of gut fungi would not only benefit for the pig health and production performances, but also provide a reference for the study of human gut fungi by using pig as an animal model.
AUTHOR CONTRIBUTIONS
Guanyue Wei designed, wrote and revised the manuscript. The author contributed to the article and approved the submitted version.
ACKNOWLEDGEMENTS
The author appreciates professor Congying Chen in the National Key Laboratory of Pig Genetic Improvement and Germplasm Innovation, Jiangxi Agricultural University for his revisions on this article. This work did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CONFLICT OF INTEREST STATEMENT
The author declares no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.




