Negative energy balance affects perinatal ewe performance, rumen morphology, rumen flora structure, and placental function
Abstract
This study investigated the effects of negative energy balance (NEB) on perinatal ewes, with a focus on changes in growth performance, serum biochemical parameters, rumen fermentation, ruminal bacteria composition, placental phenotype-related indicators, and expression levels of genes related to placental function. Twenty ewes at 130 days of gestation were randomly allocated to either the positive energy balance (PEB) or NEB groups. In the experiment, ewes in the PEB group were fed the same amount as their intake during the pre-feeding baseline period, while ewes in the NEB group were restricted to 70% of their individual baseline feed intake. The experiment was conducted until 42 days postpartum, and five double-lamb ewes per group were selected for slaughter. The results demonstrated that NEB led to a significant decrease in body weight, carcass weight, and the birth and weaning weights of lambs (P < 0.05). Additionally, NEB caused alterations in serum biochemical parameters, such as increased non-esterified fatty acids and β-hydroxybutyrate levels and decreased cholesterol and albumin levels (P < 0.05). Rumen fermentation and epithelial parameters were also affected, with a reduction in the concentrations of acetic acid, butyric acid, total acid and a decrease in the length of the rumen papilla (P < 0.05). Moreover, NEB induced changes in the structure and composition of ruminal bacteria, with significant differences in α-diversity indices and rumen microbial community composition (P < 0.05). Gene expression in rumen papilla and ewe placenta was also affected, impacting genes associated with glucose and amino acid transport, proliferation, apoptosis, and angiogenesis (P < 0.05). These findings screened the key microbiota in the rumen of ewes following NEB and highlighted the critical genes associated with rumen function. Furthermore, this study revealed the impact of NEB on placental function in ewes, providing a foundation for investigating how nutrition in ewes influences reproductive performance. This research demonstrates how nutrition regulates reproductive performance by considering the combined perspectives of rumen microbiota and placental function.
1 INTRODUCTION
Adequate nutrition is critical for both the mother and the fetus in the perinatal period. The energy requirements of sheep can rise dramatically during the perinatal period, so the organism undergoes significant energy mobilization to adapt to the challenges posed by labor, lactation, and metabolic dysfunction. However, the growth of multiple fetuses reduces rumen capacity, frequently leading to insufficient feed intake and resulting negative energy balance (NEB) in periparturient ewes. This NEB arises from a mismatch between increasing energy demands for lactation and limited energy intake, resulting in reduced performance in ewes (Du et al., 2010).
To explore the mechanisms of NEB, pathological models are often induced in ewes during the perinatal period through restricted feeding or fasting protocols (Gonzalez et al., 2011). The rumen, a unique digestive organ of ruminants, is highly sensitive to dietary changes. Inadequate nutrient intake in late gestation can perturb the rumen microbiota and epithelium. For instance, reducing feed intake to 70% of ad libitum intake has been shown to decrease rumen volatile fatty acid (VFA) absorption, a key function of the rumen epithelium (Hu et al., 2018). Likewise, 48 h of fasting impaired both VFA absorption and the barrier function of the rumen epithelium in sheep. As the rumen is critical for nutrient supply and overall health, these disruptions to the structure and function of the rumen epithelium may mediate some impacts of peripartal NEB (Doreau et al., 1997; Gabel & Aschenbach, 2002).
The rumen is a complex microbial environment comprising four major groups: bacteria, fungi, archaea, and protozoa, of which bacteria are the dominant group of rumen microorganisms (1010–1011 bacteria/mL rumen fluid) (Wright & Klieve, 2011). The major bacterial phyla are Bacteroidetes, associated with starch breakdown, and Firmicutes, linked to carbohydrate fermentation. Key rumen bacterial genera include Butyrivibrio, Prevotella, and Ruminococcus. Ruminococcus and Butyrivibrio are highly abundant in Bacteroidetes, while Prevotella is the predominant Firmicutes genus (Stevenson & Weimer, 2007). This core microbiota is adaptable and maintains a dynamic equilibrium based on diet (Weimer, 2015). Ruminal microorganisms play critical roles in the digestive metabolism of the diet. However, despite their essential functions, the effects of NEB on the rumen microbiome are largely unknown. Further characterization of rumen microbial shifts during NEB will provide insight into how this crucial microbial community responds to nutritional stress.
The placenta, a critical organ in pregnancy, serves as the primary connection between the developing fetus and the maternal uterine wall. It facilitates critical exchange of nutrients, oxygen, and waste (Cindrova-Davies & Sferruzzi-Perri, 2022). In ruminants, the placenta features an intricate cotyledonary structure characterized by numerous button-like cotyledons that play a crucial role in exchange process (Sarwalia et al., 2021). Gut microbiota and their metabolites, such as short-chain fatty acids (SCFAs), have been shown to influence placental function. For example, SCFAs contribute to placental integrity and vascularization (Wallace et al., 2019). However, the effects of the rumen microbiome specifically on ruminant placental and cotyledon development remain unclear.
This study examined changes in the rumen, rumen microbiota, and placental function in perinatal ewes experiencing nutritional stress and NEB. This study provides a theoretical basis and practical experience for improving the health of periparturient ewes, safeguarding their postpartum health, and improving the production efficiency and economic benefits of farms through nutritional control techniques. This research examine how nutrition regulates reproduction from the integrated perspectives of rumen microbiota and placental function.
2 MATERIALS AND METHODS
2.1 Management of animals and experimental design
The research site was located at the Nantong Ruipeng Farm (31°27′N–31°51′N, 121°09′E–121°54′E), situated in a subtropical monsoon climate, with an altitude of 3.5 to 4.5 m. The annual precipitation was 1128.9 mm, and the average annual temperature was 16.5°C. The sheep pens were kept clean and regularly disinfected, with natural light-dark cycles maintained and a temperature held at approximately 25°C. Twenty Hu sheep were selected with a body weight of (53.7 ± 2.77) kg, aged 2–3 years, and raised individually. Prior to mating, the ewes were regularly vaccinated and dewormed with albendazole. Then, four rams were assigned to mate naturally with the twenty ewes, and the conception days were calculated based on the time each ram spent in each ewe's enclosure.
During the pre-feeding period, all Hu sheep were allowed to feed freely on the diet shown in Table 1, with food provided at set times and quantities each day according to NRC (Nutrient Requirements of Small Ruminants, 2007) recommendations. The leftover feed was cleaned out of the troughs and weighed the next day to accurately calculate daily feed intake. This ensured the feed amounts met the ewes' nutritional requirements. On the 130th day of pregnancy, the sheep were randomly divided into two groups: positive energy balance (PEB) group or NEB group. During the trial period, PEB group continued to be fed according to the intake levels during the pre-feeding period, while the NEB group of Hu sheep was restrictively fed 70% of their pre-feeding intake level. The experiment lasted from the 130th day of pregnancy to 42 days after birth. No ewes were recorded as refusing to eat during this time.
| Items | Pregnancy period diets | Lactation period diets |
|---|---|---|
| Diet ingredients, % DM | ||
| Cron | 18.0 | 20.0 |
| Premix | 5.0 | 5.0 |
| Rice husk | 25.0 | 19.0 |
| Soybean husk | 35.0 | 22.0 |
| Corn germ meal | 10.0 | 16.0 |
| Soybean meal | 3.0 | 16.0 |
| Rice bran | 4.0 | 4.0 |
| Methionine | 0.0 | 0.0 |
| Total | 100 | 100 |
| Chemical composition | ||
| ME (Mcal, kg) | 2.16 | 2.25 |
| Crude protein | 10.50 | 13.62 |
| Fat, % DM | 3.14 | 12.22 |
| Neutral-detergent fiber, % DM | 46.66 | 42.28 |
| Acid-detergent fiber, % DM | 32.07 | 29.03 |
| Ash, % DM | 8.05 | 3.24 |
| Ca, % DM | 1.06 | 1.04 |
| P, % DM | 0.46 | 0.48 |
- Abbreviations: ADF, acid detergent fiber; DE, digestible energy; DM, dry matter; ME, metabolic energy; NDF, neutral detergent fiber.
2.2 Sample collection
Blood samples were collected from the ewes on the day of parturition as well as at 7 and 42 days postpartum. The blood samples were centrifuged for 10 min at 3000 rpm to extract the serum, which was placed into an Eppendorf tube and kept at −20°C for further analysis. The serum biochemical were evaluated by an automated analyzer (AU 5800, Olympus).
At 42 days postpartum, ewes and one of their twins were chosen for slaughter (n = 5 per treatment) 3 h after the morning of feeding. The live weight and carcass weight of each lamb were recorded prior to slaughter. Following slaughter, samples of rumen chyme were collected and filtered through four layers of gauze to obtain rumen fluid. Five milliliters of rumen fluid were preserved with the addition of 1 mL 25% (wt/vol) metaphosphoric acid and stored at −20°C for later analysis of volatile fatty acid (VFA) concentrations. Additionally, 10 mL of the filtered rumen fluid was stored at −20°C for 16 S rRNA gene sequencing. The ruminal epithelium was removed by sharp dissection from the ventral sac and washed three times in ice-cold 0.9% saline solution. These ruminal epithelia were promptly frozen at −80°C for RNA extraction. For histomorphometry analysis, sections of the ruminal epithelium measuring 0.5 × 0.5 cm were collected and preserved with 4% paraformaldehyde.
After delivery, placentas were collected. Each placenta was cleaned and weighed using a precision digital scale with an accuracy of 0.01 g. The cotyledons were then carefully separated and counted to determine the total number per placenta. Additionally, the largest diameter of each cotyledon was measured using a digital caliper with a graduation of 0.05 mm.
2.3 Analysis of rumen tissue morphology and rumen fermentation parameters
According to the approach described by Feng & Gao, (Feng & Gao, 2010) the VFA concentration in the rumen fluid was evaluated. VFAs were analyzed using gas chromatography (GC-14B, Shimadzu) with a capillary column (30 m× 0.32 mm × 0.25 μm film thickness). The column temperature was set to 130°C, the injector temperature to 180°C, and the detector temperature to 180°C. This GC system has been utilized in previous experiments for VFA determination (Wang et al., 2023). Crotonic acid should serve as the internal standard.
The rumen morphology was evaluated using the approach given by Ye (Ye et al., 2016). Briefly, rumen tissues were washed in PBS and fixed in 4% formaldehyde solution before being embedded in paraffin and stained with hematoxylin and eosin. Measurements of morphological features were taken at 40x objective magnification. For each lamb sample per treatment group, five slides were prepared with two photos per slide. The morphological criteria were quantified from the images using Image-Pro Plus software (Media Cybernetics) as previously described (Steele et al., 2011).
2.4 DNA extraction and high-throughput sequencing
The sample of lamb rumen content was taken for microbial profile study and kept at −80°C until analysis. Using MN NucleoSpin 96 Soi, the DNA was extracted. The V3-V4 portions of the bacterial 16 S RNA gene were amplified using primers 338 F (5′- ACTCCTACGGGAGGCAGCA-3′) and 806 R (5′- GGACTACHVGGGTWTCTAAT-3′) and the cycle conditions used by Hu et al. (2018) At Biomarker Technologies, high-throughput pyrosequencing was used to sequence PCR products on the Illumina MiSeq platform. Raw sequencing reads were filtered using Trimmomatic V0.33 software. The Cutadapt 1.9.1 program was used to locate and eliminate primer sequences, resulting in clean reads devoid of primer sequences. To obtain the final accurate values, they were categorized into operational taxonomy units (OTUs) with a 97% degree of similarity. Using the UCHIME V4.2 tool, the chimeric sequences were detected and removed.
2.5 RNA isolation, cDNA synthesis, and qPCR
The TRIzol method described by Liang was used to extract total RNA from ruminal tissue (Liang et al., 2020). The RNA concentration was then identified using a NanoDrop 2000 Spectrophotometer (Thermo Scientific). The absorption ratio (260/280 nm) of all samples was between 1.8 and 2.1, indicating high RNA purity.
Total RNA was used for reverse transcription using a PrimeScript® RT reagent kit with gDNA Eraser (Takara Bio). The expression of target genes was determined on the QuantStudio 5 Real-time PCR Instrument (Applied Biosystems) with fluorescence detection of AceQ qPCR SYBR Green Master Mix (Vazyme Biotech) under the standard program. The gene expression data were normalized to the housekeeping gene (actin β, ACTB) using the 2−∆∆CT method. The primers and amplicon sizes of the genes are shown in Supporting Information S1: Table S1.
2.6 Statistical Analysis
Independent samples T test was performed using Duncan's test on the fundamental data (rumen papillae, thickness, Total volatile fatty acid concentration, and pH). The data were analyzed as the mean values standard error of the mean. P < 0.05 was judged statistically significant. The analysis employed SPSS 25.0 (IBM Corp.).
3 RESULTS
3.1 Growth performance and organ index in perinatal ewes and lamb
An adverse energetic equilibrium notably diminished the body weight and carcass weight of nursing ewes (P < 0.05). There was no significant effect of NEB on organ index of ewes (P > 0.05). Moreover, NEB significantly decreased the birth weight, weaning weight, average daily gain, live weight, and carcass weight of the lambs (P < 0.05) (Table 2). In summary, the NEB compromised both ewe and lamb performance, reducing ewe body reserves and impairing lamb growth. This highlights the importance of meeting nutritional needs during lactation to support both optimal ewe and lamb health and productivity.
| Items | PEB | NEB | P-vaules |
|---|---|---|---|
| Perinatal ewe | |||
| Body weight, kg | 51.2 ± 4.09 | 44.14 ± 2.49 | 0.015 |
| Carcass weight, kg | 19.7 ± 2.35 | 16.28 ± 1.62 | 0.028 |
| Liver, kg | 1.02 ± 0.18 | 0.99 ± 0.18 | 0.815 |
| Spleen, kg | 0.07 ± 0.02 | 0.06 ± 0.02 | 0.534 |
| Lung, kg | 0.64 ± 0.17 | 0.51 ± 0.14 | 0.202 |
| Kidney, kg | 0.14 ± 0.02 | 0.13 ± 0.02 | 0.411 |
| Heart index, % | 0.59 ± 0.13 | 0.51 ± 0.15 | 0.425 |
| Liver index, % | 2.05 ± 0.16 | 2.27 ± 0.53 | 0.402 |
| Spleen index, % | 0.14 ± 0.03 | 0.15 ± 0.05 | 0.823 |
| Lung index, % | 1.30 ± 0.29 | 1.16 ± 0.35 | 0.518 |
| Kidney index, % | 0.28 ± 0.04 | 0.29 ± 0.03 | 0.579 |
| Lamb | |||
| Birth weight, kg | 3.82 ± 0.58 | 3.52 ± 0.70 | 0.029 |
| Weaning weight, kg | 13.73 ± 3.22 | 12.43 ± 2.35 | 0.023 |
| ADG, kg | 0.22 ± 0.23 | 0.20 ± 0.43 | 0.031 |
| Live weight, kg | 16.53 ± 3.65 | 11.28 ± 2.99 | 0.003 |
| Carcass weight, kg | 8.72 ± 1.99 | 5.64 ± 1.60 | 0.005 |
| Heart, kg | 0.10 ± 0.04 | 0.08 ± 0.02 | 0.563 |
| Liver, kg | 0.33 ± 0.12 | 0.23 ± 0.06 | 0.025 |
| Spleen, kg | 0.03 ± 0.01 | 0.02 ± 0.01 | 0.18 |
| Lung, kg | 0.26 ± 0.11 | 0.20 ± 0.05 | 0.635 |
| Kidney, kg | 0.06 ± 0.02 | 0.05 ± 0.00 | 0.072 |
| Heart index, % | 0.59 ± 0.13 | 0.76 ± 0.10 | 0.017 |
| Liver index, % | 1.94 ± 0.33 | 2.07 ± 0.25 | 0.556 |
| Spleen index, % | 0.17 ± 0.02 | 0.20 ± 0.05 | 0.163 |
| Lung index, % | 1.53 ± 0.34 | 1.81 ± 0.27 | 0.020 |
| Kidney index, % | 0.38 ± 0.01 | 0.42 ± 0.05 | 0.774 |
- Abbreviations: ADG, average daily gain; NEB, negative energy balance; organ index, organ weight/live weight *100%; PEB, positive energy balance.
3.2 Serum biochemical parameters of perinatal ewes
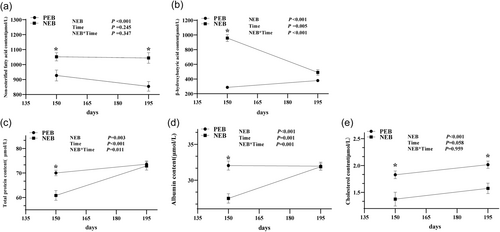
NEB significantly increased serum non-esterified fatty acids (NEFA) and β-hydroxybutyrate (BHBA) concentrations (Figure 1a,b), indicating increased fat mobilization and ketone body production, clear biomarkers of NEB. NEB significantly decreased serum total protein, cholesterol, and albumin levels in sheep (Figure 1c–e) (P < 0.05), indicating impaired liver function and nutrient deficiencies resulting from the energy deficit. At the end of lactation, although serum total protein, albumin, and BHBA levels recovered to normal (Figure 1c,d), NEFA levels in the NEB group remained significantly higher, while cholesterol levels were still significantly lower than those in the PEB group (P < 0.05), suggesting the potential for enduring metabolic sequelae in ewes exposed to prolonged and severe energy insufficiency during peak lactation.
3.3 Rumen fermentation and ruminal epithelium parameters
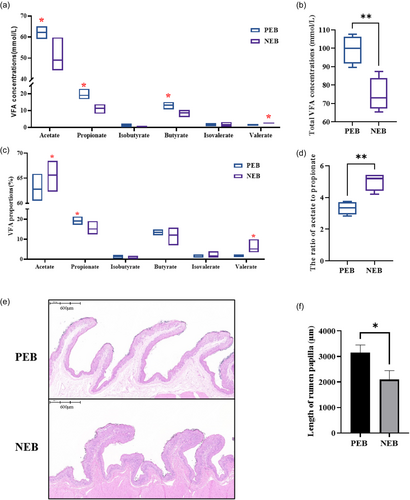
NEB in periparturient ewes resulted in significant reductions in ruminal concentrations of acetate, propionate, butyrate, and total volatile fatty acid levels (P < 0.05) (Figure 2a,b). In contrast, the percentage of acetate within the rumen was significantly increased, while the proportion of propionate was decreased in response to NEB (P < 0.05) (Figure 2c). Additionally, the ratio of acetate to propionate was markedly elevated under conditions of NEB (P < 0.05) (Figure 2d). Morphological changes in the ruminal epithelium were also observed, with NEB eliciting significant decreases in rumen papilla length (P < 0.05) (Figure 2e,f). The morphological changes likely impair absorptive capacity and VFA uptake.
3.4 NEB changed the structure and composition of ruminal bacteria in ewes
To explore the mechanisms behind the effects of NEB on rumen fermentation, we performed 16 S genome sequencing to study the changes in rumen bacterial communities. Sequencing yielded total of 1,262,053 clean reads, with an average of 229,464 reads per sample. The curves for each group were widespread along the horizontal axis, with smooth curves in the vertical direction, suggesting that sequencing saturation and maximum coverage had been reached (Supporting Information S1: Figure S1).
Analysis of alpha diversity showed that NEB significantly reduced the ACE, Shannon, and Chao1 indices of the ruminal microbiota (P < 0.05) (Figure 3a–c). Venn diagrams displayed 715 shared OTUs between groups and 294 and 219 unique OTUs in the PEB and NEB groups, respectively (Figure 3d). PCoA showed distinct clustering and clear separation of microbial composition between the two groups (P < 0.05) (Figure 3e).
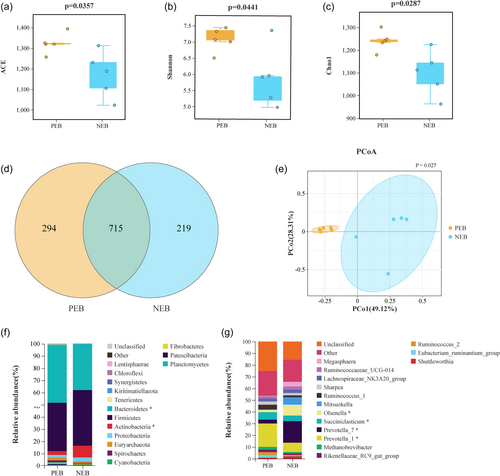
PCoA results indicated that NEB altered the composition of rumen bacterial communities in nursing ewes. Thus, rumen bacteria were further analyzed at various taxonomic levels to investigate the impact of NEB on the abundance of rumen bacterial taxa. At the phylum and genus levels, the key findings were shown in Figure 3. predominant phyla were identified across both groups: Bacteroidetes, Firmicutes, Actinobacteria, and Proteobacteria (Figure 3f). Comparative analysis indicated that NEB significantly reduced the abundance of Bacteroidetes and increased the Actinobacteria levels (P < 0.05). Further genus-level investigation was undertaken to delineate differences between groups (Figure 3g). The relative abundances of Prevotella_1 and Succimiclasticum were significantly decreased in the NEB group. In contrast, the relative abundances of Prevotella_7 and Olsenella were significantly elevated in the NEB group (P < 0.05).
3.5 Effect of NEB on mRNA expression related to ruminal papilla growth and transporters in ewe
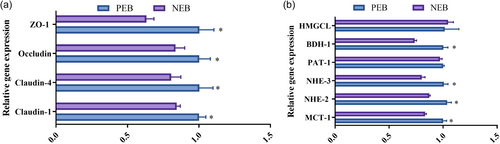
As shown in Figure 4, NEB significantly decreased the mRNA expression of tight junction components ZO-1, Occludin, Claudin-1, Claudin- 4, alongside the transporters BDH-1, NHE-3, NHE-2 and MCT-1, in comparison to the PEB group (P < 0.05). No significant differences between groups were detected in expression of HMGCL or PAT-1 (P > 0.05). The gene expression data revealed that induction of NEB altered the ruminal epithelium in ways that likely undermine the barrier, absorptive, and transport capacity of this tissue in ewes.
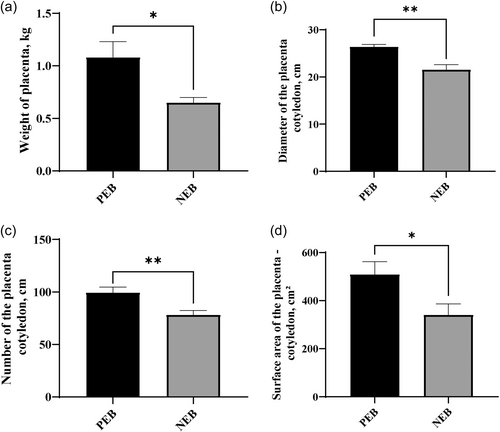
3.6 Effects of NEB on placental weight, cotyledon number, cotyledon diameter, and total cotyledon surface area in ewes
NEB during late pregnancy reduces placental growth and development in Hu sheep, as evidenced by decreased placental weight compared to control ewes (P < 0.05) (Figure 5a). Additional morphological parameters, including cotyledon diameter, cotyledon number, and total cotyledon surface area were also markedly reduced in response to perinatal energy restriction (P < 0.05). Smaller placenta with reduced exchange capacity limits nutritional supply to and waste removal from the developing fetus. This likely contributed to well-documented reductions in lamb birth weights.
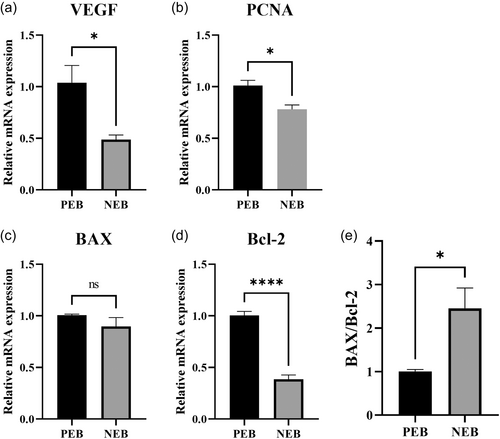
3.7 Effect of NEB on expression levels of genes involved in angiogenesis, proliferation, and apoptosis of ewe placentas
The results showed that dietary energy restriction had a significant impact on the expression levels of specific genes in the placenta of perinatal ewes (Figure 6). The mRNA expression levels of the angiogenic regulator VEGF and the proliferative marker PCNA were significantly decreased in placental tissue of undernourished ewes compared to controls (P < 0.05). Additionally, the anti-apoptotic gene Bcl-2 was also significantly decreased (P < 0.05). However, the pro-apoptotic gene BAX did not show significant changes in in transcript abundance between groups (P > 0.05). It is important to note that the ratio of BAX to Bcl-2 was significantly increased during negative perinatal energy balance (P < 0.05).
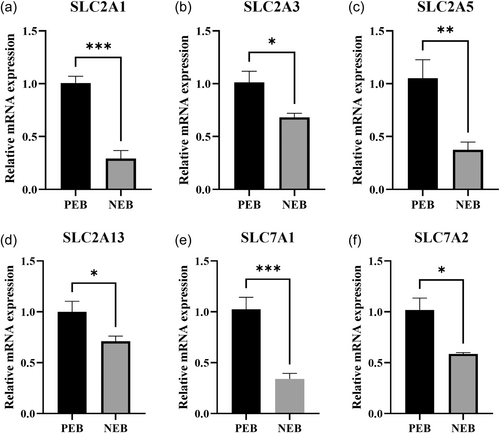
3.8 Effect of NEB on glucose and amino acid transport in ewe placentas
The results showed that NEB had significant effects on the expression of mRNA in the placenta, particularly in genes involved in glucose and amino acid transport (Figure 7). Specifically, the mRNA expression levels of the glucose transporter genes SLC2A1, SLC2A3, SLC2A5, and SLC2A13 were significantly decreased in the placenta under NEB (P < 0.05). Similarly, the mRNA expression levels of the amino acid transporter genes SLC7A1 and SLC7A2 were also significantly downregulated (P < 0.05).
3.9 The relationship between the ruminal bacterial community and placental performance
Associations between rumen bacterial composition and placental morphological parameters alongside expression of placental nutrient transporters were investigated in periparturient ewes. Several correlations between relative abundance of key rumen bacteria and placental metrics were identified (Figure 8).
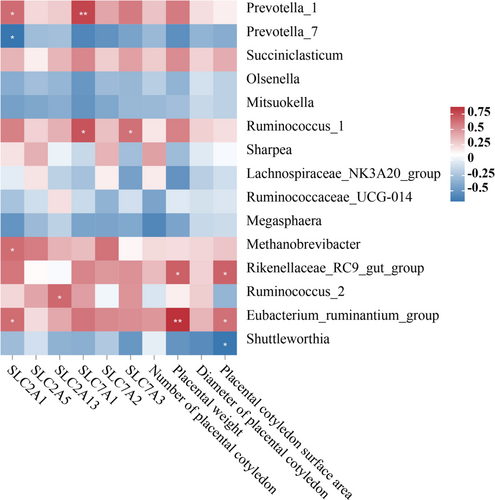
Prevotella_1 showed positive correlations with SLC2A1 and SLC7A1 (P < 0.05). Ruminococcus_1 exhibited positive correlations with SLC7A1 and SLC7A3 (P < 0.05). Methanobrevibacter had a positive correlation with SLC2A1 (P < 0.05), and Rikenellaceae_RC9_gut_group exhibited positive correlations with placental weight and cotyledon area (P < 0.05). Ruminococcus_2 had a positive correlation with SLC2A13 (P < 0.05), and Eubacterium_ruminantium_group had a positive correlation with SLC2A1, placental weight, and cotyledon area (P < 0.05). In contrast, Prevotella_7 and Shuttleworthia demonstrated a negative correlation with SLC2A1 and placental cotyledon area, respectively (P < 0.05) (Figure 8).
4 DISCUSSION
During the perinatal period, the rapid development of the fetus and demands of lactation lead to heightened nutritional requirements and decreased feed intake, resulting in ewes experiencing a NEB (Hu et al., 2018). Periparturient ewes deplete maternal crude protein and fat reserves in response to malnutrition (Luther et al., 2007). While periparturient ewes deplete maternal crude protein and fat reserves to compensate for malnutrition during NEB, (Pethick & Lindsay, 1982; Sargison et al., 1994) the impacts on tissues like the ruminal papillae and placenta and their underlying mechanisms remain unclear. In this study, a 70% energy restriction was implemented in ewes to simulate natural malnutrition and model NEB. Our results showed that when ewes were treated with NEB, serum cholesterol levels decreased, while BHBA and NEFA concentrations increased, accompanied by a significant reduction in live weight. This is consistent with the results of previous studies, (Hu et al., 2018; Xue et al., 2018) underscoring the success of the constructed model.
Rumen function plays a crucial role in the digestive capacity of adult sheep, and one measure of its effectiveness is the length of the rumen papilla (Lesmeister et al., 2004; Lv et al., 2020). Previous studies have demonstrated that malnutrition can damage the rumen's epithelial structure (Lin et al., 2019; Taschuk & Griebel, 2012). In a study conducted by Xue, it was observed that subjecting sheep to heavy restriction in late gestation reduced rumen papilla length (Xue et al., 2020). The current study's findings align with this, indicating that NEB in periparturient ewes significantly reduces rumen papilla length. These results are consistent with earlier research, reinforcing the impact of NEB on rumen morphology.
Restricted feeding has been reported to diminish rumen barrier function due to increased passive transport of 3-O-methyl-α-D-glucose in the rumen epithelium (Gabel & Aschenbach, 2002). Another study showed that short-term severely restricted feeding limited to 25% could impair barrier function (Zhang et al., 2013). In this study, NEB reduced the mRNA expression of ZO-1, Occludin, Claudin-1 and Claudin-4, suggesting that NEB may impair the tight junctions between the epithelial cells of the rumen. These findings lend support to the notion that NEB may compromise the integrity and structure of the rumen epithelial barrier, possibly leading to a reduced proliferation rate of rumen epithelial cell (Steele et al., 2015). This reinforces the concept that malnutrition can adversely affect the epithelial barrier in the rumen. Ruminal tissue plays a vital role in the transport and metabolism of VFA (Baldwin & Connor, 2017). Numerous studies have demonstrated that VFA in the rumen needs to be transported into the bloodstream via the transport function of epithelial cells (Dengler et al., 2015; Gäbel et al., 2002). Additionally, these cells convert VFA into ketone bodies to provide energy for the body (Allen, 2014). The MCT family regulates membrane-bound transporter proteins and pH homeostasis (Graham et al., 2007). MCT-1 transports VFA and its metabolites from the epithelium into the bloodstream (Muller et al., 2002). Previous studies have indicated that butyrate increased the transport activity of MCT-1 in calf and goat rumen epithelial cells (Laarman et al., 2012; Yan et al., 2014). In this study, NEB reduced the expression of MCT-1 and PAT-1 in rumen tissue, accompanied by a decrease in the concentration of butyrate in the rumen. The activity of MCT-1 in the rumen tissue in this study was positively correlated with the concentration of butyrate in the rumen, indicating that MCT-1 is involved in the regulation of butyrate in the rumen. HMG-CoA lyase (HMGCL) is implicated in synthesizing ketone molecules like acetoacetate and BHBA from HMG-CoA (Liu et al., 2019). BDH1 encodes D-β-hydroxybutyrate dehydrogenase, which is involved in the synthesis of BHBA. In the present study, NEB significantly reduced BDH-1 mRNA expression, while the difference in HMGCL expression was insignificant. This suggested that NEB may be involved in ketone body production mainly through 3-hydroxybutyrate dehydrogenase. While ketogenesis is the primary metabolic pathway of rumen VFA, increased ketone body concentrations in NEB ewes may be due to increased activity of the ketogenic pathway in the rumen epithelium due to energy deficiency, thus providing a source of energy for the organism.
In the rumen, volatile fatty acids (VFA) are the product of microbial fermentation, and is thought to have a wide range of effects on the host. Therefore, we tested the concentration of VFA. Our data showed that NEB reduced the concentrations of acetate, butyrate, and total acids in the rumen of ewes. These results demonstrated that NEB during the periparturient period significantly altered the fermentation parameters of the rumen. This is in line with previous trials conducted on sheep and cattle (Hu et al., 2018; Zhang et al., 2013). The marked impact of nutrient restriction on ruminal organic acid production highlights disturbances in fermentative capacity coupled with impaired absorption attributable to morphological changes which may undermine concomitant VFA-mediated effects on ruminal epithelium function as well as wider host metabolism and health.
We used second-generation high-throughput sequencing technology to analyze microbial diversity, aiming to explore microbial community structure changes resulting from NEB in perinatal ewes. Alpha diversity analysis showed that NEB significantly reduced the ACE, Shannon, and Chao1 indices of the rumen flora, indicating an alteration in both the diversity and abundance of the microbial community. These results suggest a pronounced impact on the bacterial community in the rumen following NEB in periparturient ewes, possibly attributed to the stress induced by the NEB. Principal Coordinates Analysis (PCoA) further identified differences in the rumen contents' taxonomic composition of the bacteria. In the present study, alterations in microbial populations within the contents may have led to a reduction in fermentation substrate and changes in rumen papilla morphology. Indeed, previous studies have shown that the rumen microbial system and rumen morphology are closely related to nutrient levels (Belanche et al., 2012; Wang et al., 2016; Xue et al., 2020). Further study should elucidate underlying mechanism driving microbial restructuring in cases of moderate nutrient scarcity, which may offer targets for maintaining ecosystem homeostasis to support optimal ruminal functionality critical for productivity.
The placenta is postulated to be the inaugural organ to commence functioning during embryonic maturation (Genbacev et al., 2000). Fetal development is inextricably reliant upon the unimpeded growth and operation of this critical organ (Waddell et al., 2000). Numerous studies have demonstrated that maternal nutritional restriction during pregnancy can lead to placental dysfunction (Edwards et al., 2020; Shao et al., 2021). Our study revealed a significant decrease in placental weight, cotyledon quantity, cotyledon diameter, and cotyledon area during pregnancy under NEB. This reduction in placental weight and cotyledon characteristics could adversely impact the exchange of nutrients and gases between the mother and the developing fetus, potentially leading to poor fetal development and growth.
The placenta plays a pivotal role in facilitating the exchange of nutrients, oxygen, and waste between the offspring and mother. This process relies on extensive angiogenesis between fetal trophoblast cells and the maternal endometrium (Grazul-Bilska et al., 2010; Reynolds & Redmer, 2001). A study indicated a significant reduction in VEGF abundance in the maternal placenta of ewes subjected to both restricted and overfed diets, suggesting impaired placental vascularization (Pillai et al., 2017). PCNA is a marker of the cell proliferation and plays a crucial role in DNA synthesis in mammalian cells (Jónsson & Hübscher, 1997). Numerous studies have shown that PCNA expression is significantly lower in growth-restricted placentas than in normal placentas (Barapatre et al., 2021; Elsamadicy & Thompson, 2022). Our study found a significant decrease in the mRNA expression levels of the angiogenic gene VEGF and the proliferative marker gene PCNA, indicating compromised blood vessel development and reduced cell proliferation in the placenta. BAX and Bcl-2 are key proteins regulating apoptosis, and alterations in their levels in the placenta have been linked to the occurrence of abnormalities (Kasture et al., 2019; Mohammadpour-Gharehbagh et al., 2019). In this study, the anti-apoptotic gene Bcl-2 was significantly decreased, while the pro-apoptotic gene BAX remained unchanged. However, an increase in the BAX to BCL-2 ratio suggested a higher propensity for cell death under negative perinatal energy balance. These results were consistent with previous studies, affirming the inhibition of placental angiogenesis and pro-apoptotic effects during NEB (Huang et al., 2024). These outcomes underscore the potential adverse consequences of dietary energy restriction on placental functionality, which may subsequently impact fetal well-being. Although the placental vasculature is essential for optimal nutrient transport, the expression of specific transporters constitutes the bottleneck for delivering crucial substances like glucose and amino acids (Jones et al., 2007). Altered transporter availability and function have been documented in humans, rats, and sheep experiencing compromised pregnancies (Edwards et al., 2020; Jansson & Pettersson, Haafiz, et al., 2006; Jansson & Powell, 2006). Our study found that the mRNA expression levels of the glucose transporter genes SLC2A1, SLC2A3, SLC2A5, and SLC2A13, as well as the amino acid transporter genes SLC7A1 and SLC7A2, were significantly reduced in the placenta under NEB. This decrease in transporter gene expression suggested that the transport of crucial nutrients, such as glucose and amino acids, to the developing fetus might be impaired under conditions of NEB (Edwards et al., 2020; Shao et al., 2021). Such impairment could contribute to poor fetal growth and development, emphasizing the necessity of sufficient energy intake during pregnancy to ensure the well-being of both the mother and her offspring.
Since both rumen microbiota and placenta have important effects on pregnant ewes, we evaluated whether the rumen microbiota is associated with placental phenotype under NEB conditions. The results indicated that certain bacterial communities, such as Prevotella_1, Ruminococcus_1, Methanobrevibacter, Eubacterium_ruminantium_group, and Rikenellaceae_RC9_gut_group, showed positive correlations with placental weight, cotyledon area, and mRNA expression levels related to glucose and amino acid transport. Previous studies have demonstrated that Prevotella could generate short-chain fatty acids, mainly acetic acid, through the degradation of carbohydrates (Balakrishnan et al., 2021) enhancing the fermentation of complex polysaccharides and glucose metabolism (Kovatcheva-Datchary et al., 2015). This may explain the aggravation of NEB in energy-restricted ewes. In addition, Balakrishnan B et al. suggested that Prevotella maintained the stability of body homeostasis by enhancing butyrate production (Balakrishnan et al., 2021). Butyrate has been shown to promote epithelial growth (Hasain et al., 2020) and improve placental function (Yu et al., 2022). In this study, the abundance of Prevotella_1 in the rumen was consistent with the trend of mRNA expression of placental transporters SLC2A1 and SLC7A1. Similarly, Methanobrevibacter, Ruminococcus_1, Eubacterium_ruminantium_group and Rikenellaceae_RC9_gut_group are also key archaea and bacteria involved in VFA metabolism and synthesis in the digestive system (Hoegenauer et al., 2022; La Reau & Suen, 2018; Shen et al., 2021; Wang et al., 2020). The abundance of these microbes was positively correlated with placental performance and transport function, suggesting that metabolites of these bacteria may be transported into the placenta through the blood to alleviate the damage of NEB on placental function. Supporting this, concentrations of these microbial metabolites decreased in the rumen fluid of NEB ewes.
5 CONCLUSIONS
The results demonstrated that NEB led to a reduction in VFA concentrations, perturbed rumen function, altered rumen microbial community composition, impaired rumen barrier and translocation functions. Additionally, the study found that NEB restricted placental development, angiogenesis, and nutrient transport, potentially leading to poor fetal growth and development. This study also suggested the influence of rumen microbes on the placental function of perinatal ewes. While these findings emphasize the importance of proper nutrition and management strategies during the perinatal period to promote the health and productivity of ewes and their offspring, certain limitations should be acknowledged. The experiment lacked a comprehensive analysis of the specific microbial species involved and their functional roles. Future studies could employ more advanced omics techniques to delineate the intricate relationships between the rumen microbiome, host metabolism, and placental function.
ACKNOWLEDGEMENTS
This research was funded by the China Agriculture Research System (CARS-38) and the Jiangsu Agricultural Industry Technology System (JATS [2023]436).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
All the data generated or analyzed in this study are included in this paper. The sequencing reads of 16S rRNA gene sequencing are available in the Sequence Read Archive (SRA) of NCBI with PRJNA952929.




