Piglets performance, nutrient digestibility and gut health in response to feeding Ulva lactuca seaweed supplemented with a recombinant ulvan lyase or a commercial carbohydrase mixture
João Pedro Bengala Freire and José António Mestre Prates contributed as equal senior authors for this study.
Abstract
Ulva lactuca, a green seaweed, may be an alternative source of nutrients and bioactive compounds for weaned piglets. However, it has a recalcitrant cell wall rich in a sulphated polysaccharide – ulvan – that is indigestible to monogastrics. The objective of this study was to evaluate the effect of dietary incorporation of 7% U. lactuca, combined with carbohydrases supplementation (commercial carbohydrase mixture or recombinant ulvan lyase), on growth performance, nutrient digestibility and gut health parameters (morphology and microbiota) of weaned piglets. The experiment was conducted over 14 days using 40 weaned piglets randomly allocated to one of four experimental diets: a control diet based on wheat–maize–soybean meal, a diet with 7% U. lactuca replacing the control diet (UL), a diet with UL supplemented with 0.005% Rovabio® Excel AP, and a diet with UL supplemented with 0.01% of a recombinant ulvan lyase. The dietary treatments had no major effects on growth performance, nitrogen balance and gut content variables, as well as histological measurements. Contrarily, dry matter and organic matter digestibility decreased with dietary seaweed inclusion, while hemicellulose digestibility increased, suggesting a high fermentability of this cell wall fraction independently of carbohydrases supplementation. Some beneficial microbial populations increased as a consequence of enzymatic supplementation (e.g., Prevotella), while seaweed diets as a whole led to an increased abundance of Shuttleworthia, Anaeroplasma and Lachnospiraceae_NK3A20_group, all related with a healthier gut. It also decreased Lactobacillus when compared to controls, which is possibly related to increased bioavailability of seaweed zinc. This study indicates that, under these experimental conditions, up to 7% dietary U. lactuca has no detrimental effect on piglet growth, despite decreasing acid detergent fibre digestibility. Carbohydrases supplementation of Ulva diets is not required at this incorporation level.
1 INTRODUCTION
Monogastric animal feeding strategies are under increasing scrutiny to circumvent issues related to feed-food-fuel competition, environmental sustainability, public health, and animal welfare and health. Such concerns originate mostly from the increasing human population, expected to reach 10 billion people by 2050, which is adding pressure on the livestock industry to increase production, while simultaneously decreasing the environmental impact inherent to animal production (FAO, 2009). This increased demand for meat, such as pork, is therefore expected to be accompanied by an increased demand for conventional feedstuffs to sustain this increased production (Komarek et al., 2021). However, feedstuffs such as maize and soybean meal are also used in human nutrition, creating food-feed competition. In addition, European countries are heavily dependent on external countries to satisfy internal food/feed demand (Galli et al., 2020). Therefore, researchers have intensified the search for alternative feedstuffs that have an adequate nutritional composition in addition to promoting environmental sustainability of animal production, in the context of a circular economy (Muscat et al., 2021). Some examples include food industry by-products (olive cake [Vastolo et al., 2019], tomato pomace [Correia et al., 2017]), insects [Dalle Zotte et al., 2018] and marine algae [Madeira et al., 2017; Ribeiro et al., 2022]). The latter include microalgae and macroalgae, also known as seaweeds.
Seaweeds are a diverse set of multicellular organisms divided into three taxa according to their pigmentation: Phaeophyceae (brown), Rhodophyceae (red) and Chlorophyceae (green). The latter includes the Ulva sp. Green seaweeds are fast-growing organisms with a variable protein content (up to 41.8% on a dry matter [DM] basis), between brown (lowest – up to 22.2% on a DM basis) and red (highest – up to 44% on a DM basis) (Costa et al., 2021). Their carbohydrate content is very high, with ulvan being the main cell wall polysaccharide, and ash content being also very high and a major constraint in using it for livestock diets (Cabrita et al., 2016). Ulva sp. has some of the highest energy densities (over 14 MJ/kg DM) reported in the literature compared with other seaweeds (Makkar et al., 2016). Ulva species are particularly efficient in accumulating starch (Kazir et al., 2021), up to 30% DM. Ulva lactuca is among the most studied green seaweeds with applications ranging from the pharmaceutical industry (Lopes et al., 2021) to pig nutrition (Ribeiro et al., 2021). Despite Ulva sp. having low crude fat content (below 7% DM basis), and low n-3 PUFA when compared to other seaweeds, it is an interesting source of other important fatty acids, such as C16:4n-3 and C18:1 (Van Ginneken et al., 2011). Furthermore, it has lysine and glutamine contents higher than those of species such as Laminaria digitata (Corino et al., 2019). These micronutrients have immunomodulatory, antioxidant, antimicrobial and anti-inflammatory activities, in addition to particularly important nutritional roles, like the role of glutamine for enteric nutrition in weaned piglets (Xiong et al., 2019) or that of lysine, which is often the first-limiting amino acid for pigs (depending on diet composition). It is thus a very interesting feedstuff to use in swine nutrition.
The weaned piglet is the centrepiece of pork production. They endure a transition phase that includes shifting from mostly liquid to mostly solid plant-based diets, together with social stress and depressed immune status that can compromise their subsequent growth, welfare and thus farm profitability (Heo et al., 2013), the post-weaning stress (PWS). Indeed, their immature digestive system is unable to adequately digest solid feeds, which leads to severe weight loss and disruption of intestinal homoeostasis, which implies increased permeability to pathogens and reduced villus heights (Pluske et al., 2018). This lack of digestive capacity can increase the amount of undigested protein reaching the large intestine, where protein fermentation takes place (Lynegaard et al., 2021). This, along with disrupted microbiota, often contributes to the occurrence of severe diarrhoea. Standard practices commonly used in the past to deal with PWS employed high levels of dietary zinc oxide (Brugger & Windisch, 2015). However, the European Union has recently restricted its use due to environmental and public health concerns (Satessa et al., 2020). Therefore, it is in the best interest of the industry to use energy-dense, high-quality feedstuffs (protein quality, nutrient digestibility, among other factors) that promote intestinal health while reducing the environmental impact of the production (Pluske et al., 2018). Seaweeds, such as U. lactuca, can play a significant role in such a context, given its nutritional composition and bioactive properties.
However, its indigestible cell wall polysaccharides elicit antinutritional effects, preventing adequate digestion in the monogastric digestive system. Carbohydrases supplementation is a putative strategy to maximise the nutritional potential of seaweeds in monogastric diets, in a similar way to what our research team has reported previously for microalgae (Martins et al., 2021, 2022). In addition, we have recently reported that a recombinant ulvan lyase can partially degrade the U. lactuca cell wall in vitro (Costa et al., 2022). The objective of this work was to evaluate the effect of high (7%) dietary incorporation of U. lactuca, with or without carbohydrases supplementation, on growth performance, nutrient digestibility and gut health parameters (morphology and microbiota) of weaned piglets.
2 MATERIALS AND METHODS
2.1 Animal welfare statement
The experimental trial took place at the Animal Production Department of the School of Agriculture (ISA) of the University of Lisbon, Portugal. It was approved by ISA Ethics Commission and by the National Veterinary Authority (process reference: 0421/000/000/2020-021337), following current legislation in Portugal and the European Union (Directive 2010/63/EU) and ARRIVE guidelines.
2.2 Live animal trial
Forty weaned piglets (Large White × Duroc) were obtained from a commercial farm, weaned at 28 days old. Upon arrival at the research facility (room with metabolic cages with stainless steel trays for sample collection, and controlled temperature), animals were weighed and divided across four experimental groups to have an even body weight distribution (Table 1). These groups were fed with control (standard diet, wheat–maize–soybean meal based), UL (with 7% U. lactuca replacing the control ingredients), ULR (UL + 0.005% Rovabio® Excel AP, a commercial carbohydrase mix bought from Adisseo) and ULU (UL + 0.01% ulvan lyase, as reported by Costa et al. [2022]). The Rovabio® product had the following activities: xylanase, β-glucanase, cellulase, pectinase, protease and others including endo-1,4 β-mannanase, β-mannosidase and α-galactosidase (https://www.adisseo.com/en/products/rovabio/rovabio-excel-the-versatile-enzyme/, accessed 6 March 2023). The seaweed was bought from Aleor in the form of a dry powder (particle size < 250 µm). No salt was added to the diets that included seaweed. Each piglet was individually housed in a metabolic cage (length: 1 m, width: 0.55 m, height: 0.55 m), equipped with nipple drinkers and heating lights. After an adaptation period of 5 days to the experimental conditions, the trial started and lasted two weeks (14 days). Piglets were weighed at the beginning and end of each week, and the first (P1) and second (P2) weeks consisted of two experimental periods. Groups were fed daily with 50 g of feed per kg of live weight (LW) to avoid differences in feed intake between control and seaweed diets. Feed refusals were recorded, and faecal and urine samples were collected daily. Faecal consistency was scored daily (0 – normal faeces, 1 – soft faeces, 2 – diarrhoea, and 3 – severe diarrhoea). At the end of the trial, piglets were slaughtered following commercial practices, with electrical stunning followed by exsanguination.
| U. lactuca | Control | UL | ULR | ULU | |
|---|---|---|---|---|---|
| Ingredients (g/kg) | |||||
| Wheat | - | 437 | 407 | 407.95 | 406.9 |
| Maize | - | 150 | 140 | 140 | 140 |
| Soybean meal 44 | - | 250 | 233.1 | 233.1 | 233.1 |
| Sweet whey powder | - | 100 | 93.4 | 93.4 | 93.4 |
| Sunflower oil | - | 30 | 28.5 | 28.5 | 28.5 |
| U. lactuca | - | 0 | 70 | 70 | 70 |
| L-Lysine | - | 5 | 4.7 | 4.7 | 4.7 |
| DL-Methionine | - | 1 | 0.9 | 0.9 | 0.9 |
| L-Threonine | - | 1 | 0.9 | 0.9 | 0.9 |
| Calcium carbonate | - | 5 | 4.7 | 4.7 | 4.7 |
| Dicalcium phosphate | - | 13 | 12.1 | 12.1 | 12.1 |
| Sodium chloride | - | 3 | 0 | 0 | 0 |
| Vitamin-mineral premixa | - | 5 | 4.7 | 4.7 | 4.7 |
| Rovabio® Excel AP | - | 0 | 0 | 0.05 | 0 |
| Ulvan lyase | - | 0 | 0 | 0 | 0.1 |
| Chemical composition (g/kg DM) | |||||
| Dry matter (g/kg original matter) | 887 | 894 | 893 | 892 | 893 |
| Ash | 317 | 59 | 72 | 75 | 73 |
| Organic matter | 683 | 941 | 928 | 925 | 927 |
| Ether extract | 29 | 56 | 55 | 57 | 53 |
| Crude protein | 282 | 180 | 183 | 187 | 186 |
| Neutral detergent fibre | 271 | 154 | 199 | 200 | 199 |
| Acid detergent fibre | - | 38 | 32 | 33 | 32 |
| Gross energy (MJ/kg DM) | 11.2 | 18.5 | 18.2 | 18.2 | 18.1 |
| Macrominerals (g/kg DM) | |||||
| Calcium (Ca) | 6.20 | 14.66 | 14.97 | 15.02 | 14.01 |
| Potassium (K) | 38.82 | 10.97 | 14.21 | 13.95 | 14.43 |
| Magnesium (Mg) | 25.89 | 1.46 | 4.19 | 3.98 | 4.02 |
| Sodium (Na) | 52.13 | 4.08 | 7.49 | 7.21 | 7.62 |
| Phosphorous (P) | 2.79 | 8.72 | 8.88 | 9.34 | 8.64 |
| Sulphur (S) | 49.27 | 3.00 | 8.91 | 8.32 | 8.72 |
| Microminerals (mg/kg DM) | |||||
| Copper (Cu) | 3.73 | 256 | 240 | 258 | 227 |
| Iodine (I) | 45.1 | 1.45 | 5.88 | 7.12 | 5.66 |
| Iron (Fe) | 537 | 265 | 258 | 294 | 253 |
| Manganese (Mn) | 39.0 | 142 | 129 | 145 | 145 |
| Zinc (Zn) | 8.96 | 257 | 261 | 256 | 281 |
- a Vitamin-mineral premix, VitaTec®, provided by Tecadi. Per 1 kg of premix: Vitamin A – 3,000,000 UI, Vitamin D3 – 500,000 UI, Vitamin E – 10,000 mg, Vitamin B1 – 500 mg, Vitamin B2 – 1000 mg, Vitamin B6 – 500 mg, Vitamin B12 – 5 mg, Vitamin H2 – 1875 mg, Vitamin K3 – 500 mg, Vitamin B5 – 3750 mg, Vitamin B3 – 6250 mg, Vitamin B9 – 62.5 mg, Choline chloride – 50,000 mg, Cu – 38,750 mg, Zn – 27,500 mg, Mn – 12,500 mg, I – 200 mg, Se – 50 mg, Fe – 25,000 mg, butyl-hydroxytoluene – 50 mg.
2.3 Sampling and analysis
Methods for sampling and analysis of gastrointestinal contents, feeds and faeces have been published before by our team (Martins et al., 2021, 2022) and are briefly mentioned here for contextual reasons. Upon slaughter, different gastrointestinal compartment contents were taken to measure pH (stomach, duodenum and jejunum, ileum, caecum and colon). Small intestine contents were also taken to measure viscosity from duodenum plus jejunum, and ileal contents. Samples of large intestine content (caecum and colon) were taken for volatile fatty acid (VFA) and microbiome analysis (colon only, from six animals per group), following previously published methodology (Martins et al., 2022). DM, organic matter (OM), ash, crude protein (CP), ether extract (EE), gross energy, neutral detergent fibre (NDF) and acid detergent fibre (ADF) were all measured on feed and faeces following EGRAN (2010) recommendations. Urine nitrogen was analysed by the Kjeldahl method, and the nitrogen balance was calculated as described (Lordelo et al., 2008). Intestinal morphology and VFA analysis were performed following the procedures described by Martins et al. (2022). Mineral profiling of feeds and faeces was carried out by Inductively Coupled Plasma – Optical Emission Spectrometry, as reported (Ribeiro et al., 2020). The total tract apparent digestibility (TTAD) coefficients were calculated as follows: (A − B)/A, where A is the ingested nutrient and B is the excreted nutrient.
2.4 Microbiome analysis
The bacterial DNA extraction was carried out using FastDNA SPIN kit for Soil (MP Biomedicals) following the manufacturer's instructions. The DNA concentration and purity (absorbance ratio 260/280 and 260/230, respectively) of the DNA isolated were checked using spectrophotometry on NanoDrop (Fisher Scientific). The V3-V4 region of the 16S rRNA gene (~460 bp) was amplified, amplicons were produced using the universal primers Pro341F: 5′-TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGCCTACGGGNBGCASCAG-3′ and Pro805R: 5′ GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGGACTACNVGGGTATCTAATCC-3′ (Takahashi et al., 2014) using Platinum™ Taq DNA Polymerase High Fidelity (Thermo Fisher Scientific) and sequenced using the Illumina MisSeq platform 300 × 2 bp. The library formation and sequencing of the 16S rRNA gene were performed with MiSeq® Reagent Kit V3-V4 on the MiSeq-Illumina® platform. Microbiota analysis was performed using the DADA2 pipeline (Callahan et al., 2016) and taxonomy was assigned using Silva Database (release number 138.1, 2019) as referenced (Quast et al., 2012).
2.5 Statistical analysis
Except for the microbiome, all data was analysed using the Mixed procedure of SAS (version 9.4; SAS Institute) (Littell et al., 1998). The piglet was used as the experimental unit and the effect of litter (11 litters in total) was introduced as a block, and statistical significance was declared when p < 0.05. The period (first – P1 and second – P2 weeks) and diet were the main effects. Because there was no significant effect of period × diet interaction, we focused on the main effects. The least-square means were compared using the Tukey post hoc test. The standard error of the means was obtained using the univariate procedure.
Regarding the microbiome, the statistical analysis of Alpha diversity and Beta diversity and taxonomics was carried out with R v4.1, using “phyloseq” (McMurdie & Holmes, 2013) v1.38, “vegan” v2.6 (Dixon, 2003) and “microbiomeutilities” v1.0 (Shetty & Lahti, 2022). For the alpha diversity, samples were rarefied to the lowest sample depth, to avoid bias linked to different sampling efforts. Differences in alpha diversity indices (Chao1, Shannon and Simpson diversity) between capsules and the other samples were tested using the Wilcoxon test. For the Beta diversity, a dissimilarity matrix using Euclidean distances of centred log ratio (clr) transformed data was constructed, and results were plotted using a PCoA plot. Differences were tested using a PERMANOVA model (Adonis) with 9999 permutations, including sample type as a factor. Pairwise contrast among sample types was carried out using pairwiseAdonis function included in the “PairwiseAdonis” package (Martinez Arbizu, 2020), p-values were then adjusted for multiple comparisons using Bonferroni correction. Linear discriminant analysis (LDA) effect size (LEfSe) algorithm at Phylum and Genus levels was applied to identify taxa differentially expressed (LDA score > 3 and p.adj < 0.05) between experimental groups.
3 RESULTS
3.1 Zootechnical parameters
There was no significant effect (p > 0.05) of diet on any zootechnical parameter (LWs, average daily gain, average daily feed intake, feed conversion ratio and faecal consistency score) (Table 2). However, litter had a significant effect on most variables, except feed conversion ratio.
| Control | UL | ULR | ULU | SEM | Litter | Diet | |
|---|---|---|---|---|---|---|---|
| Number of piglets | 10 | 10 | 10 | 10 | |||
| Initial weight (kg) | 9.4 | 9.7 | 9.6 | 9.7 | 0.17 | 0.004 | 0.787 |
| Final weight (kg) | 14.7 | 15.0 | 14.9 | 14.9 | 0.30 | 0.000 | 0.977 |
| Average daily gain (g) | 381 | 375 | 378 | 370 | 12.02 | 0.000 | 0.972 |
| Average daily feed intake (g) | 563 | 558 | 597 | 569 | 14.33 | <0.001 | 0.233 |
| Feed conversion ratio | 1.49 | 1.50 | 1.61 | 1.61 | 0.04 | 0.527 | 0.523 |
| Faecal consistency score | 0.31 | 0.31 | 0.37 | 0.38 | 0.05 | 0.028 | 0.908 |
- Abbreviation: SEM, standard error of the mean.
3.2 TTAD
TTAD, as influenced by diet, litter and period, is depicted in Table 3. The TTAD of ash, EE and CP were influenced by period (p < 0.05), all of which increased with time. Diet had a significant effect on the TTAD of DM, OM, NDF, ADF and hemicellulose. Ulvan lyase supplementation significantly decreased the TTAD of DM and OM compared to the control group (p = 0.030 and p = 0.049 respectively). In turn, NDF digestibility was significantly increased (p = 0.001) in seaweed diets compared with controls, because of the high hemicellulose digestibility of these diets (p < 0.001). The reverse relation was found for ADF, where its digestibility was decreased by at least 18 percentage points when U. lactuca was included in diets, regardless of enzyme supplementation (p < 0.001). Regarding macrominerals, the digestibility of both magnesium (Mg) and potassium (K) were significantly different (p < 0.001 and p = 0.0005, respectively) between the control and seaweed groups, with the former having lower digestibility than its counterparts. Regarding microminerals, zinc (Zn) digestibility was increased in seaweed diets compared to the control, reaching statistical significance in ULU piglets (p = 0.003).
| Control | UL | ULR | ULU | P1 | P2 | SEM | Litter | Diet | Period | |
|---|---|---|---|---|---|---|---|---|---|---|
| Number of piglets | 10 | 10 | 10 | 10 | 40 | 40 | ||||
| Dry matter | 0.875A | 0.863AB | 0.859AB | 0.852B | 0.860 | 0.865 | 0.003 | 0.056 | 0.030 | 0.245 |
| Ash | 0.804 | 0.804 | 0.798 | 0.788 | 0.723 | 0.874 | 0.009 | 0.031 | 0.244 | <0.001 |
| Organic matter | 0.883A | 0.873AB | 0.870AB | 0.863B | 0.871 | 0.874 | 0.002 | 0.060 | 0.049 | 0.294 |
| Ether extract | 0.779 | 0.779 | 0.793 | 0.777 | 0.769 | 0.795 | 0.004 | 0.011 | 0.372 | <0.001 |
| Gross energy | 0.863 | 0.855 | 0.852 | 0.844 | 0.851 | 0.856 | 0.003 | 0.054 | 0.117 | 0.149 |
| Crude protein | 0.829 | 0.816 | 0.823 | 0.810 | 0.814 | 0.825 | 0.004 | 0.017 | 0.400 | 0.040 |
| NDF | 0.726A | 0.778B | 0.767B | 0.767B | 0.759 | 0.759 | 0.004 | 0.655 | 0.001 | 0.989 |
| ADF | 0.465A | 0.284B | 0.262B | 0.243B | 0.315 | 0.312 | 0.014 | 0.422 | <0.001 | 0.853 |
| Hemicellulose | 0.810A | 0.872B | 0.867B | 0.867B | 0.855 | 0.853 | 0.004 | 0.488 | <0.001 | 0.693 |
| Macrominerals | ||||||||||
| Calcium (Ca) | 0.856 | 0.871 | 0.857 | 0.851 | 0.839 | 0.878 | 0.004 | 0.094 | 0.215 | <0.001 |
| Magnesium (Mg) | 0.332A | 0.569B | 0.490C | 0.523BC | 0.417 | 0.541 | 0.017 | 0.052 | <0.001 | <0.001 |
| Potassium (K) | 0.777A | 0.823B | 0.821B | 0.822B | 0.794 | 0.828 | 0.005 | 0.0002 | 0.0005 | <0.001 |
| Phosphorous (P) | 0.768 | 0.760 | 0.770 | 0.755 | 0.775 | 0.751 | 0.004 | 0.564 | 0.150 | 0.006 |
| Sodium (Na) | 0.822 | 0.812 | 0.789 | 0.829 | 0.800 | 0.826 | 0.007 | 0.0002 | 0.051 | 0.014 |
| Sulphur (S) | 0.798 | 0.803 | 0.786 | 0.798 | 0.795 | 0.797 | 0.004 | 0.110 | 0.459 | 0.714 |
| Microminerals | ||||||||||
| Copper (Cu) | 0.587 | 0.582 | 0.602 | 0.575 | 0.592 | 0.581 | 0.006 | 0.996 | 0.599 | 0.394 |
| Iron (Fe) | 0.541 | 0.502 | 0.555 | 0.503 | 0.524 | 0.527 | 0.008 | 0.958 | 0.085 | 0.833 |
| Manganese (Mn) | 0.540 | 0.521 | 0.545 | 0.578 | 0.550 | 0.542 | 0.008 | 0.642 | 0.123 | 0.526 |
| Zinc (Zn) | 0.376A | 0.450AB | 0.438AB | 0.493B | 0.469 | 0.410 | 0.012 | 0.063 | 0.003 | 0.002 |
- Note: Different superscripts indicate different means (p < 0.05) as a result of diet.
- Abbreviations: ADF, acid detergent fibre; NDF, neutral detergent fibre; P1, first experimental period; P2, second experimental period; SEM, standard error of the mean.
3.3 Nitrogen balance
The nitrogen balance of piglets is presented in Table 4. All variables were found to be influenced by period (increase over time) and litter. The diet significantly influenced nitrogen intake (p < 0.05), which was highest in ULR, albeit with a marginal numerical difference of 0.1 g/d/kg. Accordingly, nitrogen retention was the highest in this group compared with either control or UL (p < 0.05), but without differences to ULU (p > 0.05). Ultimately, this did not contribute to significantly different nitrogen retention coefficients (p > 0.05).
| Control | UL | ULR | ULU | P1 | P2 | SEM | Litter | Diet | Period | |
|---|---|---|---|---|---|---|---|---|---|---|
| Number of piglets | 10 | 10 | 10 | 10 | 40 | 40 | ||||
| Nitrogen intake | ||||||||||
| g/d | 14.5 | 14.6 | 15.9 | 15.2 | 11.7 | 18.3 | 0.46 | <0.001 | 0.055 | <0.001 |
| g/d/kg | 1.2A | 1.2A | 1.3B | 1.2A | 1.1 | 1.4 | 0.02 | <0.001 | 0.012 | <0.001 |
| Nitrogen retention | ||||||||||
| g/d | 10.6 | 10.5 | 11.6 | 10.8 | 8.2 | 13.6 | 0.39 | <0.001 | 0.096 | <0.001 |
| g/d/kg | 0.86A | 0.85A | 0.94B | 0.88AB | 0.75 | 1.01 | 0.02 | <0.001 | 0.009 | <0.001 |
| Nitrogen utilisation coefficients | ||||||||||
| Nitrogen retention coefficient | 0.865 | 0.870 | 0.878 | 0.869 | 0.846 | 0.895 | 0.005 | 0.058 | 0.734 | <0.001 |
| Practical nitrogen retention coefficient | 0.718 | 0.710 | 0.722 | 0.705 | 0.689 | 0.738 | 0.006 | 0.001 | 0.546 | <0.001 |
- Note: Different superscripts indicate different means (p < 0.05) as a result of diet.
- Abbreviations: P1, first experimental period; P2, second experimental period; SEM, standard error of the mean.
3.4 Gastrointestinal contents pH and viscosity
The pH and viscosity of intestine contents are presented in Table 5. No significant effect was found either for the viscosity of the small intestine contents, or for the pH of the stomach, duodenum plus jejunum, and ileum (p > 0.05). Contrarily, there was a tendency for caecum (p = 0.057) and colon (p = 0.051) pH to be lower in carbohydrase-supplemented diets, with the ULU diet reaching the lowest levels in the colon.
| Control | UL | ULR | ULU | SEM | Litter | Diet | |
|---|---|---|---|---|---|---|---|
| Number of piglets | 10 | 10 | 10 | 10 | |||
| Content viscosity | |||||||
| Duodenum + jejunum | 4.5 | 4.7 | 4.7 | 4.6 | 0.04 | 0.141 | 0.164 |
| Ileum | 4.7 | 5.0 | 4.7 | 4.6 | 0.06 | 0.563 | 0.130 |
| Content pH | |||||||
| Stomach | 4.0 | 4.2 | 4.6 | 4.6 | 0.10 | 0.624 | 0.105 |
| Duodenum + jejunum | 5.4 | 5.5 | 5.4 | 5.5 | 0.04 | 0.256 | 0.904 |
| Ileum | 6.0 | 6.1 | 6.0 | 6.0 | 0.04 | 0.809 | 0.860 |
| Caecum | 5.8 | 5.8 | 5.6 | 5.6 | 0.03 | 0.083 | 0.057 |
| Colon | 6.1 | 6.0 | 5.9 | 5.8 | 0.04 | 0.679 | 0.05 |
- Abbreviation: SEM, standard error of the mean.
3.5 Small intestine morphology
Histological measurements taken on the duodenum, jejunum and ileum of piglets are presented in Table 6. Overall, there was a lack of significant effects of diet on the villus height and width in the different segments of the small intestine. However, there was a tendency (p = 0.054) for reduced crypt depth in UL compared with control in the duodenum. This contributed to a significant increase (p = 0.026) in the villus/crypt ratio in this segment of UL compared with control, with ULR and ULU diets having intermediate values.
| Control | UL | ULR | ULU | SEM | Litter | Diet | |
|---|---|---|---|---|---|---|---|
| Number of piglets | 10 | 10 | 10 | 10 | |||
| Villus height (μm) | |||||||
| Duodenum | 375.6 | 417.2 | 381.5 | 371.8 | 13.53 | 0.436 | 0.643 |
| Jejunum | 461.2 | 424.8 | 506.1 | 483.8 | 16.94 | 0.039 | 0.257 |
| Ileum | 329.9 | 296.5 | 358.3 | 239.8 | 9.57 | 0.487 | 0.180 |
| Villus width (μm) | |||||||
| Duodenum | 163.6 | 165.2 | 179.7 | 158.1 | 3.70 | 0.809 | 0.307 |
| Jejunum | 127.7 | 133.0 | 128.0 | 127.4 | 2.57 | 0.800 | 0.880 |
| Ileum | 159.5 | 151.5 | 156 | 152.3 | 2.65 | 0.183 | 0.693 |
| Crypt depth (μm) | |||||||
| Duodenum | 479 | 383.8 | 449.7 | 428.1 | 11.87 | 0.862 | 0.054 |
| Jejunum | 309.8 | 286.5 | 330.2 | 311.1 | 6.45 | 0.699 | 0.164 |
| Ileum | 242.5 | 264.1 | 277.5 | 253.0 | 6.91 | 0.226 | 0.321 |
| Villus/crypt ratio | |||||||
| Duodenum | 0.79A | 1.12B | 0.87AB | 0.88AB | 0.04 | 0.192 | 0.026 |
| Jejunum | 1.49 | 1.55 | 1.55 | 1.58 | 0.07 | 0.144 | 0.967 |
| Ileum | 1.42 | 1.15 | 1.33 | 1.33 | 0.05 | 0.247 | 0.323 |
- Note: Different superscripts indicate different means (p < 0.05) as a result of diet.
- Abbreviation: SEM, standard error of the mean.
3.6 VFA profile of large intestine contents
The VFA profile of caecum contents is presented in Table 7. There was no significant effect of diet for most individual VFA and their sums (p > 0.05). However, there was a tendency for an increased proportion of valeric acid (C5) in UL and ULR piglets compared with the remaining groups (p = 0.078).
| Control | UL | ULR | ULU | SEM | Litter | Diet | |
|---|---|---|---|---|---|---|---|
| Number of piglets | 10 | 10 | 10 | 10 | |||
| C2 | 6.73 | 6.16 | 5.78 | 6.04 | 0.27 | 0.749 | 0.704 |
| C3 | 4.79 | 4.10 | 4.16 | 4.13 | 0.14 | 0.557 | 0.291 |
| C4 | 2.10 | 2.62 | 2.52 | 2.35 | 0.16 | 0.475 | 0.714 |
| C5 | 0.53 | 0.95 | 0.77 | 0.60 | 0.07 | 0.340 | 0.202 |
| iC5 | 0.06 | 0.02 | 0.03 | 0.01 | 0.01 | 0.808 | 0.195 |
| C2:C3 | 1.39 | 1.50 | 1.39 | 1.62 | 0.03 | 0.733 | 0.103 |
| C2:C4 | 6.53 | 2.46 | 2.97 | 2.77 | 0.84 | 0.554 | 0.315 |
| C3:C4 | 5.91 | 1.62 | 2.14 | 1.61 | 0.91 | 0.561 | 0.324 |
| C2 + C3 + C4 | 13.62 | 12.85 | 12.39 | 13.19 | 0.44 | 0.542 | 0.831 |
| Total | 14.20 | 13.82 | 13.19 | 13.80 | 0.48 | 0.468 | 0.919 |
| C2:Total | 0.48 | 0.45 | 0.44 | 0.48 | 0.01 | 0.587 | 0.165 |
| C3:Total | 0.35 | 0.30 | 0.32 | 0.30 | 0.01 | 0.478 | 0.137 |
| C4:Total | 0.14 | 0.19 | 0.18 | 0.17 | 0.01 | 0.447 | 0.193 |
| C5:Total | 0.03 | 0.07 | 0.06 | 0.04 | 0.004 | 0.291 | 0.078 |
| iC5:Total | 0.004 | 0.001 | 0.002 | 0.001 | 0.001 | 0.815 | 0.248 |
- Note: C2, C3, C4, C5 and iC5 are acetic, propionic, butyric, valeric and isovaleric acids respectively.
- Abbreviation: SEM, standard error of the mean.
Regarding the colon (Table 8), there was a strong tendency for isovaleric acid (iC5) to have the highest values in control and UL when compared with enzyme-supplemented diets (p = 0.057). Accordingly, there was a significantly decreased proportion of this VFA with these diets compared with control (p = 0.011). Conversely, there was a tendency (p = 0.097) for increased butyric acid (C4) proportion with enzyme-supplemented diets compared with the control group.
| Control | UL | ULR | ULU | SEM | Litter | Diet | |
|---|---|---|---|---|---|---|---|
| Number of piglets | 10 | 10 | 10 | 10 | |||
| C2 | 7.75 | 7.14 | 7.19 | 6.64 | 0.29 | 0.801 | 0.672 |
| C3 | 4.22 | 4.16 | 4.37 | 4.08 | 0.12 | 0.494 | 0.878 |
| C4 | 2.05 | 2.33 | 2.46 | 2.43 | 0.10 | 0.485 | 0.506 |
| C5 | 0.72 | 0.80 | 0.82 | 0.71 | 0.05 | 0.632 | 0.867 |
| iC5 | 0.15 | 0.12 | 0.09 | 0.08 | 0.01 | 0.999 | 0.057 |
| C2:C3 | 1.81 | 1.73 | 1.62 | 1.84 | 0.04 | 0.393 | 0.275 |
| C2:C4 | 4.04 | 3.13 | 3.16 | 3.19 | 0.17 | 0.247 | 0.145 |
| C3:C4 | 2.37 | 1.85 | 1.96 | 1.75 | 0.13 | 0.274 | 0.335 |
| C2 + C3 + C4 | 14.00 | 13.59 | 13.96 | 13.91 | 0.39 | 0.733 | 0.985 |
| Total | 14.87 | 14.51 | 14.86 | 14.70 | 0.41 | 0.725 | 0.991 |
| C2:Total | 0.51 | 0.49 | 0.48 | 0.50 | 0.01 | 0.487 | 0.218 |
| C3:Total | 0.29 | 0.29 | 0.30 | 0.27 | 0.01 | 0.288 | 0.570 |
| C4:Total | 0.14 | 0.16 | 0.17 | 0.17 | 0.01 | 0.073 | 0.097 |
| C5:Total | 0.05 | 0.05 | 0.05 | 0.05 | 0.003 | 0.740 | 0.862 |
| iC5:Total | 0.01A | 0.008AB | 0.006B | 0.005B | 0.001 | 0.570 | 0.011 |
- Note: Different superscripts indicate different means (p < 0.05) as a result of diet. C2, C3, C4, C5 and iC5 are acetic, propionic, butyric, valeric and isovaleric acids respectively.
- Abbreviation: SEM, standard error of the mean.
3.7 Microbiome analysis
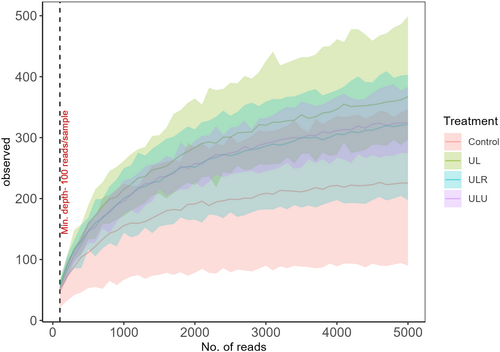
Bacterial DNA was successfully extracted and amplified from 24 samples. Overall, the sequencing procedure produced a total of 2,015,181 sequences, with an average of 83,966 per sample. After the quality check, an average of 50,919 reads were retained that produced 2841 Amplicon Sequence Variants (ASVs), following bioinformatic analysis. Rarefaction curves in Figure 1 show the number of different species observed as a function of the number of sequences. The tendency to a plateau indicates how the sequencing procedure was able to capture all the variability present in the samples.
Among the 2841 ASVs recovered, 23 phyla, 87 families and 153 genera were identified. The most abundant phyla were Firmicutes 71.83 ± 8.90%, Bacteroidota 22.72 ± 8.05% and Proteobacteria 2.42 ± 2.63%. The most abundant families were Lactobacillus (23.11 ± 14.67%), Prevotella (16.05 ± 7.91%), Streptococcus (5.72 ± 6.76%) and Megasphaera (3.85 ± 3.39%).
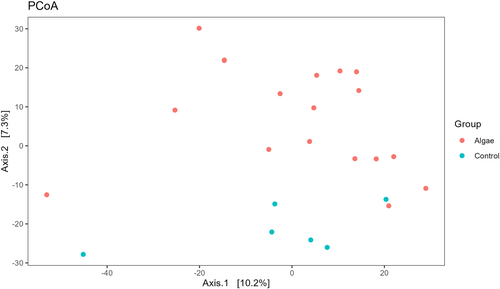
For the Beta diversity, a PCoA plot was generated using an Euclidean distance matrix based on clr transformed data Figure 2. The plot shows how seaweed diets separate from the control group. Overall, the Adonis test showed how bacterial composition was significantly affected by the diet (R2 = 0.14, p = 0.03). Moreover, we included a pairwise Adonis test, to differentiate bacterial composition between groups (Table 9). Pairwise contrast shows how UL bacterial composition is significantly different compared with Control samples (R2 = 0.13, p.adj = 0.02). Only a tendency was recovered for ULR and ULU diets compared with Control (R2 = 0.12, p.adj = 0.08 and R2 = 0.12, p.adj = 0.08 respectively).
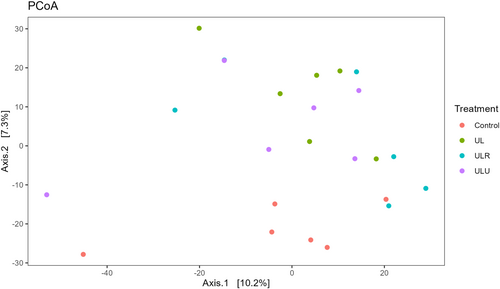
| Pairs | Df | Sums of Sqs | F model | R2 | p Value | p Adjusted |
|---|---|---|---|---|---|---|
| Control versus UL | 1 | 5868 | 1.51 | 0.13 | 0.00 | 0.02 |
| Control versus ULR | 1 | 4966 | 1.31 | 0.12 | 0.04 | 0.08 |
| Control versus ULU | 1 | 5002 | 1.25 | 0.11 | 0.03 | 0.08 |
| UL versus ULR | 1 | 4049 | 0.98 | 0.09 | 0.52 | 0.77 |
| UL versus ULU | 1 | 3998 | 0.92 | 0.08 | 0.79 | 0.79 |
| ULR versus ULU | 1 | 3934 | 0.92 | 0.08 | 0.72 | 0.79 |
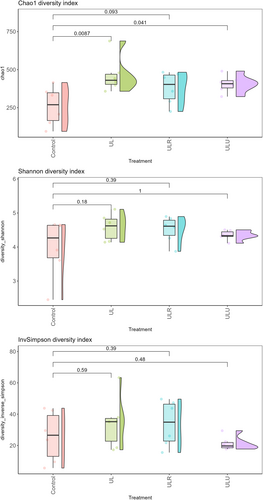
Figure 3 shows alpha diversity values for Chao1, Shannon and InvSimpson for each sample. Overall, it can be observed how bacterial richness was significantly higher in UL and ULU compared with the control group (Control vs. UL, p = 0.0087; Control vs. ULU, p = 0.041), while it tended to be higher in ULR group compared with Control (p = 0.09).
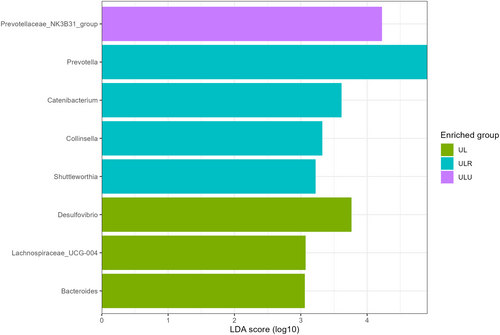
To identify specific bacterial markers that were differently expressed between groups, LEfSe analysis was conducted (Figure 4). Overall, piglets from the UL group were characterised by a higher abundance of Desulfovibrio (LDA score = 3.75, p.adj = 0.03), Lachnospiraceae_UCG-004 (LDA score = 3.14, p.adj = 0.05) and Bacteroides (LDA score = 3.07, p.adj = 0.01). Piglets from the ULU group were characterised by a higher abundance of Prevotellaceae_NK3B31_group (LDA score = 4.15, p = 0.03), while animals from ULR group had a higher abundance of Prevotella (LDA score = 4.91, p.adj = 0.03), Catenibacterium (LDA score = 3.59, p = 0.01), Collinsella (LDA score = 3.32, p.adj = 0.01) and Shuttleworthia (LDA score = 3.24, p.adj = 0.04). Therefore, U. lactuca diets significantly affected the faecal microbiota composition and richness compared with the control group.
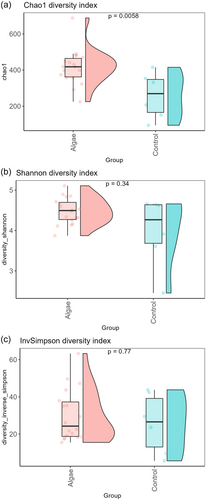
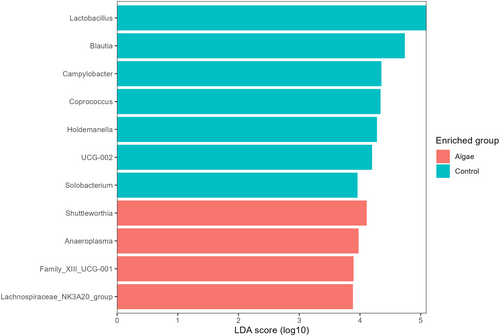
To test differences in bacterial composition between the control and U. lactuca based diet we performed the same analysis but compared control samples (n = 6) and seaweed groups altogether (UL + ULR + ULE). For the beta diversity, the plot in Figure 5 shows how seaweed groups separate from their counterparts. Overall, the Adonis test showed how bacterial composition was significantly affected by treatment (R2 = 0.06, p < 0.001). For the alpha diversity (Figure 6), the Chao1 index shows how bacterial richness was significantly higher in seaweed groups compared with the control group (p = 0.006), whereas no differences were observed for the other indices. The LEfSe analysis shows how pigs receiving U. lactuca were characterised by a higher abundance of Shuttleworthia (LDA score = 4.11, p.adj = 0.01), Anaeroplasma (LDA score = 3.98, p.adj < 0.001), Family_XIII_UCG-001 (LDA score = 3.90, p.adj = 0.04) and Lachnospiraceae_NK3A20_group (LDA score = 3.88, p.adj = 0.02). In turn, the control group was characterised by a higher abundance of Lactobacillus (LDA_score = 5.09, p.adj = 0.01), Blautia (LDA_score = 4.74, p.adj = 0.01), Campylobacter (LDA_score = 4.35, p.adj = 0.02), Coprococcus (LDA_score = 4.34, p.adj = 0.02), Holdemanella (LDA_score = 4.28, p.adj = 0.01), UCG-002(LDA score = 4.20, p.adj = 0.03) and Solobacterium (LDA_score = 3.96, p.adj = 0.02) (Figure 7).
4 DISCUSSION
Seaweeds have long been used in animal nutrition (Chapman & Chapman, 1980), but recently they have attracted the attention of the scientific community due to their potential to produce nutritionally rich biomass with low inputs, thus providing an alternative to conventional crops while promoting animal health (Costa et al., 2021; Ribeiro et al., 2021). In recent years, their bioactive properties, particularly those of brown seaweeds, have been studied mainly focusing on the use of their extracts as promoters of gut health (Corino et al., 2021; O'Doherty et al., 2021). Green seaweeds, such as U. lactuca, have nevertheless the potential to provide dietary protein while promoting gut health, which we aimed to maximise here through carbohydrase supplementation of the diets.
To the best of our knowledge, there are no results in the literature about feeding swine with green seaweeds, particularly with U. lactuca, with ingredient levels of incorporation (above 3%). However, there are some examples available for feeding other monogastric animals either in the form of extracts or intact biomass, namely poultry and rabbits (Costa et al., 2021), with higher inclusion rates, having a negative effect on growth performance. For swine in particular, Ulva sp. extracts incorporated in feed were shown to differently affect animal performance. Ulva armoricana extract (sulphated polysaccharides) has been tested in late-gestation sow diets (up to 16 g/day), not affecting litter performance (Bussy et al., 2019). Feeding growing pigs with Enteromorpha sp. enriched with copper and Zn had no significant effect on growth performance (Michalak et al., 2015, 2020). Nevertheless, Ulva prolifera extract (mainly polyphenols and unsaturated fatty acids) improved growth performance and oxidative status of weaned piglets challenged with hydrogen peroxide at 0.1% dietary supplementation (Feng et al., 2020). In the present study, there was no effect of 7% of U. lactuca in the diet on the performance of weaned piglets, regardless of carbohydrase supplementation. However, we must stress that these results were obtained in the context of a metabolic study, where piglets were individually housed and fed on a pair-feeding basis. Therefore, these conditions do not reflect commercial ones where piglets are kept in groups and are fed ad libitum, where unrestrained feed intake could generate different performance results.
Similarly to growth performance, there are few comparable data available concerning the effect of green seaweeds on feed digestibility in pigs. Michalak et al. (2020) have reported that the use of the mentioned Enteromorpha sp. biomass does not influence most TTAD or nitrogen balance parameters, except for ash digestibility, which was lower in supplemented growing pigs. Regarding weaned piglets, 5% dietary Laminaria japonica, a brown seaweed, increased DM digestibility by 2% but lowered OM digestibility by 2%, in addition to decreasing crude fibre digestibility by 9% (Brugger et al., 2020). In the present study, there was a numerical decrease of DM and OM digestibility in U. lactuca diets compared with control, which reached statistical significance in ulvan lyase-supplemented piglets. In previous studies, carbohydrase supplementation has been responsible for lower nutrient digestibility, such as CP digestibility in Arthrospira platensis-containing diets (Martins et al., 2021). This was not the case in the current study, despite possibly being related to algal starch digestibility, which is a major reserve nutrient for this seaweed (Prabhu et al., 2019) and whose resistance to weaned piglet digestion is unknown. Instead, this seems to be mostly related to the nonstarch polysaccharides. Indeed, U. lactuca inclusion lowered ADF digestibility. Ulvan has been mentioned as being undegraded by endogenous enzymes and not fermented to a large extent by colon microbiota (Corino et al., 2021), which could explain the results found in UL and ULR piglets. Unexpectedly, ulvan lyase did not improve the digestibility of the fibrous fraction of the feed. This could be explained by the circumstance that the Van Soest method (Van Soest et al., 1991) was not conceived to analyse seaweeds, thereby losing information on the effect of ulvan degradation. Therefore, we were unable to determine in which fraction it is retained or if it is retained at all during analysis. The fibre analysis of marine algae, in the context of animal production, is a field that warrants further research, and whose development is needed to accurately study these feedstuffs on ingredient levels. This has already been mentioned concerning the use of standard methods to determine the nutritional composition of microalgae (Meehan et al., 2021). It is also relevant to point out that the iodine (I) content of seaweeds is very high and may influence piglet health. However, in this particular study, we focused on a green seaweed that has a very low I accumulating capacity compared to brown seaweeds (Samarasinghe et al., 2021). The content of U. lactuca was sufficient to promote an up to 4.9-fold increase in I content in seaweed diets when compared to those in the control group. Indeed, the content of I in the diets reached a maximum of 7.12 mg/kg DM in ULR which is quite distant from the 800 mg/kg level that is mentioned as being detrimental to pig growth by the NRC (2012). Therefore, the presented data points towards an absence of the effects of dietary iodine on piglet performance.
Because seaweeds have the potential to be a rich source of minerals in the diets of domestic animals, this fraction must be understood when considering their use, because it could strongly affect its incorporation levels (Cabrita et al., 2016). This is mostly due to the possibility of compromising electrolyte balance and reaching toxic levels of other elements, which are detrimental to feed digestibility, and ultimately, animal growth and health (Guzmán-Pino et al., 2015). In our study, both Mg and K digestibility increased as a consequence of dietary U. lactuca. This caused an increased absorption of these minerals, 2.5- to 3-fold of what the needs for growing piglets (11–25 kg) according to the NRC (2012), which could explain the numerically increased urine excretion in these groups when compared to controls (data not shown). Thus, in future studies, the mineral fraction warrants special attention and may require adapting mineral premixes to their composition. Furthermore, the digestibility of Zn was increased in seaweed diets, particularly in ULU compared with control, by more than 9 percentage points. This is particularly interesting given the central role of Zn in controlling post-weaning diarrhoea in piglets and the fact that pharmacological levels of dietary zinc oxide have been recently restricted in the European Union (Brugger & Windisch, 2015). Given this relation, one might argue that its bioavailability could contribute to a healthier microbiome, which will be addressed below.
The hemicellulose fraction of the seaweed seems to be highly fermentable, which ultimately contributed to an increased NDF digestibility in seaweed-fed piglets. This is supported by the fact that calcium (Ca) and sulphur (S) digestibility, two minerals with high affinity to algal cell wall polysaccharides, were equally digestible between control and seaweed diets. Indeed, feeding piglets with fermentable carbohydrates is a strategy used to improve the microbiome and its functions (Lallès et al., 2007). This putative fermentability of dietary hemicellulose did not translate into significant effects on the VFA profile of either the caecum or colon but did modulate microbial populations. There was a reduction in isovaleric acid in the colon of piglets fed with seaweeds, which was lowest in carbohydrase-supplemented groups and a tendency for increased butyric acid proportion in the colon of seaweed-fed piglets, which was highest in those that were supplemented with carbohydrases. A recent study reports that feeding piglets with brown algae polysaccharides (laminarin) does not influence caecal VFA profile, increasing colon butyric acid instead, which was positively correlated with Prevotella bacteria (Vigors et al., 2020). Another study has reported that a sulphated polysaccharide extract from Enteromorpha clathrata promotes probiotic bacteria in the intestine of male mice, such as Bacteroides sp. and Prevotella sp. (Shang et al., 2018), which have increased as a consequence of dietary U. lactuca in the present study, regardless of enzymatic supplementation. Therefore, it seems that in this study, feeding U. lactuca to weaned piglets might limit protein fermentation (which explains the reduced isovaleric acid) in the colon, while providing substrates necessary for the proliferation of beneficial bacteria in the gut. Moreover, supplementing seaweed diets with Rovabio® resulted in the highest presence of Prevotella bacteria. Some species of this genus degrade soluble xylan that results from enzymatic activity from xylanases and β-glucanases (Flint & Bayer, 2008), which are carbohydrases that are present in the Rovabio® mix. This seemingly indicates an added beneficial effect of enzymatic supplementation. However, it is noteworthy that piglets fed with seaweed alone and supplemented with Rovabio® had a significantly high presence of Desulfovibrio and Catenibacterium respectively. These microorganisms have been related to damage to the intestinal epithelium, that is, intestinal permeability, through hydrogen sulphide production (Bi et al., 2022; Xing et al., 2022) and inflammatory processes (Namted et al., 2022) respectively. Naturally, such microbial populations are harmful to the piglet's gut health. The reason why these populations were not enriched in ulvan lyase-supplemented piglets could originate from the degradation of ulvan and the consequent release of prebiotic oligosaccharides (Corino et al., 2021) that promote the proliferation of beneficial, carbohydrate-fermenting bacteria, such as Prevotellaceae NK3B31, that also have anti-inflammatory activity (Wu et al., 2021).
Overall, control piglets had enriched populations of Lactobacillus compared with the piglets fed the other diets. These bacteria have been related to a healthier gut and the fermentation of simple carbohydrates, leading to increased production of VFA (de Vries & Smidt, 2020). In the present study, this difference can be explained by the presence of algal starch in U. lactuca diets. Tian et al. (2017) have suggested that the populations of these bacteria decrease in the piglet colon in response to resistant starch. Indeed, Ulva sp. starch has been described as having a high amylose content, which can make it resistant to enzymatic hydrolysis (Tagliapietra et al., 2022). For instance, the starch from Ulva ohnoi is found to have significantly higher amylose contents (55%) compared to either rice (34.5%) or potato (24.3%) (Kazir et al., 2021). In addition, the lower Lactobacillus populations found in these piglets are a well-documented impact of high levels of dietary Zn in weaned piglet diets (de Vries & Smidt, 2020). Furthermore, in the control piglets, there was an increased Campylobacter population, which has been reported to decrease as a consequence of 10% raw potato starch in weaned piglet diets (Yi et al., 2022). However, U. lactuca starch properties are not yet fully characterised in the context of animal nutrition, which requires further research. In contrast, the dietary seaweed promoted an increase in microbial populations such as Shuttleworthia, a butyrate producer (Miragoli et al., 2021), which could be related to the tendency found for a higher proportion of butyrate in these piglets. It also increased the population of Anaeroplasma and Lachnospiraceae_NK3A20_group which have been related to a healthier gut in piglets (Xu et al., 2018) and calves (Liu et al., 2022) respectively. This demonstrates a beneficial effect of seaweed in the weaned piglet microbiome, regardless of enzymatic supplementation, that is additionally supported by the bioavailability of seaweed Zn.
5 CONCLUSIONS AND FUTURE PERSPECTIVES
The present study demonstrates that the inclusion of 7% U. lactuca in a wheat–maize–soybean meal-based diet did not negatively affect the growth of weaned piglets. Indeed, overall digestibility parameters increased in the second experimental period, demonstrating an adaptation to all diets. However, dietary seaweed caused an ADF digestibility decrease, which was possibly compensated by the high fermentability of hemicellulose, which has beneficial effects on gut health. Furthermore, the macrominerals K and Mg were highly absorbed because of dietary seaweed, suggesting that incorporating seaweeds may require adjusting the mineral supplements provided. In turn, the seemingly high bioavailability of algal Zn may improve the gut health of piglets fed with this seaweed. In addition, our data indicate that carbohydrases supplementation of the Ulva diet is not required for this incorporation level.
In the future, further characterisation of the seaweed would improve its suitability for pig nutrition. It would be particularly interesting to characterise its starch content and digestibility for weaned piglets, test corrected premix mixtures and the effect of dietary seaweed on electrolyte balance and kidney function and test animal performance response of dietary seaweed in industry-like conditions. Finally, studying tissue metabolism, using high-throughput Omics tools, for example, to evaluate the impact of the seaweed in piglet physiology, would also help in understanding the metabolic impacts of the use of this alga in piglet nutrition. Overall, these future studies will help improve our understanding of the potential benefits and limitations of using this seaweed in piglet nutrition, ultimately contributing to the development of more sustainable and efficient pig production systems.
AUTHOR CONTRIBUTIONS
Conceptualisation: André M. de Almeida, João Pedro Bengala Freire and José António Mestre Prates. Data curation: David Miguel Ribeiro, João Pedro Bengala Freire, Paolo Trevisi and Federico Correa. Formal analysis: David Miguel Ribeiro and João Pedro Bengala Freire. Funding acquisition: José António Mestre Prates. Methodology: David Miguel Ribeiro, Daniela Filipa Pires Carvalho, Cátia F. Martins, Mónica M. Costa, Mário Pinho and Miguel Mourato. Project administration: João Pedro Bengala Freire and José António Mestre Prates. Supervision: André M. de Almeida, João Pedro Bengala Freire and José António Mestre Prates. Writing—original draft: David Miguel Ribeiro, João Pedro Bengala Freire and José António Mestre Prates. Writing—review & editing: Daniela Filipa Pires Carvalho, Cátia F. Martins, Mónica M. Costa, Federico Correa, Paolo Trevisi, Miguel Mourato, Mário Pinho, André M. de Almeida, João Pedro Bengala Freire and José António Mestre Prates.
ACKNOWLEDGEMENTS
This study was financially supported by Fundação para a Ciência e a Tecnologia (FCT, Lisbon, Portugal) through grant PTDC/CAL-ZOO/30238/2017 associated post-doc contract to M. M. C., CIISA (UIDB/00276/2020), AL4AnimalS (LA/P/0059/2020), LEAF (UIDB/04129/2020) and a PhD fellowship to D. M. R. (SFRH/BD/143992/2019).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




