N1-Acetyl-5-methoxykynuramine, which decreases in the hippocampus with aging, improves long-term memory via CaMKII/CREB phosphorylation
Abstract
Melatonin is a molecule ubiquitous in nature and involved in several physiological functions. In the brain, melatonin is converted to N1-acetyl-N2-formyl-5-methoxykynuramine (AFMK) and then to N1-acetyl-5-methoxykynuramine (AMK), which has been reported to strongly enhance long-term object memory formation. However, the synthesis of AMK in brain tissues and the underlying mechanisms regarding memory formation remain largely unknown. In the present study, young and old individuals from a melatonin-producing strain, C3H/He mice, were employed. The amount of AMK in the pineal gland and plasma was very low compared with those of melatonin at night; conversely, in the hippocampus, the amount of AMK was higher than that of melatonin. Indoleamine 2, 3-dioxygenase (Ido) mRNA was expressed in multiple brain tissues, whereas tryptophan 2,3-dioxygenase (Tdo) mRNA was expressed only in the hippocampus, and its lysate had melatonin to AFMK conversion activity, which was blocked by the TDO inhibitor. The expression levels of phosphorylated cAMP response element binding protein (CREB) and PSD-95 in whole hippocampal tissue were significantly increased with AMK treatment. Before increasing in the whole tissue, CREB phosphorylation was significantly enhanced in the nuclear fraction. In the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis, we found that downregulated genes in hippocampus of old C3H/He mice were more enriched for long-term potentiation (LTP) pathway. Gene set enrichment analysis showed that LTP and neuroactive receptor interaction gene sets were enriched in hippocampus of old mice. In addition, Ido1 and Tdo mRNA expression was significantly decreased in the hippocampus of old mice compared with young mice, and the decrease in Tdo mRNA was more pronounced than Ido1. Furthermore, there was a higher decrease in AMK levels, which was less than 1/10 that of young mice, than in melatonin levels in the hippocampus of old mice. In conclusion, we first demonstrated the Tdo-related melatonin to AMK metabolism in the hippocampus and suggest a novel mechanism of AMK involved in LTP and memory formation. These results support AMK as a potential therapeutic agent to prevent memory decline.
1 INTRODUCTION
Long-term memory (LTM) has an important role of survival in most organisms. A newly formed memory can become a long-term, stable memory if it undergoes a process known as consolidation. LTM formation is observed in Caenorhabditis elegans,1 Drosophila melanogaster,2 Aplysia californica,3 rodents,4 and humans.5 An important biological feature distinguishing short-term from LTM is that only the latter depends on a new protein synthesis. If protein synthesis is blocked before or immediately after training, LTM formation is disrupted.6 In several families of transcription factors are involved in LTM formation, notably, cAMP response element binding protein (CREB) has an essential function of memory processes in many organisms.7-9 Thus, LTM formation is highly conserved in various animal species.
Melatonin (N1-acetyl-5-methoxytryptamine) is known as the hormone of darkness because it is synthesized at night based on the length of the period of darkness.10 Melatonin is primarily synthesized by the vertebrate pineal gland, but is ubiquitous among invertebrates,11 unicellulates,11 plants,12 and even cyanobacteria.11 This hormone is involved in several functions, such as regulation of circadian rhythms,13 energy metabolism,14, 15 bone homeostasis,16, 17 and memory formation4, 18, 19; interestingly, its levels decrease by aging.20, 21
Melatonin is converted to N1-acetyl-N2-formyl-5-methoxykynuramine (AFMK) and later to N1-acetyl-5-methoxykynuramine (AMK) in the brain.22, 23 AMK may interact with calmodulin (CaM),23 which plays a crucial role in various neural functions, including cognition.24 We previously reported that melatonin and AMK administration improved LTM performance in both a melatonin-deficient strain, ICR mice4 and a melatonin-producing strain, C3H/He mice,25 using the novel object recognition (NOR) test. Interestingly, among melatonin metabolites, AMK enhance LTM at lowest dose. Previous research has shown that object recognition memory formation is enhanced during the night, when there is higher melatonin in C3H/He mice, compared with daytime.25 Since melatonin is secreted at night in all animal species, endogenous AMK may play an important role in LTM formation not only in nocturnal animals but also in diurnal animals including humans.
However, the mechanisms underlying the effect of AMK on LTM formation and the alteration of endogenous AMK levels due to aging remain unclear. In the present study, we evaluated the expression of proteins considered important for LTM, including phosphorylation of calcium/calmodulin-dependent protein kinase II (CaMKII) and CREB, in the hippocampus of AMK-treated mice. In addition, we comprehensively analyzed the expression of candidate genes involved in AMK production and the mRNA expression profiles in the hippocampus of young and old C3H/He mice, and compared the AMK levels between young and old mice. We then suggest new mechanisms for the effect of AMK on memory formation via upregulation of memory-related protein phosphorylation.
2 MATERIALS AND METHODS
2.1 Animals
C3H/He melatonin-producing mice (8-week-old male) were obtained from Sankyo Labo Service Corporation Inc., maintained under normal laboratory conditions (22°C ± 1°C, 12 h light: 12 h darkness, lights on 7:00 a.m.–7:00 p.m.). Mice participated in experiments at 2 months (young) or 22 months (old) of age. All experimental procedures received the approval from the Animal Care Committee of the Experimental Animal Center, Tokyo Medical and Dental University and the Institutional Animal Experiment Committee, Sophia University.
2.2 Measurement of melatonin, AFMK, and AMK
2.2.1 Sample preparation for the measurement of melatonin and its metabolites
Mice received the anesthesia with isoflurane and the pineal gland tissue was collected at midday (1:00 p.m.) and midnight (1:00 a.m.). Tissues from the hippocampus as well as plasma were collected from mice at midnight (1:00 a.m.). All the samples were preserved at −80°C for further analysis.
2.2.2 Liquid chromatography and mass spectrometry conditions
Melatonin, AFMK, and AMK were evaluated in tissue and plasma using an LC-MS/MS protocol.4, 17, 26 Briefly, 10-µL samples were applied into a liquid chromatography system (AC30AD; Shimadzu Corporation) having a C18 2.0 × 150-mm, 3-µm Kinetex column (Tosoh). The mobile phase used 10 µM ammonium acetate in 0.05% (v/v) acetic acid with varying concentrations. The linear gradient was performed over 20 min from 5% to 50% MeOH, thus being washed in 100% MeOH for 10 min. Further, the flow rate composed 0.3 mL/min, and the column oven and auto sampler were kept at 25°C and 4°C, respectively. Melatonin, AFMK, and AMK were identified with a triple quadrupole mass spectrometer (LCMS-8050; Shimadzu), and the quantitative analysis was performed by multiple reaction monitoring (MRM). with the transition of parent ions to product ions. The transitions for MRM were set to m/z. 233.0–130.0, m/z 265.0–136.1, and m/z 237.0–136.1 for melatonin, AFMK, and AMK, respectively.
2.3 RNA preparation and complementary DNA synthesis
Total RNA extraction from hippocampus, lateral cortex, and frontal cortex of young and old mice was carried out using a NucleoSpin® RNA kit (TaKaRa Bio). These brain tissues were collected at midday. Further, total RNA (1 μg) was reverse-transcribed to cDNA using PrimeScript Reverse Transcriptase and oligo dT primers (Takara Bio).
2.4 mRNA expression patterns of AMK synthetic enzymes in different brain tissues
Melatonin is presumed to be metabolized to AFMK by indoleamine 2,3-dioxygenase (IDO) or tryptophan 2,3-dioxygenase (TDO); then, AFMK turns into AMK by arylamine formamidase or catalase pathways. Thus, we analyzed the mRNA expression patterns of these AMK synthetic enzymes (IDO1, IDO2, and TDO) in hippocampus, lateral cortex, and frontal cortex by reverse transcription polymerase chain reaction (RT-PCR). PCR amplification was conducted using Ex Taq DNA Polymerase (Takara Bio) and primers correspondent to each mRNA of interest (see Supporting Information: Table S1). The condition of amplification included: denaturation at 95°C for 50 s followed by 30 cycles (TDO and β-actin) or 40 cycles (IDO1 and IDO2) at 95°C for 10 s, 65°C for 30 s, and 68°C for 1 min. PCR products were divided by electrophoresis on 2.5% agarose gels stained with ethidium bromide and visualized under UV.
2.5 NOR test
The NOR test was done as formerly described.4 Briefly, first, each animal was habituated to the empty arena for 5 min daily for 3 days. In the training phase, animals participated in one acquisition trials (8 s) during which they could freely explore two identical objects, which was placed symmetrically in the arena at a distance of 4 cm from the wall and 5 cm from each other. C3H/He mice have poor eyesight, because of degenerated retina due to inactivated photoreceptor cGMP phosphodiesterase 6 β-subunit (Pde6b) gene. The objects used were selected for tactile discrimination. Mice were underwent test phase after 24 h (for LTM) delay. One of the familiar objects was replaced with a new located at the same place, and the mice were given 3 min for free exploring. To eliminate bias due to preference for a particular location, the locations of novel and familiar objects were swapped in half of each experimental group. A blinded trained observer quantified the time spent exploring the objects based on the recording. The NOR test was performed in the daytime. The discrimination index (DI) was measured as follows: percentage of time spent exploring the novel object divided by the total time spent exploring both objects. Object recognition was regarded for DIs that are significantly higher chance performance (>50%).27, 28 Animals were intraperitoneally administered with AMK (0.1, 1, or 10 mg/kg body weight, Toronto Research Chemicals) at 5 min after training during the light period.
2.6 Western blot analysis
The hippocampus was collected after AMK injection (1 mg/kg bw), and homogenized in RIPA buffer (Fujifilm Wako Pure Chemical Corp.) including protease and phosphatase inhibitors. Before nuclear fraction extraction was performed, the samples were homogenized in ice-cold phosphate buffered saline, centrifuged at 500g for 5 min at 4°C, and the supernatant was removed. Nuclear protein fractionation was obtained with a cell fractionation kit following manufacturer's instructions (SF PTS kit; GL Sciences Inc.). Next, equal amount of proteins per sample were applied onto 10% SDS-PAGE gels (Bio-Rad Laboratories) and transferred to polyvinylidene difluoride membranes. Following blocking using StaringBlock™ (TBS) blocking buffer (Thermo Scientific), the membrane was incubated at 4°C overnight with rabbit anti-p-CaMKII (Thr286, 1:1000; Cell Signaling), anti-p-CREB (Ser-133, 1:1000; Novus Biologicals), anti-Lamin B1 (1:1000; PGI Proteintech, Inc.), anti-postsynaptic density-95 (PSD95) (1:1000; GeneTex), and anti-β-actin (1:1000; GeneTex) primary antibodies. Thereafter, the blots were treated with a horseradish peroxidase–conjugated secondary antibody (1:5000; Cell Signaling). Then chemiluminescence signals were detected by Clarity Western ECL Substrate (Bio-Rad Laboratories) with LuminoGraph (ATTO).
2.7 RNA sequencing
RNA-seq libraries were prepared with NEBNext® Poly(A) mRNA Magnetic Isolation Module and NEBNext® Ultra™II Directional RNA Library Prep Kit for Illumina (New England Biolabs). Then, adjusted libraries were sequenced with 150 bp paired-end reads using Novaseq. 6000. Quality check of raw read sequence was performed using FastQC version 0.11.8. Adaptors and low-quality reads were trimmed with Trimmomatic-0.39 with default parameters. The number of raw and processed reads are shown in Supporting Information: Table S2. The sequence reads were mapped to GRCh38 using STAR v.2.6.1c followed by RSEM for gene quantification. The raw read counts were normalized and the differentially expressed genes (DEGs) were analyzed using the DESeq. 2R package. The Data Base for Annotation, Visualization and Integrated Discovery (DAVID) database was used to analyze the DEGs in the hippocampus of young and old mice. The enrichment pathways were evaluated with the Kyoto Encyclopedia of Genes and Genomes (KEGG) program.
2.8 Quantitative real-time PCR (qPCR)
qPCR was carried out with primers for memory formation related genes and AMK synthesis genes (Supporting Information: Table S1). β-actin was used as an internal amplification control; no amplified products were detected in RT-missing samples as a negative control. qPCR amplification was performed with Mx3000P qPCR System (Agilent Technologies) and SYBR Ex Taq DNA Polymerase II (Takara Bio). qPCR conditions included 40 denaturation cycles at 95°C for 10 s and annealing/extension at 65°C for 40 s; then, melt curve analysis was performed.
2.8.1 Effect of TDO inhibitor on AFMK formation in hippocampus lysate
TDO was found to have metabolic activity from melatonin to N1-acetyl-N2-formyl-5-methoxykynuramine (AFMK). The activity of this enzyme was determined based on the modification of the previous method.29 The hippocampus was homogenized with ice-cold phosphate buffer (pH 7.0) containing 2 μM hematin. The homogenate was centrifuged at 20 000g for 10 min, and the supernatant was used for assay of hippocampus TDO activity. Melatonin was added to the supernatant at 10−5 M (final concentration) and incubated for 24 h at 37°C. A group was also prepared in which 5 μM of 680C91 (selective TDO inhibitor; Tocris Bioscience) was added simultaneously. After incubation, all samples were immediately analyzed by LC-MS/MS.
2.8.2 Statistical analysis
All data are provided as the mean ± standard error of the mean. Group comparisons were performed by two- or one-way analysis of variance (ANOVA) followed by Dunnett's multiple comparisons. Comparison between two groups was carried out with Student's t test.
3 RESULTS
3.1 Melatonin, AFMK, and AMK levels in the pineal gland, plasma, and hippocampus
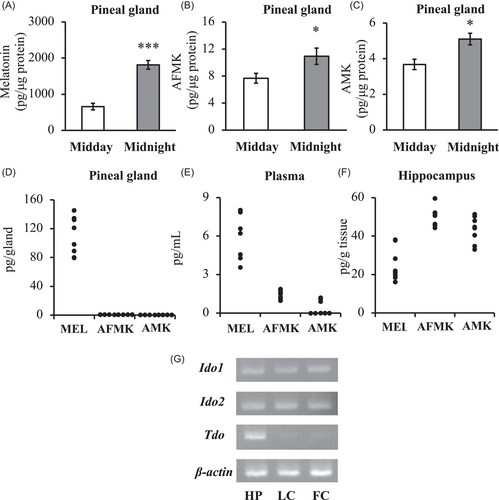
In the pineal gland, the melatonin levels were significantly higher at midnight (1812.5 ± 120.0 pg/μg protein) than that at midday (658.5 ± 92.1 pg/μg protein; Figure 1A). We also found a significant increase in AMK levels from midday (3.68 ± 0.30 pg/μg protein) to midnight (5.10 ± 0.33 pg/μg protein; Figure 1C) similar to AFMK levels (Figure 1B). The amounts of AFMK and AMK in pineal gland and plasma were very small quantity at midnight, while much higher melatonin levels observed in the same samples (Figure 1D,E). However, amounts of AFMK and AMK in hippocampus was higher than melatonin (Figure 1F). These findings indicate that AFMK and AMK is not synthesized in the pineal gland and melatonin was metabolized to AFMK and AMK in the hippocampus. The mRNA expression patterns of the metabolic enzymes implied in the metabolism of melatonin to AFMK (Ido1, Ido2, and Tdo) were analyzed by RT-PCR and agarose gel electrophoresis. Ido1 and Ido2 mRNA were detected in the hippocampus as well as lateral cortex and frontal cortex, while Tdo mRNA was only observed in the hippocampus (Figure 1G). Furthermore, hippocampus lysate samples had AFMK metabolic activities and were also blocked by the selective TDO inhibitor 680C91 (62.3%, Supporting Information: Figure S1).
3.2 Effect of AMK on LTM performance in C3H/He mice
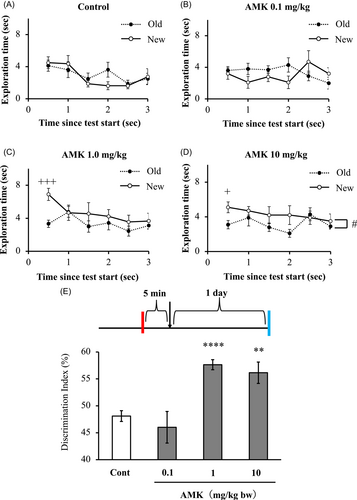
The effect of AMK was assessed in a single training session and a 24 h intertrial interval. AMK was administered 5 min after the training phase. The exploration time and time since test start in each group are shown in Figure 2A–D. In the 10 mg/kg AMK-treated group, the time employed to explore the new object was significantly longer than that used to explore the old object (two-way ANOVA, p < .05; Figure 2D). Within the first 30 s after the test start, the 1.0 and 10 mg/kg AMK treated groups explored significantly longer for the novel object compared with old object (p = .00076 and p = .021, respectively; Figure 2C,D); in contrast, there was no significant difference between novel and old objects in the control group (Figure 2A) and in the 0.1 mg/kg group (Figure 2B). The low AMK dose (0.1 mg/kg bw) treated group showed no significant changes in DI (46.0%), but a higher AMK dose (1.0 and 10 mg/kg bw) significantly increased the DIs compared with the chance level (p = .000098 and p = .010, respectively) and to the control group (p = .000031 and p = .0041, respectively; Figure 2E).
3.3 Effect of AMK treatment in the expressions of LTM formation related proteins
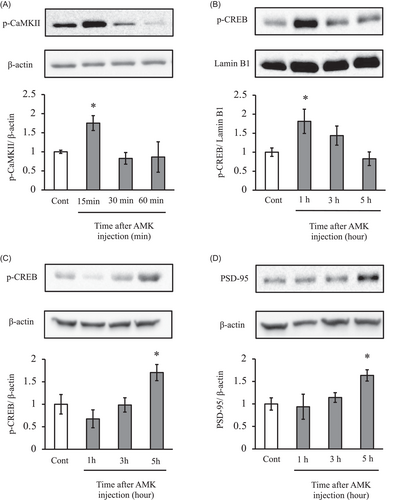
In whole hippocampus tissues lysates, AMK treatment significantly increased the p-CaMKII expression at 15 min compared with control (175.3%; Figure 3A). In the nuclear fraction, CREB phosphorylation significantly increased with AMK treatment at 1 h (181.2%; Figure 3B). At 5 h, the expression levels of CREB phosphorylation and PSD-95 (transcribed from the gene discs large homolog 4, Dlg4) in the whole hippocampus lysates significantly increased (170.4% and 163.5%, respectively; Figure 3C,D).
3.4 RNA-seq data analysis in the hippocampus of young and old mice
The raw data of sequence of reads were deposited at the DNA Data Bank of Japan (DDBJ) under the DDBJ Sequence Read Archive (DRA) accession no. DRA016891. The gene expression patterns in the hippocampus of young and old mice differed substantially (Figure 4A). In the hippocampus, we identified 5307 DEGs, of which 2940 were downregulated in old mouse samples (Figure 4B,D). GO slim overviewed the ontology content in upregulated and downregulated DEGs, respectively (Figure 4C).
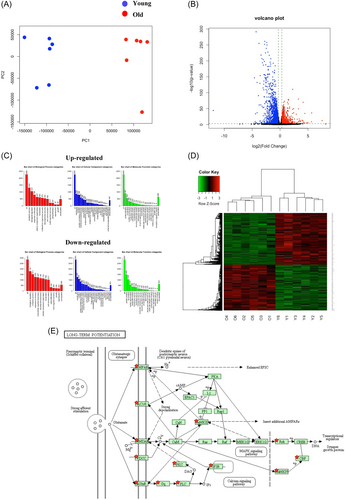
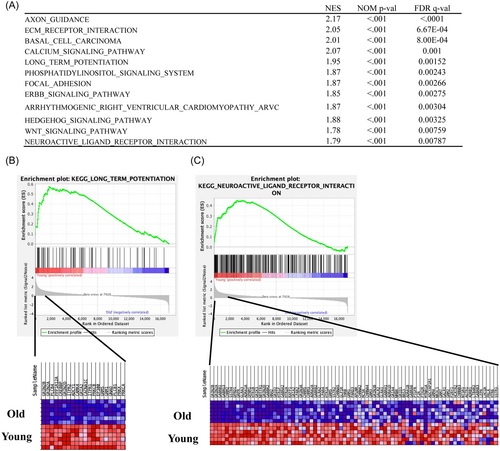
The enrichment of dysfunctional signaling pathways was screened by the KEGG pathway analysis. This analysis showed 62 pathways enriched in downregulated DEGs (FDR < 0.001; Supporting Information: Table S3). Most importantly, long-term potentiation (LTP) was significantly enriched (Figure 4E). Then, Gene Set Enrichment Analysis (GSEA) was performed using gene sets for canonical pathways derived from the KEGG pathway database to evaluate the differences in mRNA expression levels in the hippocampus of young and old mice. The gene sets in Figure 5 A correspond to genes with an FDR q value < 0.001. We observed enrichment of several gene sets, including those related to calcium signaling pathway and phosphatidylinositol signaling system. Notably, gene sets related to LTP (Figure 5B: normalized enrichment score = 1.95) and neuroactive ligand receptor interaction (Figure 5C: normalized enrichment score = 1.79) were downregulated in old mice.
3.5 Expression of memory formation related genes and AMK synthesis genes, and AMK levels in young and old mice
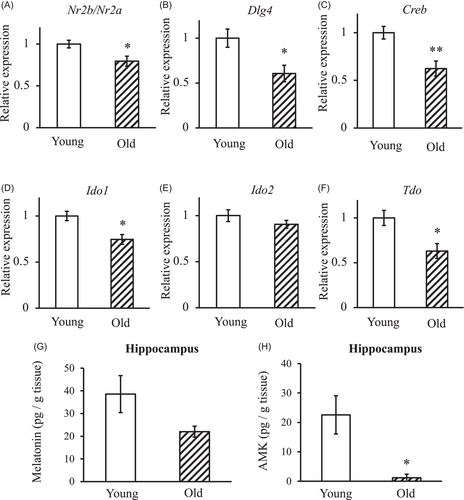
The Nr2b/Nr2a expression ratio in old mice hippocampus was significantly lower than that in young mice (80.0%; Figure 6A). Similarly, Dlg4 and Creb mRNA levels were also significantly decreased in old mice compared with young mice (60.6% and 62.4%, respectively; Figure 6B,C). The mRNA expression levels of melatonin to AFMK and AMK synthase in the hippocampus are shown in Figure 6D–F. Ido1 and Tdo mRNA expression in the hippocampus of old mice was significantly lower than that of young mice (74.6% and 63.1%, respectively) but no significant differences in Ido2 mRNA expression in the hippocampus was observed between young and old mice (Figure 6D–F). Additionally, the melatonin levels in the hippocampus tended to be lower in old mice (22.0 ± 2.4 pg/g tissue) than in young mice (38.6 ± 8.1 pg/g tissue). Interestingly, the hippocampal AMK levels in old mice (1.1 ± 1.2 pg/g tissue) were very low and significantly lower than in young mice (22.6 ± 6.5 pg/g tissue; Figure 6G,H).
4 DISCUSSION
Mild cognitive impairment (MCI) is commonly being used to refer a stage of cognitive impairment before attaining clinical dementia in Alzheimer's disease (AD). MCI is a clinical syndrome thought to represent the intermediate cognitive state between normal aging and dementia. Patients aged over 65 years diagnosed with MCI are more likely to develop dementia than normal elderly people.30 On the other hands, melatonin production level reaches highest at the age of 1–3 years and reduces to less than 10% during senescence.20 Melatonin improves learning and memory performance on impaired cognitive deficits rat,19 furthermore, melatonin supplementation could ameliorate early postoperative cognitive decline in aged patients.31 Hence, melatonin and its metabolites may possibly act a potential role of age-related memory dysfunction.
Similar to melatonin, the endogenous AFMK and AMK production in the pineal gland at midnight was significantly higher than at midday, but the AFMK and AMK levels in the pineal gland and plasma were much lower than melatonin levels. In contrast, AFMK and AMK levels in the hippocampus at midnight were higher than melatonin (Figure 1). Further, we found that Tdo (a melatonin-to-AFMK converting enzyme) was expressed only in the hippocampus; in hippocampus lysate, AFMK formation was blocked by a TDO inhibitor (62.3%), which also blocked tryptophan-to-kynurenine formation (53.6%; Supporting Information: Figure S1). Moreover, AMK levels and Tdo mRNA expression in the hippocampus of old mice were significantly lower than those of young mice (Figure 6). These findings suggest that AFMK and AMK production in the hippocampus are regulated by TDO. The memory impairment observed in old C3H/He mice,25 which might be caused by mainly downstream of Tdo mRNA expression and/or TDO enzyme activity. IDO1 and IDO2 have different affinities for tryptophan. While IDO1 has a high catalytic efficiency for tryptophan, IDO2 has very low catalytic efficiency.32-34 We found decreased Ido1 and Tdo mRNA expression in the hippocampus of old mice compared with that of young mice (74.6% and 63.0%, respectively). Previously, using ICR mice, we showed an Ido inhibitor blocked LTM enhancement by melatonin treatment.4, 25 Further studies focusing on the role of various genes in AMK synthesis are needed to elucidate the mechanisms of IDO and TDO involvement in AMK synthesis.
We previously reported that AMK (0.1 mg/kg bw) increased the DIs after single training trial in ICR mice.4 Here, a higher AMK dose (1.0 and 10 mg kg bw) could facilitate LTM in C3H mice (Figure 2). Since C3H mice, melatonin-producing strain, are constantly exposed to melatonin and AMK, the sensitivity against melatonin and AMK may be lower than that of ICR mice. Otherwise, C3H mice is thought to have poor eyesight, it is possible that AMK effects in mice was depends on visual sense rather than tactile sense. In this manner, differences of strain-specific or NOR conditions might be involved in effective dose of AMK.
In a previous study, we found that AMK administration facilitated LTM in aged mice,4 which had age-associated memory impairments. In KEGG pathway analysis, genes related with LTP were downregulated in old C3H mice. Stimulation of Ca2+/CaM-dependent protein kinase (CaMK) II via Ca2+ influx through N-methyl-d-aspartate–type glutamate receptors is one of the core signaling mechanisms that induce early LTP.35 After a single AMK administration, p-CaMKII expression was induced at 15 min (Figure 3A). Since AMK has a higher CaM affinity than melatonin23 and CaMKII is a target of Ca2+/CaM, AMK may be associated with the CaMKII signaling pathway. The entry of Ca2+ into cell causes to the formation of Ca2+/CaM complex.36 This complex binds to the regulator region of CaMKII, and leads to the phosphorylation of its substrate and holoenzyme autophosphorylation at threonine 286 (Thr286) in the isoform. Thr286 phosphorylation plays an important role in induction of LTP and it greatly promotes the activation of CaMKII and LTP.37 In addition, CaMKII binds to many proteins in the post-synaptic density. In particular, PSD-95 expression increased at 5 h after AMK treatment. This finding could result from CaMKII activation.
CREB belongs to a large family of structurally related transcription factors that bind to promoter cAMP-responsive element sites and is essential in LTM. In the present research, phosphorylation of CREB increased in the nuclear fraction 1 h after AMK administration and in the whole hippocampal lysate at 5 h thereafter (Figure 3B,C). AMK might promote the nuclear phosphorylation of CREB followed by the translocation of p-CREB from the nucleus to the cytoplasm. Recent findings propose that neuronal activity induces phosphorylated CaMKII nuclear translocation, whose main role is to shuttle Ca2+/CaM to the nucleus, leading to CREB phosphorylation and enhanced transcription. Memory-enhancing effect of AMK on LTM formation likely reflect processes that follow memory encoding. It has been well known that CaMKII is involved in memory consolidation38 and AMK may promote phosphorylation of CREB via CaMKII pathway. To the best our knowledge, this is the first report on the mechanisms of AMK effects on learning and memory in mice.
Melatonin has been shown to affect learning and memory. Several studies showed high fat diet (HFD) induces memory impairment.39, 40 In HFD fed rat, melatonin prevented this impairment by preventing alteration of oxidative stress in the hippocampus.41 Scopolamine, a nonselective antimuscarinic agent, leads to progressive memory impairment by blocking central cholinergic signaling,42 however, melatonin attenuated scopolamine-induced synaptic dysfunction and memory impairments by ameliorating oxidative brain damage and neuroinflammation.43 Acute melatonin treatments can improve cognitive performance. A single melatonin administration before training trials facilitated LTM assessed 24 h posttraining in the NOR tasks,44 which is consistent with our previous findings.4 Together, it has been demonstrated that a single melatonin administration facilitates cognitive performances in human.45 Furthermore, melatonin improved memory deficits of the AD model mice.46 A systematic review showed night-time melatonin levels in cerebrospinal fluid and blood of AD patients were lower compared with healthy controls.47 Considering our results, these melatonin effects on memory formation may be causable by a melatonin metabolite, AMK.
AD accounts for 50%–75% of all dementia cases.48 With a rapidly aging world population, the incidence rate of AD is estimated to increase to 115.4 million cases by 2050.49 It has been reported that melatonin secretion gradually decreases with the progression of AD.50 In patients with MCI, melatonin treatment showed positive effects on improving sleep, cognition, depression, and behavioral symptoms.51 This effect on cognition may also be inherent in the metabolites of melatonin, AMK. AMK is a potential therapeutic agent to improve quality of life in individuals with MCI patients and the elderly.
In conclusion, this study of C3H/He mice (a melatonin-producing strain) is the first to demonstrate that endogenous AMK production in the brain dramatically decreases with age and that AMK ameliorates age-related memory impairments though changes in CaMKII and CREB phosphorylation levels. The present data strongly suggest that reduced endogenous AMK production is associated with memory decline in the elderly. Thus, the present results indicate that AMK plays as a potentially promising drug for the prevention of MCI.
AUTHOR CONTRIBUTIONS
Kazuki Watanabe performed most of the experiments and wrote first draft. Haruyasu Kato, Hikaru Iwashita, and Jun Hirayama assisted in some studies. Yusuke Maruyama provided advice on measurement of melatonin metabolites using the LC-MS/MS. Atsuhiko Hattori supervised all studies and drafting of the manuscript.
ACKNOWLEDGMENTS
The authors would like to thank Atsuhiko Chiba, Department of Materials and Life Sciences, Faculty of Science and Technology, Sophia University, and Yukihisa Matsumoto, Department of Biology, College of Liberal Arts and Sciences, TMDU for their kind support, as well as the staff at TMDU's experimental animal center. The super-computing resource was provided by Human Genome Center (the Univ. of Tokyo), through the usage service provided by M&D Data Science Center, Tokyo Medical and Dental University. This work was supported by JSPS KAKENHI Grant Number JP22K11823 to Atsuhiko Hattori and JP22J01508 to Kazuki Watanabe.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The original contributions presented in the study are included in the article/supplementary material. The RNA-seq raw data are deposited at DNA Data Bank of Japan (DDBJ; https://www.ddbj.nig.ac.jp/index-e.html) Sequence Read Archive (DRA), under the accession number DRA016891.




