Melatonin for prevention of placental malperfusion and fetal compromise associated with intrauterine inflammation-induced oxidative stress in a mouse model
Funding information
This research was in part supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (NRF-2019R1C1C1008223, 2016R1D1A1B03931905) and Johns Hopkins University's Integrated Research Center for Fetal Medicine Fund.
Abstract
Melatonin has been shown to reduce oxidative stress and mitigate hypercoagulability. We hypothesized that maternally administered melatonin may reduce placental oxidative stress and hypercoagulability associated with exposure to intrauterine inflammation (IUI) and consequently improve fetoplacental blood flow and fetal sequelae. Mice were randomized to the following groups: control (C), melatonin (M), lipopolysaccharide (LPS; a model of IUI) (L), and LPS with melatonin (ML). The expression of antioxidant mediators in the placenta was significantly decreased, while that of pro-inflammatory mediators was significantly increased in L compared to C and ML. The systolic/diastolic ratio, resistance index, and pulsatility index in uterine artery (UtA) and umbilical artery (UA) were significantly increased in L compared with other groups when analyzed by Doppler ultrasonography. The expression of antioxidant mediators in the placenta was significantly decreased, while that of pro-inflammatory mediators was significantly increased in L compared to C and ML. Vascular endothelial damage and thrombi formation, as evidenced by fibrin deposits, were similarly increased in L compared to other groups. Maternal pretreatment with melatonin appears to modulate maternal placental malperfusion, fetal cardiovascular compromise, and fetal neuroinflammation induced by IUI through its antioxidant properties.
1 INTRODUCTION
Oxidative stress induces vasoconstriction, hypercoagulation, and pathologic vascular remodeling, which are all implicated in the pathophysiology of obstetric complications, including miscarriage, preeclampsia, and fetal growth restriction.1 Also, oxidative stress is an inevitable component of inflammation as pro-inflammatory cytokines activate mediators of oxidative stress which then further induce cytokines in a vicious cycle.1-3 The pro-inflammatory cytokine response induced by intrauterine inflammation IUI) during pregnancy is thought to be a major cause of preterm birth and fetal inflammatory response syndrome FIRS), including fetal brain injury.2, 4, 5 In a recent study, our group showed that exposure to IUI leads to placental malperfusion and fetal vessel resistance, which ultimately predisposes the fetus to cardiac dysfunction and brain damage.6 Ultimately, this type of brain injury can lead to a spectrum of adverse neurobehavioral outcomes, such as cerebral palsy, autism, schizophrenia, and cognitive delay.2, 7-10 Furthermore, preterm birth constitutes up to one-third of perinatal mortality and more than one-half of long-term morbidity.11, 12
Melatonin, a major secretory hormone of the pineal gland, has antioxidant and anti-inflammatory effects. While melatonin is primarily produced in the pineal gland, the placenta acts as one of the major extrapineal sources of melatonin during pregnancy.13, 14 Melatonin easily crosses the placenta and the blood-brain barrier and is considered to be important for normal placental function as well as fetal growth and brain development.15, 16 Furthermore, current studies demonstrate that melatonin supplementation is relatively safe for the mother and the fetus.13, 17-19
As an antioxidant and anti-inflammatory agent, melatonin inhibits lipopolysaccharide (LPS)-induced release of reactive oxygen species (ROS) and inflammatory cytokines and chemokines.20-22 Previous studies have shown that melatonin protects mice from LPS-induced fetal death, intrauterine fetal growth restriction, and preeclampsia through its antioxidant and anti-inflammatory effects.23, 24 In preeclampsia, melatonin therapy was assessed to be a safe and effective adjuvant therapy by improving endothelial function and prolonging pregnancy although no significant results could be seen with its antioxidative effects against the excessive placental oxidative stress in preeclampsia.25 Our group recently demonstrated that melatonin pretreatment in LPS-treated mice resulted in reduced levels of pro-inflammatory cytokines in the uterus and the placenta.26 We also showed that melatonin may prevent adverse neuromotor outcomes in offspring exposed to maternal inflammation.26
Despite the vast research on the overall anti-inflammatory qualities of melatonin, there have only been few reports on the antioxidative effects of melatonin on pathologic vascular changes of placenta and fetal sequelae as a result of IUI-induced oxidative stress.24, 25, 27-29 Based on the antioxidant features of melatonin, we hypothesized that maternally administered melatonin may reduce oxidative stress, pathologic vascular changes of the placenta, and fetal complications caused by IUI-induced oxidative stress.
2 MATERIALS AND METHODS
2.1 Animal preparation
All animal care and treatment procedures were approved by the Animal Care and Use Committee of Johns Hopkins University Hopkins-IACUC Protocol No. MO14M326. CD1 pregnant dams Charles River were used for this study. Pregnant dams were subjected to a well-established model of IUI as per a previously described protocol on embryonic day 17 (E17).30-32 Mice were anesthetized with isoflurane (Baxter # NDC 10019-360-60) before undergoing a mini-laparotomy. After dressing the abdominal area, a 1.5-cm midline incision was performed in the lower abdominal wall. Mice were randomized to intrauterine injections of either lipopolysaccharide (LPS), a model of IUI, or phosphate-buffered saline (PBS) at E17 as follows: (a) control (C); n = 44 dams and 70 pups; (b) melatonin (M); n = 24 dams and 50 pups; (c) LPS (L); n = 51 dams and 83 pups; and (d) LPS with melatonin (LM); n = 50 dams and 82 pups. LPS (Sigma-Aldrich) from E. coli O55:B5, 25 μg in 100 μL PBS in L and LM groups or 100 μL of vehicle (PBS) by itself in C and M groups, was injected between the first and second amniotic membranes of the lower right uterine horn with care to not enter the amniotic cavity. Routine laparotomy closure was performed, and the dams recovered. Melatonin (Sigma-Aldrich), 10 mg/kg in 100 µL PBS for M and LM groups or 100 µL of vehicle (PBS in C and L groups), was injected intraperitoneally 30 minutes before the LPS injection. Pregnant mice were sacrificed 2 or 6 hours after the LPS injection to collect tissue samples of placenta and fetal brain for biochemical and histological analysis.
2.2 Doppler examination
Doppler examination was performed for the uterine artery (UtA), placental and fetal sides of the umbilical artery (UA), and fetal Myocardial Performance Index Tei index) 6 hours after LPS exposure in the IUI group and PBS exposure in the control group right before the mice were sacrificed, in the same way as a previously described protocol.6 Briefly, general anesthesia was induced by inhalation of 5% isoflurane and oxygen at 3 L/min and maintained with 1.5% isoflurane and oxygen at 2 L/min. The average amount of isoflurane used per mouse is 2.38 ± 0.66 mL. Doppler waveforms of the UtA were obtained at the level where the main UtA branches from the internal iliac artery and were measured on both the right and left sides. The right and left Doppler waveforms were not different. The pulsatility index (PI) and the presence of early diastolic notch were evaluated in UtA. An early diastolic notch in the uterine arteries is defined as the lowest velocity just after systolic flow but before maximum diastolic flow. To evaluate the depth of the notch, the diastolic notch index was used. The diastolic notch index was quantified by measuring the ratio between the early diastolic flow (A) and peak diastolic flow (B). This A/B ratio allowed measuring of the notch depth. We considered that an early diastolic notch was present when the diastolic notch index was 0.7 or greater, based on the previous study.33 Doppler waveforms of the placental and fetal sides of UA were obtained at the placental cord insertion sites and fetal abdominal cord insertion sites, respectively. Doppler waveforms of UA and Tei index were obtained from both sides of the uterus (1 to 2 fetuses on the right, 1 to 2 fetuses on the left). PI and the presence of absent end-diastolic flow (AEDF) were evaluated in the UA. PI was calculated as [(peak systolic velocity − end-diastolic velocity)/mean velocity]. To measure the Tei index, tissue Doppler imaging (TDI) of the mitral annulus was obtained from the apical four-chamber view. The Tei index was calculated as [(isovolumic contraction time + isovolumic relaxation time)/ejection time]. All Doppler waveforms were taken at an angle as close as possible to 0°. Vevo 770 (VisualSonics) with a 22-83 MHz, the RMV 708 probe (VisualSonics), was utilized for the Doppler evaluation.
2.3 RNA extraction and quantitative polymerase chain reaction
After euthanizing the mice 2 or 6 hours after the LPS or PBS injection, quantitative polymerase chain reactions (qPCRs) were completed based on protocols described in the previous study.34 Placentas were isolated from the first through fourth gestational sacs of the right uterine horn and fresh frozen on dry ice, followed by long-term storage at −80°C. Initially for each placental specimen, 2 µg of RNA was used for complementary (c)DNA synthesis in a 40 µL reaction, using Bio-Rad iScript™ cDNA Synthesis Kit (Bio-Rad, Cat. No. 170-8891). The primers for actin (Assay ID Mm.PT.39a.22214843.g), NF-κB1 (Mm.PT.58.30400172), including interleukin (IL)-1β (Mm.Pt.58.44004828), IL-6 (Mm.PT.58.13354106), and tumor necrosis factor alpha (TNF-α) (Mm.PT.58.12575861) were obtained from Integrated DNA Technologies. The primer for 18S (Cat. No. 4310893E) was obtained from Applied Biosystems (Thermo Fisher Scientific).
2.4 Western blot analysis
After euthanizing the mice 2 or 6 hours after the LPS or PBS injection, placentas were isolated from the first through fourth gestational sacs of the right uterine horn and fresh frozen on dry ice, followed by long-term storage at −80°C. Proteins from homogenized placenta lysed in RIPA buffer (containing proteases inhibitors) were quantified using Bradford's method (Bio-Rad). The segregated proteins (10 μg/lane) were isolated by 8% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred onto nitrocellulose membranes (Bio-Rad), and blocked with 5% skim milk (Bio-Rad) in Tris-buffered saline (TBS; Bio-Rad) with 0.1% Tween-20 (Sigma-Aldrich). Membranes were incubated with primary antibodies against 4-hydroxy-2-nonenal (4-HNE; Abcam), silent information regulator transcript-1 (SIRT1; Abcam), nuclear factor erythroid 2-related factor 2 (Nrf2; Abcam), phosphorylated nuclear factor kappa beta (pNF-kB; Abcam), or β-actin (Abcam) diluted in TBS-Tween-20 with 5% skim milk at 4°C overnight. Membranes were then washed with PBS containing 0.1% Tween-20 (Sigma-Aldrich) and incubated for 1 hour with their respective secondary Abs conjugated to HRP (Sigma-Aldrich). An enhanced chemiluminescent Western blotting substrate kit (Bio-Rad) was used for detection. The signals were imaged with a ChemiDoc XRS + Imager (Bio-Rad) using Image Lab software (Bio-Rad), and densitometric analysis was performed using ImageJ (National Institutes of Health; http://rsb.info.nih.gov/ij/). Resulting values were normalized first with β-actin and then as a ratio of the control samples.
2.5 Histochemistry and immunohistochemistry
Six hours after LPS or PBS injection, fresh placentas and whole fetal brains were dissected and fixed in 4% paraformaldehyde (PFA) (Affymetrix Inc) for histology. Tissue was then immersed in 30% sucrose (Sigma-Aldrich). Using Leica CM1950 cryostat (Leica Biosystems Inc), the specimens were cut at 20 µm thickness and mounted on positively charged slides (Fischer Scientific). Placentas were cut transversally, and fetal brains were cut coronally. For immunohistochemistry (IHC), slides were incubated in PBS solution containing 0.05% Triton X-100 (Sigma-Aldrich) and 5% normal goat serum (Invitrogen) for 30 minutes. Placental tissues were incubated with endothelial markers, rabbit anti-vimentin antibody 1:200, Abcam),35 and rat anti-CD31 (1:200, Abcam).36 Ionized calcium-binding adaptor molecule 1 IBA1) is a marker of fetal brain injury.37 Fetal brains were incubated with IBA1 antibody (1:400, Wako Chemicals).38 Donkey anti-rabbit Alexa Fluor 568 and donkey anti-rat Alexa Fluor 488 fluorescent secondary antibodies were utilized (1:500, Life Technologies) along with DAPI counterstaining. All images analyzed were obtained from Zeiss AxioPlan 2 Microscope System. Placental data were obtained at the middle level. Fetal brain data were collected from the cortical area, including the cortex plate, subplate, intermediate zone, and ventricular zone. Analyses were performed with ImageJ by evaluators blinded to group identification.
2.6 Fibrinogen and fibrin measurement
Histochemical staining (rapid phosphotungstic acid hematoxylin, PTAH, Polysciences Inc) was applied to the harvested placenta for fibrin staining, according to the manufacturer's protocol. Analyses were performed with ImageJ by evaluators blinded to group identification.
2.7 Statistical analyses
Discrete data were analyzed using chi-squared test and continuous variables using a one-way ANOVA with Tukey post-HSD test or Kruskal-Wallis test with Bonferroni-Dunn correction. Continuous data were tested for normality, and outliers were identified using Grubb's test. When outliers were identified in data following normal distribution, they were excluded from the analysis. Statistical significance was set at P < 0.05. Data analyses were performed with GraphPad Prism version 5 (GraphPad Software).
3 RESULTS
3.1 Melatonin prevented uterine and umbilical artery insufficiency in dams and pups exposed to IUI-induced oxidative stress
PI at the UtA in C (0.93 ± 0.05), M (1.03 ± 0.10), L (1.34 ± 0.07), and ML (0.80 ± 0.05) showed that the value was significantly increased in L compared to C (P < 0.001) and ML (P < 0.001). The rate of early diastolic notch in the UtA in C (0.0%), M (0.0%), L (17.2%), and ML (0.4%) showed significant increase in L than ML (P < 0.001). And PI at both the placental and fetal sides of the UA in C (1.38 ± 0.05, 1.51 ± 0.05), M (1.25 ± 0.14, 1.34 ± 0.13), L (1.74 ± 0.03, 1.76 ± 0.03), and ML (1.58 ± 0.03, 1.55 ± 0.04) showed that values were significantly increased in L compared to C (P < 0.001, P < 0.001), M (P < 0.001, P < 0.001), and ML (P < 0.001, P < 0.001). The rate of absent end-diastolic flow (AEDF) in both the placental and fetal sides of UA in C (0.0%, 0.0%), M (0.0%, 0.0%), L (42.3%, 17.2%), and ML (0.0%, 0.0%) showed significant increase in L than ML (P < 0.05, P < 0.01). (Figure 1).
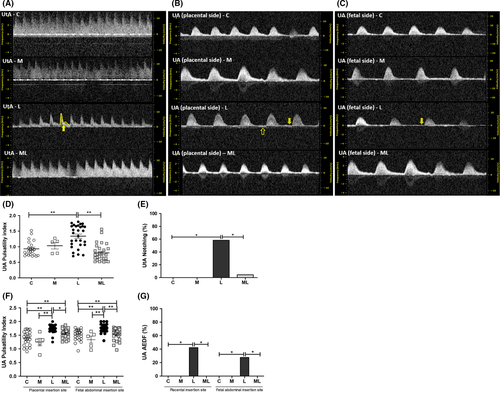
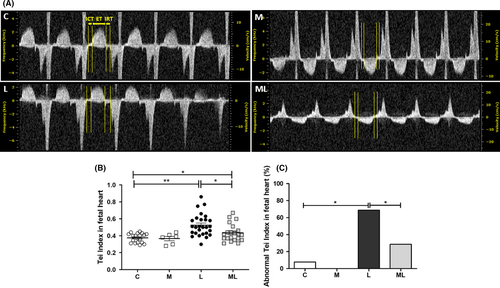
3.2 Melatonin prevented the increase in left ventricle Tei index in fetuses exposed to IUI-induced oxidative stress
Tei indices in C (0.38 ± 0.01), M (0.37 ± 0.03), L (0.53 ± 0.03), and ML (0.43 ± 0.02) showed that the value was significantly increased in L compared to C (P < 0.001) and ML (P = 0.012). The rate of abnormal Tei indices (>0.44) in C (7.7%), M (0.0%), L (68.8%), and ML (28.6%) showed significant increase in L compared with C (P < 0.01) or ML (P < 0.05) (Figure 2).
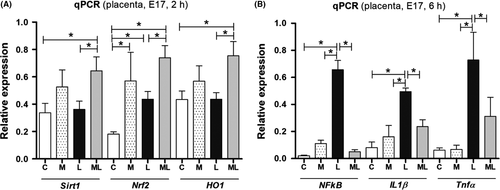
3.3 Melatonin activated precursors of anti-inflammatory mediators and prevented the increase in pro-inflammatory mediators in placentas exposed to IUI-induced oxidative stress
The expression of anti-inflammatory mediators, SIRT1, Nrf2, and HO-1, in the placenta was significantly increased in ML compared to C and L, 2 hours after PBS or LPS exposure. However, these changes were not observed in M. There were no significant changes in SIRT1, Nrf2, and HO-1 between all four groups at 4 and 6 hours (Figure S1). On the contrary, the expression of pro-inflammatory mediators, NF-κB, TNF-α, and IL-1β, was significantly increased in L compared to C and M, 6 hours after PBS or LPS exposure. However, these changes were not observed in ML. Instead, the expression of pro-inflammatory mediators was significantly decreased in ML than L (Figure 3). While changes in the relative quantity of NF-κB, TNF-α, and IL-1β showed consistent trends between all four groups (ie gradually increasing expression of all three markers in L compared to all other groups), there was no statistical difference noted at 2 and 4 hours (Figure S1).
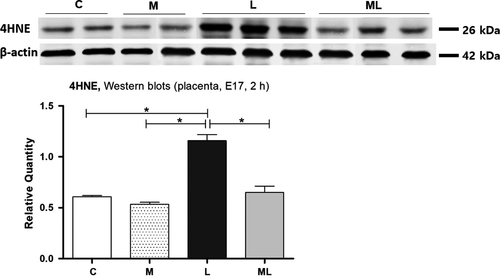
3.4 Melatonin prevented the increase in oxidative stress markers in placentas exposed to IUI-induced oxidative stress
Western blot analysis in C (0.61 ± 0.01), M (0.53 ± 0.02), L (1.16 ± 0.06), and ML (0.65 ± 0.06) showed significant increases of the amount of 4-hydroxy-2-nonenal (4-HNE) in L compared to C (P < 0.05) and M (P < 0.05), 2 hours after PBS or LPS exposure. However, this increase was not observed in ML (P > 0.05), and the amount of 4-HNE protein was significantly decreased in ML than L (P < 0.05) (Figure 4).
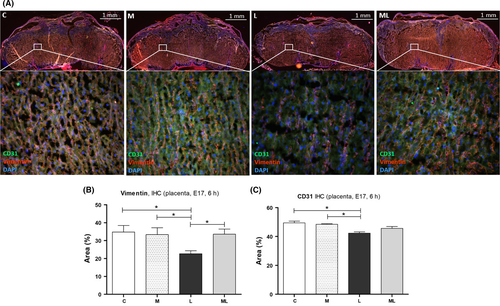
3.5 Melatonin prevented the decrease in vimentin in placentas exposed to IUI-induced oxidative stress
IHC staining for vimentin and CD31 was performed for the placenta 6 hours after PBS or LPS exposure in C (34.80 ± 3.67%, 49.48 ± 1.15%), M (33.30 ± 3.92%, 48.48 ± 0.38%), L (22.68 ± 1.67%, 42.39 ± 0.89%), and ML (33.57 ± 2.90%, 45.68 ± 1.28%). IHC staining for vimentin demonstrated less vimentin in the labyrinth of LPS-exposed placentas compared to those in C (P < 0.05), M (P < 0.05), and ML (P < 0.05). And IHC staining for CD31 demonstrated less CD31 expression in the labyrinth of LPS-exposed placentas compared to those in C (P < 0.05) and M (P < 0.05) (Figure 5B,C). However, melatonin prevented decreased vimentin in placentas exposed to IUI-induced oxidative stress, in ML (P > 0.05). The area of positive vimentin expression in L was significantly smaller than the positive vimentin area in ML (P < 0.05) (Figure 5B).
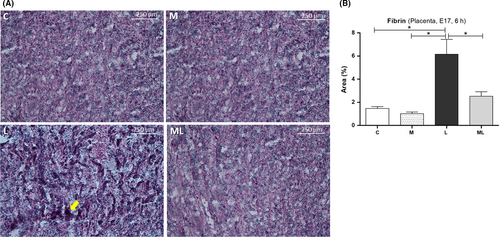
3.6 Melatonin prevented coagulation in placentas exposed to IUI-induced oxidative stress
PTAH staining in the placenta 6 hours after PBS or LPS exposure in C (1.47 ± 0.17%), M (1.02 ± 0.14%), L (6.16 ± 1.27%), and ML (13.53 ± 7.39%) showed increased deposit of fibrin in L compared to C, M, or ML (Figure 6). The area for fibrin staining was significantly higher in L than C (P < 0.05) and M (P < 0.05). However, this increase in staining was not observed in ML (P > 0.05). Instead, the area of fibrin staining was significantly lower in ML than L (P < 0.05).
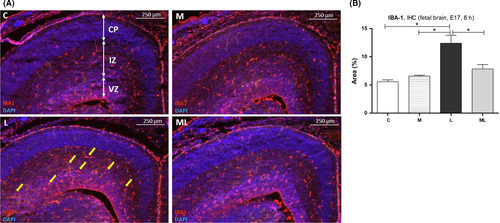
3.7 IBA1 expression increased in brains of fetuses exposed to IUI-induced oxidative stress
IBA1 expression 6 hours after PBS or LPS exposure was increased in fetal brains in L compared to C, M, and ML (Figure 7A). The area of IBA1 expression in C (5.59 ± 0.32%), M (6.57 ± 0.18%), L (12.42 ± 1.39%), and ML (7.84 ± 0.78%) showed significantly increased deposit of IBA1 in L compared to C (P < 0.05) and M (P < 0.05). However, this change was not observed in ML (P > 0.05) (Figure 7B).
4 DISCUSSION
Our study is the first to examine the beneficial effect of antenatal maternally administered melatonin on fetoplacental hemodynamic changes and fetal neuroinflammation caused from IUI-induced oxidative stress. This finding is supported by our results showing a reduction in placental gene expression and proteins of pro-inflammatory mediators and oxidative stress markers, 4-HNE.39 We also observed that melatonin pretreatment induced an increase in anti-inflammatory precursor, SIRT1, Nrf2, and HO-1, suggesting a beneficial neuroprotective effect against an inflammatory insult.
A previous study has demonstrated an association between inflammatory markers and thrombotic pathology.40 Elevated levels of C-reactive protein have been associated with an increased risk of venous thromboembolism,41 and increased levels of certain pro-inflammatory substances, such as IL-6, have been linked with venous thrombosis.42 Also, maternal placental malperfusion is known to lead to fetal metabolic and cardiovascular sequelae, which have been linked to fetal brain injury.43 Our study has shown the possibility that these perinatal sequelae associated with maternal inflammation may be prevented with antenatal maternal melatonin.
In a previous study, we evaluated how fetoplacental malperfusion following exposure to IUI contributed to fetal cardiac dysfunction and neuroinflammation.6 We also demonstrated ultrasonographically detectable hemodynamic changes in uterine and umbilical blood flow, which were confirmed by changes in placental volume and were associated with endothelial damage and thrombi formation. Furthermore, we demonstrated that fetal microglial activation, a sign of fetal neuroinflammation as evidenced by increased IBA1 expression, exhibited a tight association with placental thrombotic disruption in the passage of nutrients, gases, and toxins across the placenta and believed to be a result of hypoxic-ischemic injury.6 Our current study showed that pretreatment with melatonin alleviated the risk for hypoxic-ischemic injury in the fetus by showing improvement in fetoplacental hemodynamic changes and significantly decreasing the level of IBA1, a marker of fetal neuroinflammation, in pups of dams with the melatonin pretreatment. Our results are in agreement with past studies that used fetal sheep models from pregnancies complicated by fetal growth restriction. These past studies showed that antenatal treatment with melatonin could reduce oxidative stress to decrease neurodevelopmental deficits in newborn lambs and protect their cerebral vascular endothelium to maintain the blood-brain barrier.44, 45 Furthermore, other studies have shown that melatonin administration even after an ischemic insult to the fetus, such as following hypoxia-ischemia 46 or brief asphyxiation via cord occlusion,47 can provide neuroprotection for the fetus. Our results, along with these past studies, show the plausibility of using adjuvant melatonin for the prevention and/or partial treatment of fetal neurodevelopmental deficits induced by IUI.
Many studies have demonstrated the role of melatonin in several physiological processes and therapeutic functions related to immune functions beyond the regulation of circadian rhythm.48, 49 Melatonin has powerful antioxidant and anti-inflammatory effects and improves the clinical course of illnesses with an inflammatory etiology.50 A previous study showed that melatonin decreased the permeability of monolayer endothelial cell induced by IL-1β and the expression and activity of MMP9 by a NF-kB-dependent pathway in HUVECs induced by IL-1β.51 Another study showed that melatonin modulates macrophages to produce less TNF-α and IL-6 and depresses the pro-inflammatory response under inflammatory conditions.52 Other studies have reported that melatonin prevents immune activation by interfering with the cell cycle and differentiation of T cells.53, 54 Furthermore, melatonin's therapeutic role has been studied in different perinatal conditions apart from general inflammatory conditions. For pregnancies complicated by preeclampsia, Hobson et al showed that melatonin improved the endothelial function in placentas that were damaged by pro-oxidant mediators.25 These studies support the biologic plausibility of melatonin's beneficial effect during pregnancy, since a successful pregnancy depends on maternal placental immune cells and their maintenance of fetomaternal tolerance.53
CD8 + T cells, with their cytotoxic role and increased infiltration of the placenta from the maternal circulation in response to pro-inflammatory cytokine secretion during maternal inflammation, have been implicated in chorioamnionitis.55, 56 The ability of melatonin to alter these inflammatory T-cell responses may be one of the mechanisms by which melatonin exerts its positive effects on pregnancy. Additionally, the presence of numerous melatonin receptors in the placenta and the fact that melatonin is synthesized by the uterus and the placenta during pregnancy suggest that melatonin may also have a direct effect on these organs that have yet to be fully uncovered.17, 57-59
Similarly, in our study, we found that melatonin treatment during pregnancy repressed LPS-induced up-regulation of NF-kB and other pro-inflammatory mediators, such as IL-1β, IL-6, and TNF-α, in the placenta. These results suggest that melatonin improved maternal and fetal inflammation by attenuating the LPS-induced up-regulation of the pro-inflammatory pathway involved in the recruitment of immune cells to the maternal-fetal interface.
Melatonin also plays a role in improving blood flow through anti-coagulation and vasodilatory action. A previous study indicated that melatonin stimulates vascular endothelial cells to secrete free tissue factor pathway inhibitor TFPI), and they suggested that melatonin could have potential clinical implications in both prophylaxis and treatment of thromboembolic events.60 Another study found lower levels of coagulation measures FVIII:C and fibrinogen 1 hour after oral intake of a single dose of 3 mg of melatonin compared to placebo medication. And they suggested potential implications for the use of melatonin as a therapeutic agent in patients at risk of atherothrombotic events, such as patients with coronary artery disease or systemic hypertension.61 Other studies also showed that melatonin has emerged as a promising resource for improving neurological recovery from stroke and ischemic brain damage in adults.62-65 Torres et al showed that melatonin reduces oxidative stress and improves vascular vasodilator function of small pulmonary arteries in pulmonary hypertensive newborn sheep.66 The mechanisms by which melatonin prevents placental malperfusion remains uncertain, but research has focused on melatonin's antioxidative and anti-inflammatory effects and its immune modulation. Okatani et al showed that melatonin significantly suppressed the vasospastic effect of homocysteine Hcy) in a concentration-dependent manner.67 These findings suggest that Hcy potentiates 5-hydroxytryptamine (5-HT)-induced vasoconstriction in the human umbilical artery, possibly by suppressing bioavailable NO.67 Melatonin protects against the vasospastic effect of Hcy, most likely by scavenging ·OH arising from Hcy auto-oxidation. Stokkan et al showed that melatonin reduces noradrenaline-induced vasoconstriction in the uterine artery of pregnant hooded seals.68 Other studies demonstrated that melatonin significantly improved placental efficiency and birth weight by up-regulating placental antioxidant enzymes.69-71 Similar results were found in our study. When melatonin was administered, the blood flows of the uterine and umbilical artery were improved, and Tei index, a useful predictor of global cardiac function,72, 73 in the fetal heart was also improved.
We believe that the beneficial effect of antenatal maternally administered melatonin on fetoplacental hemodynamic changes caused by IUI-induced oxidative stress occurred because melatonin reduced the pro-inflammatory and oxidative stress mediators by promoting the anti-inflammatory SIRT1/Nrf2 pathway.74 We showed that melatonin reduced oxidative stress markers, 4-HNE, as well as pro-inflammatory mediators and that melatonin activated the gene expression of SIRT1, Nrf2, and HO-1 in the placenta. Moreover, a recent study has shown that beyond the ability to induce antioxidant enzymatic expression and activity, melatonin can also modulate the production of pro-oxidant reactive oxygen species from their sources, such as the mitochondria, NADPH oxidase, and xanthine oxidase.75 Therefore, melatonin may be achieving its antioxidant effects in our murine model of IUI through both increases in antioxidant enzymatic activities and decreases in pro-oxidant production. Further studies need to be done to discern the exact pathophysiology.
In conclusion, our study shows that maternal administration of melatonin prior to LPS-induced IUI results in amelioration of maternal placental malperfusion, fetal cardiovascular compromise, and fetal neuroinflammation induced by intrauterine oxidative stress. Current results suggest that melatonin could serve as a new preventive and/or therapeutic agent to prevent fetal injury from IUI-induced oxidative stress.
CONFLICT OF INTEREST
The authors report no conflict of interest.
AUTHORS’ CONTRIBUTIONS
Ji Yeon Lee designed and performed the experiments, analyzed the data, and wrote the manuscript. Su Li, Quan Na, Jie Dong, Bei Jia, Maide Ozen, Jun Lei, and Michael W. McLane performed the experiments. Na E. Shin and Kimberly Jones-Beatty assembled the data and wrote the manuscript. Irina Burd designed and directed the experiments, analyzed the data, and wrote the manuscript. All authors read and approved all changes made by the final manuscript.




