Melatonin attenuates neutrophil inflammation and mucus secretion in cigarette smoke-induced chronic obstructive pulmonary diseases via the suppression of Erk-Sp1 signaling
Abstract
The incidence of chronic obstructive pulmonary disease (COPD) has substantially increased in recent decade. Cigarette smoke (CS) is the most important risk factor in the development of COPD. In this study, we investigated the effects of melatonin on the development of COPD using a CS and lipopolysaccharide (LPS)-induced COPD model and cigarette smoke condensate (CSC)-stimulated NCI-H292 cells, a human mucoepidermoid carcinoma cell. On day 4, the mice were treated intranasally with LPS. The mice were exposed to CS for 1 hr per day (8 cigarettes per day) from day 1 to day 7. Melatonin (10 or 20 mg/kg) was injected intraperitoneally 1 hr before CS exposure. Melatonin markedly decreased the neutrophil count in the BALF, with reduction in the proinflammatory mediators and MUC5AC. Melatonin inhibited Erk phosphorylation and Sp1 expression induced by CS and LPS treatment. Additionally, melatonin decreased airway inflammation with a reduction in myeloperoxidase expression in lung tissue. In in vitro experiments, melatonin suppressed the elevated expression of proinflammatory mediators induced by CSC treatment. Melatonin reduced Erk phosphorylation and Sp1 expression in CSC-stimulated H292 cells. In addition, cotreatment of melatonin and Erk inhibitors significantly limited the proinflammatory mediators with greater reductions in Erk phosphorylation and Sp1 expression than that observed in H292 cells treated with Erk inhibitor alone. Taken together, melatonin effectively inhibited the neutrophil airway inflammation induced by CS and LPS treatment, which was closely related to downregulation of Erk phosphorylation. These findings suggest that melatonin has a therapeutic potential for the treatment of COPD.
Introduction
The incidence of chronic obstructive pulmonary diseases (COPD) has continuously increased in the recent decade. In 1990, COPD was ranked as the sixth cause of death worldwide, and it was predicted to become third by 2020 1. COPD is characterized by airflow limitation that is usually progressive and associated with an abnormal inflammatory response of the lungs to various particles or gases 2. Of these factors, cigarette smoke (CS) is considered as the most important risk factor in the development of COPD. Cigarette smokers account for more than 80% of patients with COPD 3. CS contains toxic chemicals that can induce abnormal airway inflammation. This, in turn, triggers the release of chemoattractants, promoting the recruitment of neutrophils and other inflammatory cells 4, 5. In particular, neutrophils are key players in the development of COPD, as demonstrated by the marked increase in the number of neutrophils observed in sputum from patients with COPD 6. Neutrophils can produce reactive oxygen species (ROS), proinflammatory cytokines, chemokines, and tissue lysis enzymes such as neutrophil elastase and matrix metalloproteinases (MMPs) 7-9. These mediators augment airway inflammation and contribute to the development of emphysema. Therefore, controlling neutrophil airway inflammation is believed to be an important therapeutic target for treating COPD.
Melatonin (N-acetyl-5-methoxytryptamine) among a number of organs is synthesized by the pineal gland with maximum secretion at night. Melatonin is well known as a transmitter for seasonal time and for favoring adaptation to environmental light/dark situations. With these properties, increasing evidence has demonstrated that melatonin has a various physiological and pharmacological properties including antioxidant, anticancer, anti-inflammatory, and immunomodulatory effects 10-15. In particular, anti-inflammatory effect of melatonin is supported by many in vivo and in vitro experiments. Melatonin has exhibited anti-inflammatory effects in various diseases animal models such as crude venom-induced inflammation 16, 17, indomethacin- or aspirin-induced gastric ulcers 18, 19, and mechlorethamine-induced nephrotoxicity 20, which is associated with the modulation of many inflammatory-related signaling including NF-κB, MAPKs, SIRTs, and HO-1 10, 21-23. Considering this evidence, we hypothesized that melatonin might have therapeutic potential for treating COPD. There has been a study of melatonin in COPD 24.
In this study, we investigated the effects of melatonin on cigarette smoke and lipopolysaccharide (LPS)-induced lung inflammation by measuring neutrophil counts and assessing the expression of proinflammatory cytokines and proteins. In addition, we further explored the mechanism for the protective effects of melatonin on development of COPD using cigarette smoke condensate-treated H292 cells with a focus on its role in the modulation of Erk/Sp1 signaling.
Materials and methods
COPD mouse model
Specific pathogen-free male C57BL/6N mice (6 wk old, weight 20–25 g) were purchased from Koatech Co. (Pyeongtaek, Korea) and used after 2 wk of quarantine and acclimatization. The mice were given sterilized tap water and standard rodent chow. All experimental procedures were approved by the Institutional Animal Care and Use Committee of Daejeon University (Daejeon, Korea) and were performed in compliance with the National Institutes of Health Guidelines for the care and use of laboratory animals and Korean national laws for animal welfare.
The mice were divided into five groups as follows: normal control (NC), COPD (CS exposure with LPS intranasal instillation), ROF (CS exposure with LPS intranasal instillation + roflumilast (10 mg/kg, p.o.), and Mel 10 or 20 (CS exposure with LPS intranasal instillation + melatonin 10 or 20 mg/kg, i.p., respectively). LPS was instilled intranasally on day 4 at a dose of 10 μg dissolved in 50 μL distilled water, while the mice were under anesthesia. The CS was generated from 3R4F research cigarettes (Kentucky reference cigarette, University of Kentucky, USA), containing 11.0 mg of total particulate matter, 9.4 mg of tar, and 0.76 mg of nicotine per cigarette. The exposure of mice to the CS (one puff/min, 35 mL puff volume over 2 s, every 60 s, 8 cigarettes per day) was conducted using cigarette smoke generator (Daehan Biolink, Inchun, Korea). The mice received 1 hr of CS exposure in an exposure chamber (50 cm × 30 cm × 30 cm) for 7 days. Melatonin (Sigma Aldrich, Carlsbad, CA, USA) was administered to the mice for 7 days, at doses of 10 and 20 mg/kg by intraperitoneal injection, 1 hr before cigarette smoke exposure.
Inflammatory cell count in bronchoalveolar lavage fluid (BALF)
Twenty four hours after the last CS exposure, the mice were sacrificed by the intraperitoneal injection of pentobarbital (50 mg/kg; Hanlim Pharm. Co., Seoul, Korea) and a tracheostomy was performed. To obtain the bronchoalveolar lavage fluid (BALF), ice-cold PBS (0.5 mL) was infused into the lung and withdrawn via tracheal cannulation thrice (total volume 1.5 mL). To determine differential cell counts, 100 μL of BALF was centrifuged onto slides using a Cytospin (Hanil Science Industrial, Seoul, Korea) (200 g, 4°C, 10 min). The slides were dried, and the cells were fixed and stained using Diff-Quik® staining reagent (IMEB Inc., Deerfield, IL, USA), according to the manufacturer's instructions. The supernatant obtained from the BALF was stored at −70°C prior to subsequent biochemical analysis.
Measurement of reactive oxygen species (ROS), neutrophil elastase activity, and contents in BALF
The induction of oxidative stress was monitored using 2',7'-dichlorofluorescein diacetate (DCF-DA; Sigma-Aldrich), which is converted into highly fluorescent DCF by cellular peroxides, including hydrogen peroxide. Briefly, the cells from the BALF were washed with PBS, and the total numbers of cells (5 × 103) were counted. The BALF cells were treated with 20 μm DCF-DA for 10 min at 37°C. Intracellular ROS activity was measured by measuring the fluorescence at 488 nm excitation and 525 nm emission on a fluorescence plate reader (Perkin-Elmer, Waltham, MA, USA).
To measure neutrophil elastase activity, the BALF was reacted with N-succinyl-(Ala)3-p-nitroanilide (Sigma-Aldrich) in 37°C for 90 min, according to a protocol described in a previous study 25. The absorbance was measured at 405 nm using an ELISA reader (Molecular Devices, Sunnyvale, CA, USA). In addition, the BALF contents were evaluated using a protein assay kit (Bradford Assay, Bio-Rad, Hercules, CA, USA). The absorbance was measured at 595 nm using an ELISA reader (Molecular Devices).
Measurement of the levels of proinflammatory cytokines in the BALF
The levels of IL-6 (BD Biosciences, San Jose, CA, USA), TNF-α (BD Biosciences), and MUC5AC (MyBioSource, San Diego, CA, USA) in the BALF were quantified by ELISA, according to the manufacturer's protocols. The absorbance was measured at 450 nm using an ELISA reader (Molecular Devices).
Histology and immunofluorescence
After the BALF samples were obtained, the lung tissue was fixed in 10% (v/v) neutral buffered formalin. The tissues were embedded in paraffin, sectioned at 4 μm thickness, and stained with hematoxylin and eosin (H&E) solution to estimate inflammatory response. For the immunofluorescence staining, sections were deparaffinized and hydrated, incubated in a solution of normal goat serum (Vector ABC Elite Kit, Vector Laboratories, Burlingame, CA, USA) for 60 min, reacted with rabbit anti-MPO (1:500 dilution; Dako, Glostrup, Denmark) overnight at 4°C, and washed with phosphate-buffered saline containing 0.1% Tween 20. After washing, the bound primary antibodies were tagged with tetramethyl rhodamine isothiocyanate-labeled anti-rabbit IgG for 1.5 hr at room temperature. After washing, the sections were counterstained with DAPI and mounted. Subsequently, the sections were observed under a fluorescence microscope (Nikon Eclipse 80i; Nikon Corporation, Tokyo, Japan).
Immunoblotting
Lung tissue was homogenized (1/10 w/v) using a homogenizer with a tissue lysis/extraction reagent (Sigma-Aldrich) containing a protease inhibitor cocktail (Sigma-Aldrich). The protein concentration of each sample was determined using Bradford reagent (Bio-Rad), according to the manufacturer's instructions. Equal amounts of total cellular protein (30 μg) were resolved by 10% SDS-polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane. The membrane was incubated with blocking solution (5% skim milk), followed by an overnight incubation at 4°C with the appropriate primary antibody. The following primary antibodies and dilutions were used: anti-β-actin (1:2000 dilution; Cell Signaling, Denver, MA, USA), anti-pErk (1:1000 dilution; Cell Signaling), anti-Erk (1:1000 dilution; Cell Signaling), and anti-Sp1 (1:1000 dilution; Santa Cruz, Dallas, TX, USA). The blots were washed thrice with Tris-buffered saline containing Tween 20 (TBST) and then incubated with a 1:3000 dilution of a horseradish peroxidase (HRP)-conjugated secondary antibody (Jackson Immuno Research, West Grove, PA, USA) for 30 min at room temperature. The blots were again washed thrice with TBST and then developed using an enhanced chemiluminescence kit (Thermo Scientific, Waltham, MA, USA). To determine relative ratio of protein expression, we measured the densitometric band values using ChemiDoc (Bio-Rad).
Gelatin zymography
Lung protein was loaded for gelatin zymography. SDS-PAGE zymography was performed to determine the gelatinase activity according to the methods described by Heussen and Dowdle 26. Briefly, equal amounts of total cellular protein (30 μg) were resolved by the zymogram gels consisting of 10% SDS–PAGE containing 1% gelatin as an MMP substrate. The gels were washed in 2.5% Triton X-100 for 1 hr to remove the SDS and then incubated at 37°C for 16 hr in a developing buffer (1 m Tris–HCl, pH 7.5 with CaCl2). The gels were stained with 25% methanol/8% acetic acid containing Coomassie brilliant blue. The gelatinase activity was visualized as white bands on a blue background, which represented areas of proteolysis. Quantitative analysis for MMP-9 activity (arbitrary unit) was evaluated using Chemi-Doc (Bio-Rad).
Cell culture
NCI-H292 cells, a human airway epithelial cell line, were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). The cells were maintained in RPMI 1640 supplemented with 10% fetal bovine serum (FBS) in the presence of penicillin (100 U/mL), streptomycin (100 μg/mL), and HEPES (25 mm) and incubated in a 5% CO2 incubator at 37°C. The cells were seeded on 96-well plates at a density of 5 × 104 cells/well and incubated in serum-free medium in the presence various different concentrations of melatonin. After incubation for 24 hr, the cell viability was evaluated via the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay. All of the experiments were performed in triplicate.
Preparation of cigarette smoke condensate (CSC)
CM6 (CORESTA approved Monitor No. 6) reference cigarette was conditioned by ISO conditioning at a temperature of 22 ± 2°C and relative humidity of 60 ± 5% for 48 hr or more. The reference cigarettes were smoked under ISO conditions (one puff/min., 35 mL puff volume over 2 s, every 60 s) using an automatic smoking machine (Borgwaldt RM20, Borgwaldt KC, Hamburg, Germany). CSC was trapped in a 92-mm Cambridge filter pad and then extracted with methanol for 3 days at room temperature. After extraction, the solvent was evaporated under vacuum and then stored at −80°C 27.
The cells were seeded on 6-well plates at a density of 5 × 105 cells/well, treated with a nontoxic concentration of melatonin, and incubated in the presence of CSC (10 μg/mL). To investigate the effect of melatonin on proinflammatory mediator and MUC5AC expression, the cells were treated with melatonin (50, 100, 200, and 400 μm). The cells were harvested 24 hr after melatonin treatment. Total RNA was isolated using TrizolTM reagent (Invitrogen, Carlsbad, CA, USA) as instructed by the manufacturer, and a reverse transcription reaction was performed using a cDNA kit (Qiagen, Hilden, Germany) to investigate the effect of melatonin on expression of MUC5AC mRNA. Polymerase chain reactions were performed using specific forward and reverse primers (MUC5AC, forward, 5'-TGA TCA TCC AGC AGC AGG GCT-3', reverse, 5'-CCG AGC TCA GAG GAC ATA TGG-3'; IL-8, forward, 5'- ATG ACT TCC AAG CTG GCC GTG GCT-3', reverse, 5'-TTA TGA ATT CTC AGC CCT CTT CAA AAA-3', β-actin, forward, 5'-CAT GTA CGT TGC TAT CCA GGC-3', reverse, 5'-CTC CTT AAT GTC ACG CAC GAT-3') and a premixed solution according to the manufacturer's instructions (Bioneer, Korea). MUC5AC and IL-8 protein levels were measured using commercial ELISA kit (MyBioSource). The absorbance at 450 nm was measured using a microplate reader (Bio-Rad).
Effects of melatonin on the expression of Erk/Sp1 proteins in CSC-stimulated H292 cells
The cells were treated with various concentrations of melatonin, followed by incubation in the presence of CSC (10 μg/mL) for 6 hr. The cells were collected via centrifugation, washed twice with PBS, and resuspended in an extraction lysis buffer (Sigma-Aldrich) containing protease inhibitors. The protein concentration was determined using a protein assay reagent (Bio-Rad) according to the manufacturer's instructions. Western blotting was performed as described above, and the levels of Erk phosphorylation and Sp1 expression were determined.
The effects of melatonin on Erk/Sp1 signaling
To investigate the effects of melatonin on Erk/Sp1 signaling in EGF-stimulated cells, the cells were pretreated with melatonin (400 μm) and PD098059 (ERK inhibitor, 10 μm; Millipore Co., Billerica, MA, USA) and incubated for 6 hr or 24 hr in the presence of CSC (10 μg/mL). The proteins were extracted as described above. Erk/Sp-1 signaling was investigated via Western blot. The levels of MUC5AC and IL-8 were evaluated via RT-PCR and ELISA.
Statistical analysis
The data are expressed as the mean ± standard error of the mean (S.E.M.). Statistical significance was determined using analysis of variance (ANOVA) followed by a multiple comparison test with Dunnett adjustment. A P-value <0.05 was considered significant.
Results
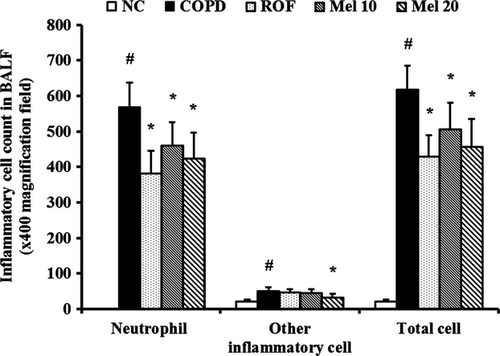
COPD mice exhibited a significant increase in the number of inflammatory cells in the BALF, compared with the normal controls (Fig. 1). In contrast, the roflumilast-treated group had markedly decreased numbers of inflammatory cells, compared with the COPD group. The melatonin-treated mice also had significantly lowered numbers of inflammatory cells, particularly neutrophils, compared with the COPD mice. This decrease was dose dependent.
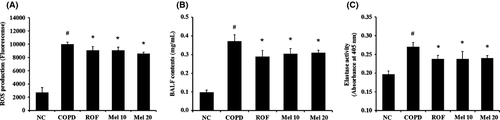
The COPD mice showed markedly increased ROS production compared with the normal controls (Fig. 2A). In contrast, the roflumilast-treated mice had significantly decreased ROS production compared with the COPD mice. The melatonin-treated mice had significantly reduced ROS production compared with the COPD mice, which occurred in a dose-dependent manner. The results of elastase activity and BALF contents were consistent with that of ROS production. The COPD mice showed markedly increased elastase activity and BALF contents compared with the normal controls, whereas the melatonin-treated mice showed significant decreases in a dose-dependent manner, compared with the COPD mice (Fig. 2B,C).
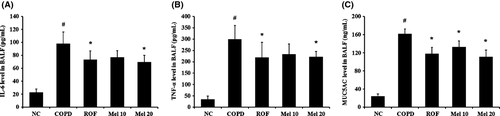
Next, we investigated the effects of melatonin on the production of proinflammatory cytokines in COPD mice. The COPD mice showed a significant increase in IL-6 in the BALF, compared to the normal controls (Fig. 3A). However, the melatonin-treated mice showed markedly reduced levels of IL-6, in a dose-dependent manner, compared with the COPD mice. In particular, 20 mg/kg melatonin-treated mice had similar effects as the roflumilast-treated mice. The results of TNF-α and MUC5AC were consistent with that of IL-6. The COPD mice showed significantly increased levels of TNF-α and MUC5AC compared with the normal controls, whereas the melatonin-treated mice showed marked decreases, in dose-dependent manner, compared with the COPD mice (Fig. 3B,C).
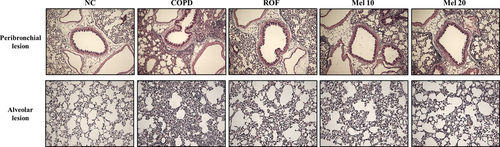
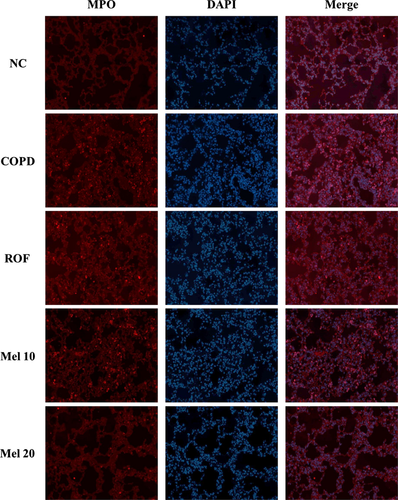
In histological analysis, the COPD mice exhibited extensive inflammatory infiltration into the lung tissue (Fig. 4). However, the melatonin-treated mice showed significantly decreased inflammatory cell infiltration, compared with the COPD mice. In addition, the COPD mice showed higher MPO expression in lung tissue, compared with the normal controls (Fig. 5). In contrast, roflumilast-treated mice showed reduced MPO expression compared with the COPD mice. The melatonin-treated mice also showed decreased MPO expression in lung tissue compared with the COPD mice.
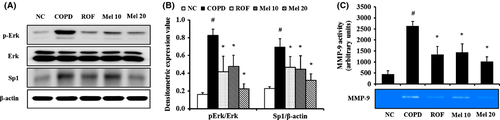
We also investigated the actions of melatonin on Erk phosphorylation and Sp1 expression in COPD mice. The COPD mice exhibited increased phosphorylation of Erk in the lung tissue, compared with the normal controls. However, the melatonin-treated mice showed a significant reduction in Erk phosphorylation, which occurred in a dose-dependent manner, compared with the COPD mice (Fig. 6A,B). In addition, Sp1 expression markedly increased in the COPD mice compared with the normal controls. In contrast, the melatonin-treated mice showed markedly decreased Sp1 expression, compared with the COPD mice (Fig. 6A,B). The results of MMP-9 activity were consistent with those of Erk and Sp-1. The COPD mice showed markedly increased MMP-9 activity, compared with the normal controls, whereas the melatonin-treated mice showed significant reductions, which occurred in a dose-dependent manner, compared with the COPD mice (Fig. 6C).
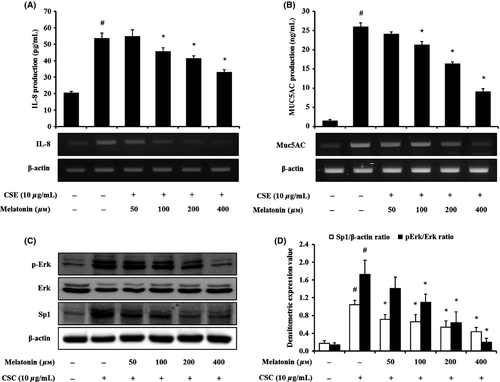
Based on these results, we evaluated the effects of melatonin on inflammatory responses and MUC5AC expression in CSC-stimulated H292 cells. CSC-stimulated H292 cells showed the elevated levels of proteins and mRNA of proinflammatory cytokines, compared with the controls. However, melatonin-treated H292 cells significantly reduced the elevation of IL-8 production stimulated by CSC (Fig. 7A). MUC5AC production was also markedly increased by CSC stimulation, which was significantly reduced by melatonin treatment, in comparison with the CSC-stimulated H292 cells (Fig. 7B). In addition, CSC-stimulated H292 cells showed an increase in the phosphorylation of Erk compared with the normal controls (Fig. 7C,D). However, treatment with melatonin suppressed the phosphorylation of Erk in H292 cells induced by CSC stimulation. Sp1 expression was also markedly increased by CSC stimulation, which was significantly reduced by melatonin treatment.
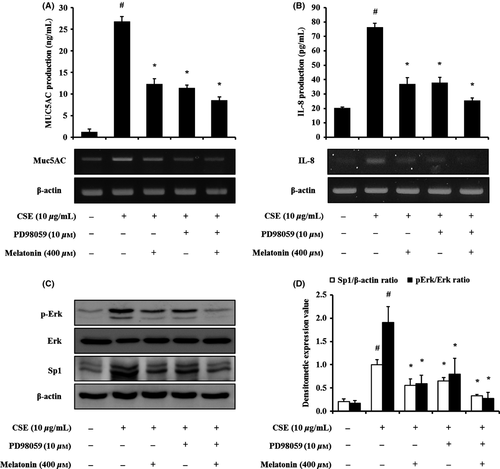
Finally, we investigated whether melatonin regulates the CSC-stimulated phosphorylation of Erk using an Erk inhibitor (PD098059). Treatment with the Erk inhibitor reduced the mRNA and protein expression of IL-8 and MUC5AC induced by CSC stimulation (Fig. 8A,B). Treatment with melatonin also decreased the expression of IL-8 and MUC5AC. Notably, the cotreatment of melatonin and Erk inhibitor also reduced IL-8 and MUC5AC expression, compared with the CSC-stimulated H292 cells, and this occurred to a larger degree than that with the treatment with the Erk inhibitor alone (Fig. 8A,B). In addition, Erk inhibitor treatment reduced the phosphorylation of Erk induced by CSC stimulation, with a reduction in Sp1 expression (Fig. 8C,D). Furthermore, the cotreatment with melatonin and Erk inhibitor caused larger decreases in Erk phosphorylation and Sp1 expression than the treatment with the Erk inhibitor alone.
Discussion
The worldwide prevalence of COPD has substantially increased, and it has become an important global health problem, threatening human life. COPD is a progressive and largely irreversible airway limitation due to chronic airway inflammation or emphysema induced by chronic stimulus such as CS, irritants, chemicals, and air pollutants 28. In this study, we investigated the effects of melatonin on the development of COPD using CS and LPS-induced COPD models and CSC-stimulated H292. Melatonin significantly reduced neutrophils counts, ROS, IL-6, TNF-α, MUC5AC, and tissue lysis enzymes, including elastase and MMP-9 with the consequent suppression of airway inflammation and MPO expression in lung tissue. In addition, melatonin significantly lowered the elevation of proinflammatory cytokines and MUC5AC, which was accompanied with the reduction in Erk phosphorylation and Sp1 expression in CSC-stimulated H292 cells.
Melatonin administration resulted in significant reductions in the number of neutrophils in the BALF of COPD mice induced by CS and LPS. In the development of COPD, neutrophils act as important players, which has been described previously 9. Neutrophils are a source of ROS, proinflammatory cytokines, and tissue lysis enzymes including elastase and MMP-9 7, 8. ROS and proinflammatory cytokines aggravated airway inflammation via the activation of inflammatory signaling pathways, such as NF-κB and MAPKs. Elastase and MMP-9 damage the normal alveolar structure, resulting emphysema 29, 30. Our study showed that melatonin effectively suppressed the proinflammatory cytokines, ROS, elastase, and MMP-9, induced by CS and LPS. In particular, the inhibitory effects of melatonin that we observed were consistent with previous studies. Melatonin reduced the production of proinflammatory cytokines, such as TNF-α, IL-1β, and IL-8, via the suppression of NF-κB and MAPKs signaling 10. In in vitro studies, melatonin suppressed inflammatory responses in LPS-induced various cells 10. Additionally, recent studies have demonstrated that melatonin suppresses the infiltration of inflammatory cells into lung tissue 31, 32. Patients with COPD showed reduced oxidative stress and improved dyspnea following the administration of melatonin 24. Based on previous reports and our current findings, melatonin is considered to effectively inhibit airway inflammatory responses induced by CS and LPS. These were strongly supported by histological analysis. Melatonin attenuated airway inflammation and mucus production induced by CS and LPS. Additionally, the elevation of MUC5AC and MPO expression was suppressed by melatonin administration. MPO is released by neutrophils and is an indicator of the activation, adhesion, and recruitment of neutrophils into the lung 33. MPO released into extracellular lesions markedly increases ROS production, which leads to the oxidative damage of proteins and cellular structures 34. These pathophysiological changes eventually alter the alteration of normal alveolar structure. Thus, the inhibitory effects of melatonin on MPO expression reflect the attenuation of infiltration of inflammatory cell into the lung.
MAPKs have been implicated as playing key regulatory roles in inflammatory responses including the production of proinflammatory cytokines and chemokines via the regulation of downstream signaling leading to inflammatory responses 35. MAPKs, including Erk, JNK, and p38, mediate proinflammatory gene transcription via their phosphorylation, which is induced by stimuli, such as cytokines, growth factors, and LPS 36. In particular, Erk is phosphorylated by Raf/MEK induced by cytokines or other inflammatory mediators 37. Phosphorylated Erk induces the expression of Sp1, leading to the production of inflammatory cytokines 38. CSC is a stimuli that can lead to the phosphorylation of MAPKs 39. According to Carter 40, CSC induced a concentration-dependent increase in the phosphorylation of Erk and led to the production of proinflammatory cytokines, including IL-1β, IL-6, and IL-8. In this study, CSC-stimulated H292 cells showed significant increases in the production of proinflammatory mediators with elevation of Erk phosphorylation and Sp1 expression. However, melatonin treatment caused a marked reduction in the expression of proinflammatory cytokines and decreases in Erk phosphorylation and Sp1 expression in CSC-stimulated H292 cells. These results were consistent with that obtained in our in vivo experiment. The administration of melatonin suppressed Erk phosphorylation and Sp1 expression in CS- and LPS-exposed mice. Thus, these findings indicated that the melatonin attenuated the inflammatory response induced by CSC via the suppression of Erk phosphorylation. Furthermore, in CSC-stimulated H292 cells, the cotreatment of melatonin and an Erk inhibitor decreased the levels of proinflammatory mediators to a greater degree than treatment with the Erk inhibitor alone. This result support that the effects of melatonin on CSC-stimulated H292 cells are closely related to the inhibition of Erk phosphorylation.
Mucus production is an important feature in the development of COPD as excess mucus production limited airflow, which causes decreased pulmonary function. It is well known that exposure to CS induces mucus production in many in vivo experiments 41, 42. In this study, the exposure to CS resulted in mucus secretion in the airway, which was accompanied by an increase in MUC5AC expression. However, melatonin reduced the mucus secretion induced by CS and LPS exposure and MUC5AC expression in CSC-stimulated H292 cells. These finding indicated that melatonin effectively inhibited the mucus secretion induced by CS. As described earlier, the reduction in mucus secretion is accompanied by decreases in Erk phosphorylation and Sp1 expression. This result reflected that the suppression of melatonin on mucus secretion is associated with the reduction in Erk phosphorylation. During mucus production, Erk is phosphorylated by various stimuli such as cytokines, elastase, growth factor, LPS, and phorbol esters, following which it activates Sp1, resulting MUC5AC expression 37, 43. CSC also leads to MUC5AC production 44, 45. Thus, MUC5AC production induced by CSC is closely related to Erk phosphorylation. This evidence was confirmed in our experiments. CSC-stimulated H292 cells markedly increased MUC5AC production with the elevation of Erk phosphorylation and Sp1 expression. In addition, in CSC-stimulated H292 cells, the cotreatment of melatonin and an Erk inhibitor decreased MUC5AC production to a greater degree than that observed with treatment with the Erk inhibitor alone. Thus, these findings indicate that suppression of MUC5AC production by melatonin is closely associated with the inhibition of Erk phosphorylation. This effect of melatonin was also described in our previous study 32. Melatonin suppressed MUC5AC production via the inhibition of Erk phosphorylation in EGF-stimulated H292 cells. Additionally, the suppressive effects of melatonin on MAPKs phosphorylation have been previously described in many other reports 10, 46, 47. Based on these evidences, the pharmacological effects of melatonin involve the inhibition of MAPKs phosphorylation and melatonin is considered to have therapeutic potential for MAPKs-related inflammatory pulmonary diseases, such as chronic bronchitis and pneumonitis induced by bacteria.
Taken together, melatonin significantly decreased the production of proinflammatory mediators and mucus production in COPD mice and H292 cells stimulated with CSC. These effects were closely associated with the inhibition in Erk/Sp1 signaling. Thus, our results suggest that melatonin may effectively inhibit the of COPD.
Acknowledgements
This study was supported by the KRIBB Research Initiative program (KGM1221413) of Republic of Korea.
Conflict of interests
There are no competing financial interests.




