Melatonin improves insulin sensitivity independently of weight loss in old obese rats
Abstract
In aged rats, insulin signaling pathway (ISP) is impaired in tissues that play a pivotal role in glucose homeostasis, such as liver, skeletal muscle, and adipose tissue. Moreover, the aging process is also associated with obesity and reduction in melatonin synthesis from the pineal gland and other organs. The aim of the present work was to evaluate, in male old obese Wistar rats, the effect of melatonin supplementation in the ISP, analyzing the total protein amount and the phosphorylated status (immunoprecipitation and immunoblotting) of the insulin cascade components in the rat hypothalamus, liver, skeletal muscle, and periepididymal adipose tissue. Melatonin was administered in the drinking water for 8- and 12 wk during the night period. Food and water intake and fasting blood glucose remained unchanged. The insulin sensitivity presented a 2.1-fold increase both after 8- and 12 wk of melatonin supplementation. Animals supplemented with melatonin for 12 wk also presented a reduction in body mass. The acute insulin-induced phosphorylation of the analyzed ISP proteins increased 1.3- and 2.3-fold after 8- and 12 wk of melatonin supplementation. The total protein content of the insulin receptor (IR) and the IR substrates (IRS-1, 2) remained unchanged in all investigated tissues, except for the 2-fold increase in the total amount of IRS-1 in the periepididymal adipose tissue. Therefore, the known age-related melatonin synthesis reduction may also be involved in the development of insulin resistance and the adequate supplementation could be an important alternative for the prevention of insulin signaling impairment in aged organisms.
Introduction
Over the past decades, a great variety of studies have been demonstrating the correlation between aging and the enhanced incidence of chronic metabolic diseases such as cardiovascular disease and type 2 diabetes 1-3. In aged rats, the insulin signaling pathway is impaired in tissues that play a role in glucose homeostasis such as liver, muscle 4, 5, adipose tissue 6, 7, and central nervous system (CNS) 8. In addition, the aging process is associated with a reduction in pineal melatonin 9-12, which is synthesized and released in a circadian manner at night, and is also linked to the putative biological rhythmicity disruption showed in diabetes 13, 14.
The adipose tissue is also an important target for several hormones, including melatonin. In this tissue, it was already demonstrated that melatonin supplementation is able to revert age-related changes upon the adiposity 15. In fact, Rasmussen et al. 16 demonstrated that nocturnal administration of melatonin reduces visceral fat, insulin, and leptin levels to similar values found in younger animals. Furthermore, other experimental evidences indicate a direct effect of melatonin in the mitochondria, regulating the brown and white adipose tissue mass in both animal models and isolated adipose cells 17.
It was demonstrated that the melatonin receptor type 1B encoding gene is an important locus which influences the fasting plasma glucose levels in humans 18-21. In this regard, melatonin is known to potentiate insulin cell signal transduction in the CNS and peripheral tissues 22-25 and also regulates the daily rhythm of insulin secretion and action 26-28. Furthermore, the glucose transporter 4 (GLUT4) content of skeletal muscle and adipose tissue is regulated by melatonin both in the absence of circulating pineal melatonin, such as in insulin-resistant pinealectomized rats 26 and in the melatonin supplementation to hyperthyroid rats 29. Therefore, the available data clearly indicate the fundamental role of melatonin in improving insulin signaling and the amelioration of insulin resistance condition.
Thus, the reduced pineal melatonin synthesis and the impaired insulin signaling of important metabolic tissues observed in aged animals gave us support to investigate, in old and obese rats, the effects of melatonin supplementation on the status of the insulin signaling pathway in the hypothalamus, liver, skeletal muscle, and visceral (periepididymal) adipose tissue.
Materials and methods
Animals
Male Wistar rats (12 months old) were kept under a 12 h:12 h light/dark cycle in a temperature-controlled room (21 ± 2°C), with water and food ad libitum. Ethics approval was granted by the Committee of Ethics in Animal Experimentation of the Institute of Biomedical Sciences, University of São Paulo, São Paulo, Brazil. The animals were randomly assigned to 2 experimental groups. Animals from both groups received tap water during the light phase. During the daily dark phase, rats in the control group received tap water containing 0.01% ethanol (vehicle), while the animals in the melatonin group received melatonin supplementation in the drinking water (0.5 mg/kg, Sigma Biochemical CO, St. Louis, MO, USA) for either 8- or 12 wk.
On the day of the experiment the animals, fasted for 12 h, were anesthetized with thiopental (25 mg/Kg, i.p.), and the abdominal wall was excised to permit a bolus injection of saline as control and/or insulin [10 μm] through the portal vein. After insulin stimulus, the rats were decapitated, all studied tissues (hypothalamus, liver, skeletal muscle, and periepididymal adipose tissue) were collected and the total protein content was extracted as previously described 30. The time for the collection of each tissue was based on previous pilot studies on the time course of the phosphorylated status of the proteins of the insulin signal transduction pathway as follows: 30 sec after the insulin injection for liver, 120 s for skeletal muscle, 120 s for adipose tissue, and 10 min for hypothalamus (data not shown).
Short-term insulin tolerance test–glucose disappearance rate (KITT)
Regular insulin (0.75-U/Kg body weight) was intravenously injected (penile vein). Blood glucose levels were measured on samples obtained from tail vein at 0, 4, 8, 12, and 16 min after insulin injection using a glucometer (Accu-check Advantage II Control, Roche Diagnostics, Indianapolis, IN, USA). The corresponding 4- to 16-min values were used to calculate the glucose disappearance constant (kITT) as previously described 31.
Immunoblotting and immunoprecipitation
Immunoblotting analysis was performed as previously described 4. Protein concentration was determined using the Bradford dye binding method 32 and protein loaded into the SDS-PAGE was normalized by Ponceau staining 33. The reagents for sodium dodecyl sulfate-polyacrylamide gel eletrophoresis (SDS-PAGE), immunoprecipitation, and immunoblotting were obtained from Bio-Rad (Bio-Rad, Richmond, CA, USA). Human recombinant insulin (Biohulin and Iolin R) was purchased from Biobrás (Biobrás, Montes Claros, MG, Brazil). Anti-IR (beta-subunit), anti-IRS-1, anti-IRS-2, anti-AKT1, and anti-p42/44MAPK (also known as ERK 1 and 2) were bought from Santa Cruz Technology (Santa Cruz, CA, USA). Anti-PI 3-kinase (p85 subunit) was obtained from Upstate Biotechnology Incorporated (Lake Place, NY, USA). Anti-phosphoserine AKT (Ser473), anti-phosphothreonine AKT (Thr308), anti-phospho MAPK (Thr202∕Try204), and anti-phospho JNK antibodies were purchased from Cell Signaling (Beverly, MA, USA). The enhanced chemiluminescence reagent kit, ECL, and protein A Sepharose 6 MB were bought from Amersham-Pharmacia Biotech (Buckinghamshire, UK).
Statistical analysis
Results were plotted as mean ± S.E.M. The samples from positive controls were assumed as 100% due the correspondence with the maximal phosphorylation value indicated by acute bolus of insulin. One-way ANOVA followed by Bonferroni's post hoc test was used and Student's one-tailed unpaired t-test was performed when appropriate. All analyses were done using GraphPad Prism (GraphPad Software version 5.01, San Diego, CA, USA).
Results
Melatonin supplementation for 8 wk had no impact in body weight; however, a reduction in body weight was evidenced after 12 wk of treatment followed by an associated periepididymal fat mass reduction. Food and water intake and fasting blood glucose remained unchanged in both treatment schedules (Table 1).
| t | Control 8 wk (n) | Melatonin for 8 wk (n) | Control 12 wk (n) | Melatonin for 12 wk (n) |
|---|---|---|---|---|
| KITT – Glucose disappearance rate (%/min) | 2.84 ± 0.6 (6) | 5.96 ± 0.2a (6) | 3.39 ± 0.40 (6) | 7.11 ± 0.17a (6) |
| Initial body weight (g) | 387 ± 15 (14) | 383 ± 18 (14) | 394 ± 11 (21) | 391 ± 16 (21) |
| Final body weight (g) | 405 ± 10 (14) | 388 ± 11 (14) | 424 ± 17 (21) | 383 ± 9a (21) |
| Periepididimal adipose tissue (g) | 3.13 ± 0.78 (7) | 2.76 ± 0.65 (7) | 3.94 ± 0.17 (9) | 2.58 ± 0.18a (9) |
| Food intake (g/day) | 20 ± 0.8 (7) | 19 ± 0.8 (7) | 23 ± 1.2 (21) | 20 ± 0.9a (21) |
| Water intake – day time (mL/day time) | 11 ± 1.4 (7) | 10.1 ± 0.6 (7) | 10 ± 0.5 (21) | 9.1 ± 0.7 (21) |
| Water intake – night time (mL/night time) | 28 ± 1 (7) | 30 ± 1.5 (7) | 31 ± 1 (21) | 27 ± 2 (21) |
- a P < 0.05 comparing the supplemented group with its respective control.
On the other hand, the insulin sensitivity, analyzed by the percentage of glucose disappearance induced by the short-term insulin tolerance test (kITT), in melatonin-supplemented animals, presented a 2.1-fold increase prior to the body mass reduction, at 8 wk of treatment (Table 1).
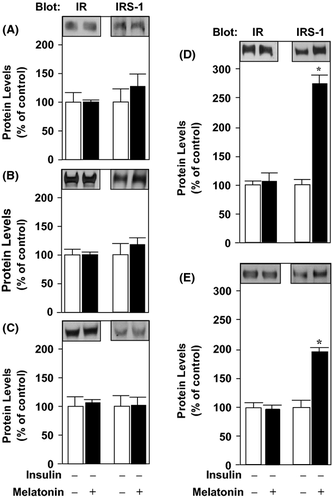
The total protein content of the insulin receptor (IR), IRS-1, and IRS-2 remained unchanged in the hypothalamus, liver, and skeletal muscle of 8- and 12-wk-treated animals (Fig. 1A–C, respectively). However, in the adipose tissue there was an approximately 2.5-fold increase in IRS-1 protein expression at 8- and 12 wk of melatonin supplementation (Fig. 1D, E).
On the other hand, the protein phosphorylation was increased in the tissue samples from melatonin-supplemented rats compared to control ones (Figs 2-5).
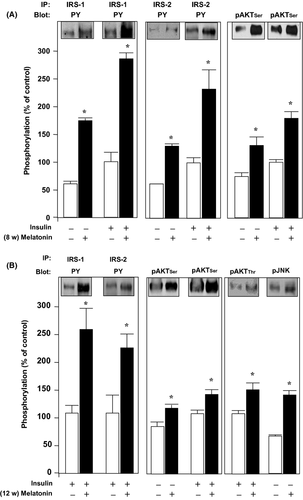
In the hypothalamus (Fig. 2), melatonin induced a 2.9-fold increase in tyrosine phosphorylation (PY) of IRS-1 both in the basal condition and after acute insulin stimulus at 8 wk of melatonin supplementation (Fig. 2A, left panel). In a similar fashion, IRS-2 tyrosine phosphorylation increased approximately 2.0-fold in both conditions, basal and after acute insulin infusion (Fig. 2A, middle panel). The IRS proteins downstream AKT also presented increased serine phosphorylation in the samples from melatonin-treated animals, approximately 1.8-fold, in both basal and after acute insulin infusion (Fig 2A, right panel). The increased phosphorylation induced by melatonin and also by the acute insulin bolus associated with melatonin treatment had a similar pattern of fold increase at 8 wk of treatment (Fig. 2B, left and middle panels). Furthermore, there was a 1.3-fold increase in the insulin-induced threonine phosphorylation AKT and a 1.4-fold increase in the phosphorylation of JNK after 12 wk of melatonin treatment (Fig. 2B, right panel).
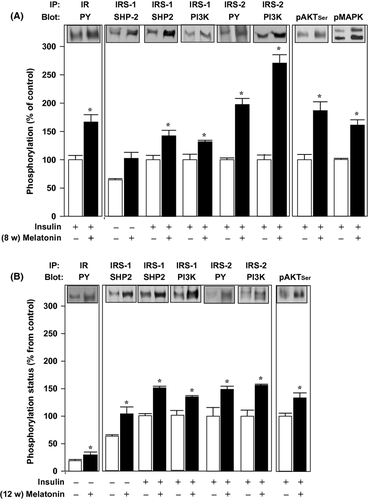
We found enhanced insulin-induced phosphorylation of proteins related to the insulin signaling cascade not only in the hypothalamus, but also in the liver (Fig. 3), skeletal muscle (Fig. 4), and periepididymal adipose tissue of 8- and 12-wk melatonin-supplemented rats (Fig. 5).
In the liver samples, we detected an approximately 1.5-fold increase in tyrosyl phosphorylation of IR (Fig. 3A,B, left panels) and IRS-2 (Fig. 3A,B, middle panels) from both 8- and 12-wk melatonin-treated rats both before and after acute insulin stimulation. The associations of IRS proteins to SHP2 and PI3K proteins were also increased in the melatonin-treated animals compared to controls, both after 8- and 12 wk of treatment. There was a 1.6-fold increase in the IRS-1/SHP2 association, a 1.3-fold increase in the association of IRS-1/PI3K, and an approximately 1.5-fold increase in the association of IRS-2/PI3K (Fig. 3A,B, middle panels).
The downstream protein AKT showed an approximately 1.7-fold increase in the phosphorylation status both at 8- and 12 wk of melatonin supplementation (Fig. 3A,B, right panel).
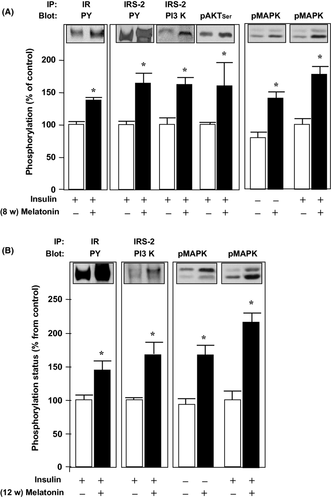
In the gastrocnemius muscle, we also detected an approximately 1.7-fold increase in the phosphorylation of IR, IRS-2, AKT, and MAPK before and after acute insulin stimulus and both at 8-and 12 wk of melatonin treatment (Fig. 4).
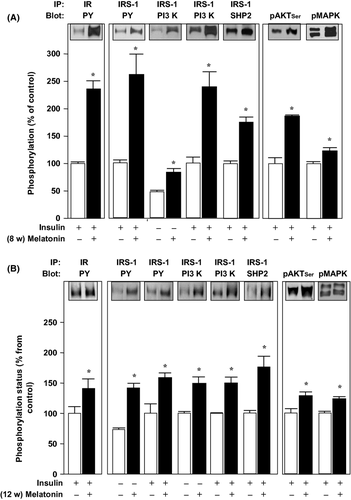
The adipose tissue revealed that 8 wk of melatonin treatment induced an approximately 2-fold increase in the acute insulin stimulated tyrosine phosphorylation of IR and IRS-1, followed by an approximately 1.6-fold increase in the association of IRS-1 to PI3K and to SHP2, and also an approximately 2-fold increase in the phosphorylation of AKT and MAPK (Fig. 5A). A similar pattern was detected after 12 wk of melatonin treatment, although the magnitude of the fold increases was not >1.9 (Fig. 5B).
Discussion
According to our results, the old and obese rats that received melatonin supplementation in the drinking water at night (for 8- and 12 wk) demonstrated an improvement of the insulin signaling pathway in central- and peripheral tissues prior to the weight loss.
The main cause of age-related increased insulin resistance 34 is the reduced insulin sensitivity within the organism 35, 36. In accordance, the insulin signaling cascade is affected by the aging process in a tissue-specific manner 4, 37. Pineal and plasmatic melatonin levels reduce in parallel with aging, and it was demonstrated that melatonin supplementation can partially restore the biochemical (e.g., reduces insulin and leptin concentration) and behavioral profile in old animals 16, 38. Moreover, our research group already demonstrated the effect of melatonin on the modulation of insulin secretion by pancreatic islets as well as the potentiating effect of insulin on norepinephrine-mediated melatonin synthesis in the pineal gland, showing therefore an intimate interdependence of both hormones in the modulation of their syntheses 28, 30, 39.
In the present study, melatonin treatment improved insulin sensitivity by increasing the glucose decay constant (kITT), which is in agreement with previous studies 40. It is known that fat mass reduction per se is able to improve insulin sensitivity 41, 42 and that melatonin supplementation promotes body weight reduction 43, 44. To the best of our knowledge, this is the first report showing that melatonin supplementation was able to increase central and peripheral insulin sensitivity prior to significant fat mass reduction.
Melatonin supplementation did not change the total amount of IR or IRS-1/2 in the evaluated tissues, except in the periepididymal adipose tissue that presented an increase in IRS-1 levels. However, despite the similarity of the protein content, the phosphorylation levels of the insulin signaling cascade components were affected by melatonin in a tissue-specific manner. These differences were expected considering the particular role played by each tissue on the intermediate metabolism.
Insulin exerts several pleiotropic actions in hypothalamus 45, 46 influencing, therefore, the central regulation of energy metabolism 46, a pivotal role of this organ 47. Aging is accompanied by a reduction in the number of insulin receptors 48 as well as by an increase in insulin resistance in the central nervous system 49. Our data demonstrated that in hypothalamus, melatonin supplementation increased the phosphorylation levels of IRS-1/2 and of their downstream proteins such as AKT/PKB after acute insulin stimulus.
Adipose tissue is a crucial site for the increase in insulin resistance observed with aging, and several evidences suggest that this tissue is one of the first sites to develop the metabolic disorders that contribute to glucose intolerance 50. In vitro 51, 52 and in vivo 53 studies suggested that the changes observed in adipose tissue are related to the reduction in the activation of insulin receptors. Additionally, in aged rats, the insulin signaling pathway is markedly impaired in adipocytes as demonstrated by the reduced activation of AKT/PKB and GLUT4 translocation 50-54. The importance of insulin action in adipose tissue was further evidenced in mice, while GLUT4 knockout adipose tissue-specific mice present a glucose intolerance 55, fat-specific insulin receptor knockout mice (FIRKO) 56 present a greater total life span in comparison to their wild type counterparts.
Our data demonstrated that melatonin supplementation increased IRS-1 tyrosine phosphorylation and protein level in adipose tissue. Additionally, the association of IRS-1 with downstream components of the insulin cascade, PI3-K, and SHP-2 protein was increased by melatonin. Previous studies suggested that the increase in IRS-1 phosphorylation is pivotal to the metabolic actions of insulin in the adipose tissue 57, 58. This statement was further reinforced by the demonstration of an increased GLUT4 translocation by the increased expression of IRS-1 in adipocytes 59 or by the reduced cellular growth and DNA synthesis of fibroblasts treated with anti-IRS-1 antibody and stimulated with insulin 60. Besides, studies performed with knockout mice to SHP-2 suggested that this protein is able to improve insulin action, being essential to maintain the glucose homeostasis during the aging process 61. Another deleterious condition involved in the insulin resistant adipose tissue from obese animal models is the endoplasmic reticulum (ER) stress. A recent evidence using chemical chaperones demonstrated alleviation of the ER stress in adipocytes and the improvement of the insulin signaling throughout IRS-1 protein level and phosphorylation 62. In this regard, the adipose tissue specific and selective increase in the IRS-1 protein expression detected herein could be related to the known effect of melatonin as a scavenger of ROS and other free radicals 63, 64 that could be involved with the ER stress in the adipose tissue of obese animals. Furthermore, data from the literature showed that melatonin enhances leptin expression in the presence of insulin 23, 25 in adipocytes, reinforcing the importance of melatonin for this tissue.
Insulin sensitivity impairment associated with aging is also observed in the liver 65 as a possible consequence of improper insulin transduction signaling 4 because neither the protein nor the phosphorylation levels of IR or the ability of IR to bind to the insulin are altered in aged Wistar rats. Our results demonstrated that melatonin increased IR and IRS-2 phosphorylation levels as well as the association between IRS-1 and IRS-2 with PI3-K. Considering the differential role for these substrates in the liver 66, our data suggest that melatonin increased both glucose homeostasis and lipid metabolism in liver of aged Wistar rats. This is reinforced by the increased phosphorylation of AKT/PKB because the higher activation of such protein is related with a reduced transcription of phosphoenolpyruvate carboxykinase (PEPCK), a key enzyme of gluconeogenesis, and a consequent reduction in hepatic glucose production 53, 67. In addition, our research group also demonstrated the role of melatonin in the regulation of hepatic insulin homeostasis, because pinealectomized rats present a night-time hepatic insulin resistance and increased gluconeogenesis due to stimulation of nocturnal unfolded protein response 68.
Skeletal muscle, the main tissue involved on the insulin dependent glucose uptake, is not uniformly affected by the aging process, for example, some muscles become more insulin resistant than others 69. It was previously demonstrated in this tissue that IRS-1 is involved with glucose uptake while IRS-2 contributes to the mitogenic actions of insulin by activating ERK-1 and 2 37. Because melatonin treatment increased the phosphorylation levels of IR, IRS-2, and the downstream components AKT/PKB and ERK-1 and 2, our results suggest that this hormone improved the mitogenic actions of insulin without affecting their effects on glucose uptake. It is important to note that it was demonstrated that the chronic activation of AKT/PKB in skeletal muscle could promote a negative feedback in IRS-1 without changing the muscular glucose uptake 70. Although, a reduction in GLUT4 expression was also demonstrated in skeletal muscle of 12-month-old obese rats when expressed as g/tissue or g/body weight, but when the expression was showed in μg protein no difference was observed 71.
In summary, our results demonstrate that melatonin supplementation to old and obese rats was able to increase insulin sensitivity prior to the expected body weight and fat mass reduction, preventing the insulin resistance observed with aging. This insulin signaling improvement involved the phosphorylation status of the insulin cascade proteins in a particular extent depending on the studied tissue, which is in accordance with the pleiotropic effects of insulin.
Therefore, the melatonin synthesis reduction with aging may also be involved in the development of insulin resistance and the adequate supplementation could be an important alternative for the prevention of insulin signaling impairment in aged organisms.
Acknowledgements
We thank Mrs. Julieta Helena Scialfa Falcão for technical assistance. This work was supported by grants from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) and Conselho Nacional de Pesquisa (CNPq).




