Potato bacterial wilt suppression and plant health improvement after application of different antioxidants
Abstract
Bacterial wilt caused by Ralstonia solanacearum is a devastating disease that often threatens potato production and exportation. The potential of four antioxidants (seaweed extract (SWE), yeast, chitosan and ascorbic acid (ASA)) in controlling the disease was evaluated in vitro, under glasshouse and field conditions. The field experiment was conducted in two naturally infested locations: Wardan, Giza (sandy soil), and Talia, Minufiya (silty clay soil). Only chitosan showed antibacterial properties against the pathogen in vitro. SWE, yeast and chitosan showed disease suppression under both glasshouse and field conditions. The disease suppression was accompanied by an increase in the ratio of soil copiotrophic to oligotrophic bacteria. The three antioxidants increased plant nitrogen content, decreased soil OM content and decreased C/N ratio. Disease suppression after chitosan application was clearly observed only in Wardan area, which was characterized by a higher soil alkalinity. A high percentage of antagonistic fluorescent strains similar to Pseudomonas putida group were detected for chitosan-treated plants in Wardan area (sandy soil). ASA drastically decreased the count of the pathogen in soil, but was conducive to the pathogen in plant tissues. A remarkable increase in microbial (bacterial and fungal) soil and rhizosphere diversity as indicated by PCR-DGGE analysis for bacterial 16S rRNA and fungal 18S rRNA was recorded. In Talia area (silty clay soil), the soil microbial community was more stable and was in general resistant to the disease where the soils were characterized by high electrical conductivity. SWE, yeast and ASA significantly increased crop production in Talia area only.
1 INTRODUCTION
Potato is the third most important crop for human consumption after rice and wheat. Egypt is considered the top potato producer and exporter in Africa. In 2013, Egypt exported 427,907 ton to Europe, Russia and Arabic countries as compared to 738,594 ton exported by whole Africa (FAOSTAT, 2013). Potato brown rot disease (bacterial wilt) is one of the most important threats for potato production and international trading. It is a systemic bacterial disease caused by Ralstonia solanacearum race 3 biovar 2 phylotype II sequevar 1 (Prior & Fegan, 2005).
In Egypt, potatoes are grown either in the traditional production fields in the Nile Delta or in the newly reclaimed areas, mainly in the desert. Since 1998 and according to FAO standards and EU regulations, potatoes are to be produced in pest-free areas (PFAs), which are mostly located in the newly reclaimed areas (NRAs). This change in production area shifted the benefits of exporting potatoes to bigger companies. However, farming at small-scale levels is more concentrated in the Nile Delta, representing a total of approximately 85% of all potato farmers. Controlling the disease is considered a difficult task; chemicals such as soil fumigants, antibiotics and copper compounds were used to control bacterial wilt without much success (Hartman & Elphinstone, 1994). In addition, these methods have a negative environmental impact. A sustainable environmental method for controlling the disease is required; for example, inducing plant systemic resistance against phytopathogens is the target of many researchers nowadays (Elhalag, Messiha, Emara, & Abdallah, 2016). Antioxidants were used in the last few years with promising success in controlling plant disease (Seadh & El-Metwally, 2015).
An important signal of a successful plant infection with pathogens is the production of reactive oxygen species (ROS) by the host due to oxygen consumption, and this process is called oxidative burst (Torres, Jones, & Dangl, 2006). Continuous production of ROS can damage cell lipids, proteins and nucleic acids by initiating chemical chain reactions. The antioxidants (free radical scavengers) are molecules that are capable of inhibiting the oxidation of other molecules (Vertuani, Angusti, & Manfredini, 2004). Examples of these antioxidants are seaweed extract (SWE), yeast, chitosan and ascorbic acid (ASA).
Seaweed extract (SWE) was reported to contain phytohormones and osmoprotectants such as cytokinins, auxins, polyamines and betaines (Zhang, Ervin, & Schmidt, 2003). Spraying potato plants (Solanum tuberosum) with seaweed extract gave a 25% yield increase similar to that with kinetin, which is a cytokinin (Blunden & Wildgoose, 1977). Cytokinin or growth regulators can inhibit the activity of free radical groups, which are the major elements for chlorophyll degradation during senescence (Yan, 1993).
Natural yeast isolates are potentially useful antagonists (Ghaouth, Wilson, & Wisniewski, 2003) because they are biodegradable, cost-effective and not toxic to humans or other animals (Raacke, Rad von, Mueller, & Berger, 2006). Yeasts can also enhance defensive enzymes such as chitinase, (CHI), b-1, 3-glucanase, phenylalanine ammonia lyase (PAL), peroxidase (POD), polyphenoloxidase (PPO), superoxide dismutase (SOD) and catalase (CAT) (Zhao, Tu, Shao, Jing, & Su, 2008).
Chitosan, deacetylated chitin, is currently present in the outer shell of crustaceans such as crabs, krills and shrimps (Sandford & Hutchings, 1987). It is a polymer having high molecular weight; it is non-toxic, bioactive agent and has become a useful compound due to its fungicidal effects and its defence mechanisms in plant tissues (Terry & Joyce, 2004). The antibacterial effect of the chitosan oligomers may be due to its DNA transcription inhibitory effect (Liu, Guan, Yang, Li, & de Yao, 2001).
Ascorbic acid (ASA) is a naturally occurring organic compound in plant tissues with antioxidant properties and is associated with resistance against many plant diseases (Zacheo, Lambertif, Arrigoni-lisor, & Arrigonio, 1977). Ascorbic acid can terminate the chain reactions of ROS by electron transfer forming its own radical ion called “semidehydroascorbate.” It acts as an antioxidant by removing hydrogen peroxide (chloroplasts lack catalase) formed by oxygen photoreduction in PSI (Mehler reaction). This is catalysed by ascorbate peroxidase (AP), some of which is bound to thylakoids where it can scavenge the generated hydrogen peroxide (Miyake & Asada, 1992).
The aim of this research was to maintain the PFAs and to decrease time needed to release accidently infested areas from quarantine in order to enable Egyptian producers to sustainably serve the international markets with disease-free potatoes. For that purpose, an environmentally friendly programme for controlling potato brown rot was employed. The relationship between the four antioxidants and potato brown rot suppression as well as potato health was evaluated under glasshouse and field conditions. The disease suppression was correlated with plant growth parameters, plant chemical compounds (enzymatic and non-enzymatic) as well as microbial biodiversity.
2 MATERIALS AND METHODS
Three experiments were conducted to evaluate the effect of four antioxidants against Ralstonia solanacearum, the causal agent of potato bacterial wilt disease in vitro, in vivo under glasshouse conditions as well as in field experiments (naturally infested). Three virulent isolates (RS2, RS7 and RS9) of R. solanacearum were tested at PBRP from infected potato tubers with clear symptoms. The three isolates were identified by immunofluorescent antibody staining (IFAS), real-time PCR (RT-PCR) and pathogenicity test on tomato (Janse, 1988). The four antioxidants were ascorbic acid (ASA) (Roth, Cat. A0104, lot 63524); brown seaweed extract (SWE) (Shanghai Redbrillian chemical, Batch no. 20140920) containing 43% alginic acid (natural polysaccharide) and 43% OM; yeast (commercial pressed baker's yeast); and chitosan, from crab shells (Roth, Cat. C0108, Lot 133115).
2.1 The effect of the four antioxidants on inhibition of the pathogen in vitro
Antimicrobial activities of the four antioxidants against the bacterial pathogen, R. solanacearum, were determined in vitro (Farag, 2013). Three virulent isolates RS2, RS7 and RS9, previously isolated from infected potato tubers and identified by IFAS, RT-PCR and pathogenicity test on tomato at Potato Brown Rot Project (PBRP), were used as 2-day-old cultures grown at 28°C on nutrient agar medium. The concentrations of the antioxidant materials were 125, 250 and 500 mg/L for ASA and 0.5, 1 and 2 g/L for SWE, yeast and chitosan each.
Four discs per plate with R. solanacearum lawns including a control plate (filter paper discs soaked in sterilized water) were used. The plates were incubated upside down at 28°C for 48 hr. Antibacterial activity was evaluated by measuring the diameter of the cleared inhibition zone.
2.2 The effect of the four antioxidants on disease incidence, growth parameters and induced resistance under glasshouse conditions
The experiment was carried out in the quarantine glasshouse at PBRP. Soil was collected from an area with no disease history in Ismailia governorate at random 10 sampling points down to a depth of 15-25 cm using augers and mixed well to ensure homogenized soil. Pots of 25 cm diameter were filled with 3.5 kg/pot of unsterilized sandy soil. Soil inoculation was made using the same three virulent isolates (RS2, RS7 and RS9) of R. solanacearum as described previously. The inoculum density was adjusted to give a final concentration of 107cfu/g dry soil. Glasshouse conditions were adjusted to 25°C during the day and 20°C during the night, with a relative humidity of 75% to 80% and 14 hr light/day. Six replicates (pots) for each treatment were made with inclusion of uninoculated controls as well as positive control (pathogen only). Three eye pieces of susceptible potato variety Nicola were planted per pot. The antioxidants were applied at one concentration as follows: ASA at 250 mg/L and SWE, yeast and chitosan at 1 g/L. The antioxidants were applied by three different methods, either by presoaking the potato tuber only for 30 min, by foliar spray only and by presoaking the tuber with foliar spray for each antioxidant. The foliar spray was applied twice, 30 days and 45 days after planting. The plots were arranged in a complete randomized block. The main plots were the application method and the subplots are the treatments. Watering of plants was made regularly throughout the growth period.
2.2.1 Disease incidence
Soil samples were collected 21 days after planting to determine the level of soil infestation. The pathogen content was evaluated as (log CFU+1) (colony-forming unit) count per g dry soil and in crown area (CFU/g fresh weight, FWt) at the end of the experiment (60 days) as detected on SMSA medium (Elphinstone, Hennessy, Wilson, & Stead, 1996). Random typical and suspected colonies were confirmed as R. solanacearum by real-time PCR using broad primer and probe (RS) which detects all biovars without distinguishing between them (Weller, Elphinstone, Smith, Boonham, & Stead, 2000).
2.2.2 Growth parameters, chemical constituents, photosynthetic pigment and endogenous non-enzymatic antioxidants
The growth parameters were evaluated by measuring plant height, fresh weight, dry weight as well as ratio of leaf weight to the total plant weight at the end of the experiment.
The effect of the four antioxidant treatments on plant chemical constitution was determined at the end of the experiment, 60 days after planting. Total photosynthetic pigments (chlorophylls a, b, total chlorophyll and carotenoids) as well as endogenous non-enzymatic antioxidants (photosynthetic pigments, ascorbic acid (ASA), phenols (mg/g FWt) and proline (mg/g FWt) were determined.
For measuring photosynthetic pigments, fresh leaf samples from the third terminal leaf (0.5 gm) were extracted by methanol for 24 hr at laboratory temperature after adding a trace of sodium carbonate; then, chlorophyll a, chlorophyll b and carotenoids were determined by spectrophotometer (Jenway, 6300, Essex, UK) at absorbance wave lengths of 650, 665 and 452 nm, respectively, and then calculated by equations of McKinney (1941).
Endogenous ascorbic acid (ASA) content was determined using the dye 2, 6, dichlorophenol indophenol for mg/100 g fresh weight according to the method of Ranganna (1979). Total phenol was measured according to Singleton, Orthofer, and Lamuela-Raventos (1999). Proline was extracted and quantified spectrophotometrically at 520 nm (Bates, Waldren, & Teare, 1973).
2.2.3 Estimation of antioxidant enzyme activities
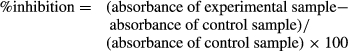
Activity of POD (EC 1.11.1.7) was estimated according to Verma and Mishra (2005). The absorbance was measured at OD520. One unit of enzyme activity is defined as the amount of H2O2 decomposed per min per mg protein.
Activity of CAT (EC 1.11.1.6) was estimated according to Kato and Shimizu (1987). The rate of H2O2 decomposition was calculated at optical density of 240 nm.
2.3 The effect of the four antioxidants (SWE, yeast, chitosan and ASA) on disease incidence, growth parameters and induced resistance under field conditions
Two separate field experiments were carried out in two naturally infested areas: Wardan, Giza (sandy soil; 30°56′8.93″E, 30°20′29.31″N), and Talia, Minufiya (silty clay soil; 30°58′28.69″E, =30°15′49.54″N). Potato seeds of the susceptible potato variety Lady Rosetta were planted in rows with approximately 90 cm between rows and approximately 30 cm between plants within the row. NPK fertilizer was applied for the whole experiment at a rate of 3.3: 1: 4 and total nitrogen was added at a rate of 170 kg/ha according to the EU regulations. Nitrogen, phosphorous and potassium were added in the form of ammonium sulphate (20% N), phosphoric acid (60% P) and potassium sulphate (50% K), respectively. Fertilizer was divided into three equal doses that were applied during the three growth stages except for potassium sulphate, which was applied during the second and third potato growth stages only. The three growth stages were as follows: vegetative growth (20–30 days after planting), tuber formation (40-50 days) and tuber bulking (60–70 days). Trace elements (Fe, Mn and Zn) were applied at a rate of 25 mg/m2 each during the three application times. The four antioxidants were applied in separate plots; control plots received NPK and trace elements only. Each application was represented by three plots each of 100 m2 with complete randomized block design. Antioxidants were applied by presoaking seed potatoes and foliar spray till saturation in the three time intervals. SWE, yeast and chitosan were added at the rate of 100 mg/m2 (1 g/L), while ASA was added at the rate of 25 mg/m2 (250 mg/L).
2.3.1 Disease incidence
Disease incidence was determined by calculating the pathogen density in bulk and rhizosphere soil (CFU/g dry weight for both) as well as in fresh plant tissues (CFU/g fresh weight, FWt) and tubers (10 tubers/plot). Level of infection in tubers was determined by IFAS (Janse, 1988) and real-time PCR (Weller et al., 2000).
2.3.2 Growth parameters
Growth parameters were plant length, shoot fresh weight, percentage of leaf weight to total shoot weight, percentage of plant dry weight and crop productivity (tuber weight per plot).
2.3.3 Estimation of antioxidant enzyme activities
Activities of CAT (EC 1.11.1.6) and PPO (EC 1.10.3.1) were determined as mg/g per min (FWt/min) at the mature plant stage (75 days) for fresh shoots. The analysis was made at the Central laboratory of Biotechnology, Plant Pathology Research Institute (PPRI, ARC). CAT activity (H2O2 decomposition) was determined by measuring the change in colour density at 240 nm absorbance (UV) per time unit (Aebi, 1984). PPO activity was determined by measuring the increase in absorbance at 400 nm per time unit according to the method described by Benjamin and Montgomery (1973). The increase in absorbance was recorded each 30 s (10 readings), with a Beckman Coulter DU 800 spectrophotometer (Beckman Coulter International, Nyon, Switzerland). The rate of catalysed enzyme reaction is proportional to the amount of the enzyme in the leaf extract.
2.3.4 Effect of different antioxidants on soil and plant chemical characteristics
Soil physical and chemical characteristics as well as percentage of N, P and K in potato leaves were determined at the end of the experiment at the National Research Centre (NRC), Cairo. Soil characterization was performed at the Agricultural and Biological Research Department, National Research Centre (NRC). Fifty-one grams of each soil sample were air-dried at room temperature. Fractions of different soil particle sizes were determined according to Bouyoucos (1927). Particle sizes of <2 mm were considered clay, 2–50 mm was considered silt, and 50–2,000 mm was considered sand. Soil of area (A) was a sandy soil (coarse sand 43.5%, sand 44.2%, silt 8.3% and clay 4%). Soil of area (B) was a silt clay soil (coarse sand 8%, sand 14.6%, silt 39.5% and clay 37.9%).
Electrical conductivity and pH were determined in soil extract (1:2.5 soil/water). Total nitrogen in soil and plant tissues was determined using the semi-micro-Kjeldahl procedure, phosphorous content was measured using Perkin-Elmer Model UVNIS-Lambada 2 (Überlingen, Germany), and potassium content was measured by Perkin-Elmer Model 1100 atomic absorption spectrometer (Überlingen, Germany). Organic matter content was determined using the chromic acid titration method according to Walkely and Black (1934).
2.3.5 Effect of different antioxidants on cultural biodiversity
The effect of different antioxidants on copiotrophic, oligotrophic bacteria, heterotrophic, endospore bacteria, fluorescent pseudomonads and actinomycetes was studied. Ratios of and percentages of cultures before and after treatment were estimated by growing and counting the bacteria using standard media according to Termorshuizen et al. (2006).
The dominating antagonistic species in different treatments was detected by random screening of colonies developed on the media plates of the diluted rhizosphere samples. The antagonistic colonies were purified and identified by 16S rRNA gene sequencing (Applied Biosystem 3500 genetic analyzer HITACHI (8-capillary) 8 ch Ruo 622-0010, Tokyo, Japan).
2.3.6 Identification of antagonistic bacteria
Two bacterial colonies (2 mm diameter) were suspended in 100 ul of lysis solution (0.05 m NaOH, 0.25% sodium dodecyl sulphate [SDS]) and were incubated for 15 min at 100°C. The suspension was centrifuged for 1 min at 13,800 g and diluted 20-fold in DNA-free distilled water (pellet discarded). One microlitre of the diluted suspension was used in each reaction. The V6 to V8 region of the 16S rRNA gene was amplified from the extracted DNA using the primers 968 f and 1401 r as described in Hiddink, Termorshuizen, Raaijmakers, and van Bruggen (2005). The amplified PCR products were purified using Pure Link TM quick gel extraction kit (Invitrogen, Life Technologies, Löhne, Germany).
Twenty ng from each purified PCR product was added to 20 μl PCR and amplified according to the diagnostic procedure by ABI Prism® BigDye® Terminator v3.1 Cycle Sequencing Kits (Applied Biosystems, Foster City, CA, USA). The sequencing process was conducted at the PBRP laboratories (Giza, Egypt) using an 8-capillary Genetic Analyzer (Applied Biosystem). The partial 16S rRNA gene sequences (containing a sequence between U968-f and U1401-r) were compared with the sequences of the GenBank DNA database by using the BLASTN algorithm (http://blast.ncbi.nlm.nih.gov/Blast.cgi), and nucleotide blast was selected. bioedit software was used to refine forward and reverse DNA sequences.
2.3.7 Effect of different fertilizers on soil microbial biodiversity
Total bacterial and fungal biodiversities of soils and rhizospheres of plants exposed to different antioxidants were determined. DNA extraction was made using NucleoSpin ®Soil kits (MACHEREY-NAGEL, GmbH & Co. KG., Düren, Germany) following the manufacturers’ protocol with some adjustments. For the analysis of the eubacterial soil population, the V6 to V8 region of the 16S rRNA gene was amplified from total soil DNA using the primers 968 f-GC and 1401 r (Heuer & Smalla, 1997). Table 1 shows the primers used in this study. One hundred ng of DNA was added to 50 μl PCR and amplified using the scheme of Rosado, Duarte, Seldin, and van Elsas (1998) with small modifications. Each reaction contained 38 μl Millipore water, 5 μl Dynazyme buffer (10×), 1 μl dNTP mix (10 μlM), 1 μl U968-GC clamps (10 μm), 1 μl L1401 (10 μm), 0.5 μl formamide 100% or DMSO and 1.5 μl Dynazyme DNA polymerase (2 U/μ). Dynazyme was replaced by Mytaq HS Red Mix, 2x (Bioline, London, UK, Lot No. PM348-B032280), and showed higher sensitivity than the Dynazyme. The reaction was conducted in a Tpersonal thermocycler (Biometra GmbH, Göttingen, Germany) using the following programme for 16S rDNA Eubacteria amplification: initial denaturation at 94°C for 5 min, followed by 35 cycles of denaturation at 94°C for 30 s, primer annealing at 50°C for 20 s, elongation at 72°C for 40s and final extension at 72°C for 7 min. The PCR programme for the fungal SSU rDNA primers was as follows: 94°C (4 min), 92°C (0.5 min), 55°C (1 min), 68°C (2 min) (two cycles), then the temperature dropped 2°C every two cycles until 47°C was reached. The programme was run for an additional 29 cycles: 92°C (0.5 min), 47°C (1 min), 68°C (45 s + 1 s cycle-1) and a final extension step at 68°C (10 min). A reagent control in which DNA was replaced with DNA-free water was included in every experiment. The amount and quality of the PCR products were examined prior to DGGE analysis by standard gel electrophoresis using 1.2% (wt/vol) agarose in 0.5 TBE, RedSafe™ Nucleic Acid Staining Solution and UV transillumination, to confirm product integrity and to estimate yield. The expected product size was approximately 450 bp for 16S Eubacteria and 390 bp for 18S rDNA (Vainio & Hantula, 2000). The amount and quality of the obtained PCR products were checked on 1.2% agarose gel prior to DGGE analysis.
| PCR target | Primer name | Sequence 5′-3′ | Primer position | Reference |
|---|---|---|---|---|
| Bacterial 16S | U968-f containing GC clamp | 5′-CGC CCGGGG CGC GCC CCG GGC GGG GCG GGG GCA CGGGGG GaAAC GCG AAG AAC CTT A-3′ | 16S-968 | Felske, Engelen, Nübel, and Backhaus (1996) |
| U968-f | 5′ACCGCGAAGAACCTTAC3′ | 16S-968 | Felske et al., (1996) | |
| L1401-r | 5′-CGG TGT GTA CAA GACCC-3′ | 16S-1401 | Heuer and Smalla (1997) | |
| Fungal 18S | FR1 | 5′-AICCATTCAATCGGTAIT-3′ (I=inosine) | 18S-1664 | Vainio and Hantula (2000) |
| FF390 | 5′-CGATAACGAACGAGACCT-3′ | 18S-1317 | Vainio and Hantula (2000) | |
| FR1 containing GC clamp | 5′-CGCCCGCCGCGCGCGGCGGGCGGGGCGGGGGCACGGGGGGa AICCATTCAATCGGTAIT-3′ | 18S-1664 | Vainio and Hantula (2000) |
- a Primers containing GC clamps were used only for PCR-DGGE soil microbial analysis (Felske et al., 1996).
Microbial biodiversity in each soil and rhizosphere was determined at least in duplicate samples per treatment and at least twice per sample using the Dcode system (Bio-Rad Laboratories, Hercules, CA, USA). The DGGE gels were prepared as described by Hiddink et al. (2005) with small modification, and the Model 475 Gradient deliver System was used instead of the gradient mixer pump. Gels were stained with Bio-Rad's Silver Stain (Bio-Rad Laboratories) according to the manufacturer's protocol. Gels were dried for 2 days at room temperature.
The gels were scanned using a resolution of 600 dots per inch. Scanned gels were analysed with Phoretix 1Dpro (NonLinear Dynamics Ltd., Newcastle upon Tyne, UK). Only bands with pixel intensity above two were included in the analysis. The bacterial and fungal diversities were estimated as species richness S and as the Shannon–Wiener index of bacterial diversity, H’. Species richness S was determined by the number of DGGE-detected bands per soil type, while the H’ was determined by: H0 = _PilogPi based on the relative band intensities as formulated by Eichner, Erb, Timmis, and Wagner-Döbler (1999). Pi is defined as ni/N, where ni is the area of the peak in intensity and N the sum of all peak areas in the lane profile (Van Diepeningen, de Vos, Korthals, & van Bruggen, 2006).
2.4 Statistical analysis
As variables were generally not normally distributed, nonparametric analyses (NPar tests, Mann–Whitney test) were conducted for log-transformed data (CFU + 1) in soil, log(CFU + 1) in rhizosphere soil and log(CFU + 1) in plant tissue using spss v 12 (SPSS Inc., Chicago, Illinois, USA). Cluster analysis was carried out on the DGGE band intensities of all lanes on individual gels, representing the bacterial composition for different treatments within the same soil type using the phoretix software (1D-pro) (Nonlinear Dynamics Ltd).
3 RESULTS
3.1 The effect of the four antioxidants on inhibition of the pathogen in vitro
The paper disc diffusion assay showed the absence of antibacterial activities for SWE, yeast and ASA as indicated by the absence of an inhibition zone. On the other hand, chitosan gave clear inhibition zones of 23.1 ± 2.4, 43.1 ± 1.9 and 39.4 ± 2.8 mm (mean ± SE) for concentrations of 0.5, 1 and 2 g/l, respectively (Figure 1). Chitosan showed no significant increase in inhibition zone by increasing the concentration from 1 g/l to 2 g/l.
3.2 The effect of the four antioxidants on disease incidence, growth parameters and induced resistance under glasshouse conditions
3.2.1 Disease incidence under glasshouse conditions
The effect of four antioxidants (SWE, yeast, chitosan and ASA) on potato bacterial wilt disease was evaluated as shown in Table 2. The nonparametric Mann–Whitney test showed that the pathogen densities in soil were not significantly different in antioxidant treatments as compared to the positive control after 21 days. However, all antioxidants decreased the pathogen densities (log (CFU+1)/g) in the crown area with the most significant inhibition in the presoaking in combination with spraying treatment of the yeast (91% decrease) followed by the SWE (88% decrease) presoaking treatment (p <.01).
| Treatments | Applications | Day 21 Cfua/g dry soil mean ± SEf | Final cfu/g dry soil mean ± SE | Crown area, cfu/g mean ± SE | AUDPCg mean ± SE | Wilt severity % mean ± SE |
|---|---|---|---|---|---|---|
| PCb | 6.57 ± 0.12 | 5.97 ± 0.14 | 5.98 ± 0.14 | 273 ± 31 | 51.7 ± 8.3 | |
| Seaweed extract |
Presoaking Spraying presoaking+ spraying |
6.51 ± 0.08 6.46 ± 0.09 6.56 ± 0.06 |
2.83 ± 1.26* 2.46 ± 0.09* 2.64 ± 0.08* |
0.71 ± 0.71** 1.25 ± 0.80** 1.34 ± 0.88* |
33.33 ± 33.46** 0** 0** |
3.33 ± 7.9** 0** 0** |
| Yeast |
Presoaking Spraying presoaking+ spraying |
6.30 ± 0.11 6.17 ± 0.12 6.29 ± 0.09 |
5.82 ± 0.17 5.82 ± 0.15 2.86 ± 1.2* |
2.37 ± 0.77 1.09 ± 0.70* 0.56 ± 0.5** |
0** 76.6 ± 32** 8.33 ± 5.27* |
0** 23.33 ± 12.01* 21.66 ± 14.20 |
| Chitosan |
Presoaking Spraying Presoaking+ spraying |
6.55 ± 0.15 6.41 ± 0.23 6.46 ± 0.14 |
3.34 ± 0.13* 4.77 ± 0.42 5.53 ± 0.17 |
3.40 ± 0.39** 1.77 ± 0.50 2.60 ± 0.51* |
0** 33.33 ± 33.46** 0** |
0** 6.66 ± 6.69** 0** |
| Ascorbic acid |
Presoakingc Sprayingd presoaking+ sprayinge |
6.44 ± 0.20 6.39 ± 0.06 6.63 ± 0.12 |
5.83 ± 0.24 5.76 ± 0.24 3.83 ± 1.2* |
0.87 ± 0.86** 2.91 ± 0.97 2.13 ± 0.97 |
62.5 ± 42.2** 0** 0** |
11.66 ± 7.4** 0** 0** |
- acolony-forming unit; bpositive control (pathogen only); cpreplanting eye piece treatment only; dfoliar spray only; epreplanting eye piece treatment and foliar spray; fstandard error; and garea under disease progressive curve. *.01 < p <.05, **p <.01 as compared to positive control (PC). Disease incidence was expressed as wilt severity, AUDPC and CFU of R. solanacearum per g dry soil and crown area of potato plants.
SWE decreased the pathogen density in soil up to 50% at the end of the experiment (100 days) using different application methods (from 5.97 ± 0.14 CFU/g in the control to 2.83 ± 1.26 (p =.029), 2.46 ± 0.09 (p =.016) and 2.64 ± 0.08 (p =.046) CFU/g in the presoaked and sprayed treatments and a combination of these treatments, respectively). It significantly decreased the pathogen density in the crown area from 5.98 ± 0.14 in the control to 0.71 ± 0.71, 88% (p =.002); 1.25 ± 0.80, 79% (p =.007); and 1.34 ± 0.88, 78% (p =.002) in the presoaked and sprayed treatments and a combination of these treatments, respectively. There was a significant decrease in AUDPC and wilt severity (approximately 90% decrease after presoaking to complete absence of wilt symptoms after spraying and a combination of presoaking and spraying), p <.01 (Table 2).
Yeast caused a significant decrease in the pathogen density in soil only after presoaking in combination with spraying (50% decrease, p =.031). The pathogen density in the crown area was significantly decreased as compared to positive control by the spraying application (83% decrease, p =.02) and the combination of presoaking and spraying (91% decrease, p =.002). The presoaking application decreased the wilt symptoms to 0, while spraying only caused 72% decrease (p =.004) in AUDPC. The combination of presoaking and spraying resulted in a 97% decrease in AUDPC (p =.012) (Table 2).
Chitosan treatment was also effective in reducing the disease progress, although only the presoaking application reduced the pathogen density in soil to 56% (p =.015) as compared to the positive control (from 5.97 ± 0.14 CFU/g to 3.34 ± 0.13). All chitosan applications resulted in a significant decrease (or at least a trend) in the pathogen in the crown area as compared to the positive control (43%, p =.004; 70%, p =.096; and 57% decrease, p =.022) in the presoaking and spraying treatments and a combination of these two, respectively. Wilt symptoms were absent after presoaking and the combination of presoaking and spraying of chitosan, while spraying resulted in 87% decrease in AUDPC and wilt severity (p =.002) (Table 2).
Ascorbic acid significantly decreased the pathogen density in soil (36% decreases, p =.045) but only when the presoaking and spraying methods were combined. Only the presoaking application of ASA caused a significant pathogen decrease in the crown area (85%, p =.002). Wilt symptoms were absent after spraying and for presoaking combined with spraying of ASA, while 77% (p =.009) and 87% (p =.008) decreases in AUDPC and wilt severity, respectively, were observed for presoaking application (Table 2).
3.2.2 Growth parameters under glasshouse conditions
All parameters of vegetative growth were significantly reduced in the positive control treatment (PC) compared to the negative, non-inoculated control (NC) (p <.001) (data not shown). Seaweed extract did not enhance growth parameters as compared to the PC. Yeast caused a significant increase in total leaf weight, approximately three times the values of the PC (from 1.13 ± 0.06 to 3.06 ± 0.06 g (mean ± SE)), but only in the combined spraying and presoaking treatment. Chitosan (presoaking application) significantly increased plant height compared with the PC (23% increase, p <.001) but the value was still less than that of the NC. Also, chitosan significantly increased potato leaf fresh weight (50% increase, p <.000) in the presoaking plus spraying treatment as compared to the positive control (still less than the NC). Chitosan resulted in 32% increase in shoot fresh weight in the spraying and the spraying plus presoaking treatments. Chitosan increased shoot dry weight approximately five times compared to the PC (p =.007, .010 and .045 for the presoaking, spraying and presoaking plus spraying applications, respectively). A trend of 50% increase in dry weight of the shoot system was observed after ascorbic acid application as compared to positive control (data not shown).
3.2.3 Chemical constituents
The effect of the four antioxidant treatments on endogenous non-enzymatic antioxidants (photosynthetic pigments, ascorbic acid (ASA), phenols (mg/g FWt) and proline (mg/g FWt)) was evaluated in infected potato plants. The endogenous ASA level was increased by 36% in the PC (9.5 ± 0.02 μg/g FW) as compared to the NC (7.00 ± 0.04 μg/g FW). Application of the four antioxidants slightly increased the ASA content by an average of 9% (10.3 ± 0.1 μg/g FW) as compared to the PC. The endogenous phenols increased twice in the PC (2.39 ± 0.29 mg/g FWt) compared to NC (1.14 ± 0.02 mg/g FWt). Application of any of the four antioxidants decreased the endogenous phenols by approximately one-third (1.6 ± 0.01) mg/g FWt) as compared to the PC. Endogenous proline increased from 0.21 ± .0.02 for the NC to 0.3 ± .0.02 mg/g FWt for the PC. The proline content almost doubled after the application of different antioxidants as compared to the PC. The highest proline content was observed after presoaking with ASA (0.68 ± 0.05 mg/g FWt).
Total photosynthetic pigments decreased by 50% for infected plants as compared to NC plants (from 2.26 ± 0.04 mg/g FWt to 1 ± 0.05 mg/g FWt). Application of SWE and yeast increased total photosynthetic pigments as compared to infected potato leaves (still less than the NC). The most noticeable increase was observed for presoaking plus spraying of SWE (1.8 ± 0.06 mg/g FWt) and spraying of yeast (1.91 ± 0.21 mg/g FWt). Chitosan treatment showed an increase in photosynthetic pigments (chlorophylls a, b, total chlorophyll and carotenoids) as compared to PC, but the values were still less than those of the NC (data not shown).
3.2.4 Enzymatic activity
Activity of endogenous SOD decreased from 120 to 95 U/g FWt and of POD from 120 to 95 U/g FWt for the PC as compared to the NC, while CAT activity increased from 0.15 to 0.25 U/g FWt for the PC as compared to the NC. Application of antioxidants (SWE, yeast, chitosan and ASA) caused an increase in endogenous SOD, POD and CAT activities in most cases compared with control plants. The most noticeable increase in SOD enzyme activity was observed after the application of different antioxidants (twice and half) as compared to positive control.
SWE and yeast increased POD activity when applied by spraying in combination with presoaking. SWE decreased the activity of CAT (EC 1.11.1.6) as compared to the NC level when applied as a spray in combination with presoaking. On the other hand, yeast increased the activity of CAT by 37.5% over the PC, especially when applied as a spray in combination with presoaking. Chitosan increased POD activity over the PC using any of the application methods and its level exceeded that in the NC. On the other hand, CAT activity decreased as compared to the PC and reached a similar level as in the NC. Application of ASA did not affect POD (EC 1.11.1.7), but decreased CAT activity in most cases compared to PC plants to a similar or lower level than in the NC (data not shown). Chitosan increased SOD, POD and CAT activities in infected potato plants. Application of chitosan (presoaking and/or spraying) was the most effective in increasing SOD and POD activities.
3.3 The effect of the four antioxidants (SWE, yeast, chitosan and ASA) on disease incidence, growth parameters and induced resistance under field conditions
3.3.1 Disease incidence under field conditions
Wilt severity and pathogen densities in soil, potato rhizosphere, potato crown area and tubers were determined at the end of the experiment, 105 days after planting.
Area A (Wardan village, sandy soil)
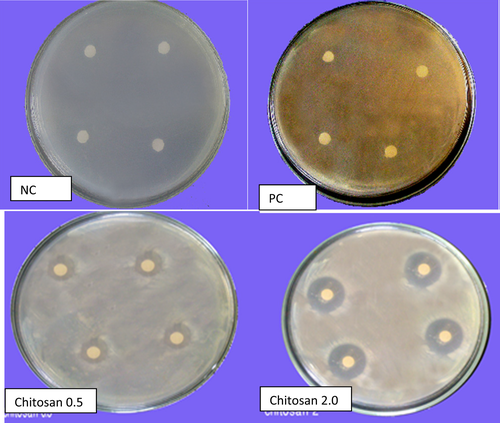
Only ASA affected the level of the pathogen in soil where the pathogen was under the detection level (0.0 ± 0.0) as compared to the control (1.35 ± 0.7) (Figure 2a). A significant decrease in R. solanacearum CFUs was found in harvested potato tubers after the application of chitosan (1.7 ± 1.7) as compared to the control (2.87 ± 1.55) (41% decrease) (Figure 2a). Effects of other treatments were not significant.
Area B (Talia village, silty clay soil)
Ralstonia solanacearum was not isolated from plant tissues after SWE (100% decrease, p =.037, as compared to the control (2.6 ± 0.5)). A 60% increase in pathogen CFUs was found in potato plant tissues (4.17 ± 0.2, p =.046) after ASA application (Figure 2b). Thus, only SWE suppressed the disease in plant tissues, but not in soil, while ASA may enhance the pathogen in plant tissue, but suppress it in soil. Chitosan had no effect in area B, but slightly suppressed the pathogen in area A (Figure 2b).
3.3.2 Growth parameters under field conditions
Area A (Wardan village, sandy soil)
Nonparametric analysis revealed no significant increase in fresh leaves, fresh shoot system and dry weight after different antioxidant treatments as compared to the control (NPK only).
Area B (Talia village, silty clay soil)
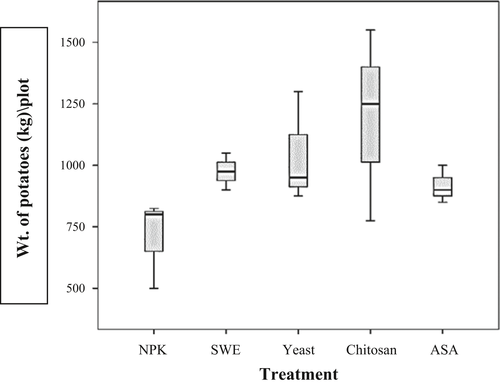
Most of the antioxidants except for chitosan increased crop production (tuber weight per plot of 100 m2) significantly (p =.05): SWE (975 ± 43) kg/plot (38% increase), yeast (1,042 ± 130) kg/plot (47% increase), ASA (917 ± 44) kg/plot (30% increase) as compared to control (708 ± 104) (Figure 3).
3.3.3 Effect of different antioxidants on soil and plant chemical characteristics
Area A (Wardan village, sandy soil)
The highest percentage of plant nitrogen was observed in the seaweed treatment (75% increase), followed by the yeast treatment (27% increase) and the chitosan treatment (25% increase) as compared to control. However, the percentage of organic matter (OM) in soil decreased after the application of the three antioxidants (0.71%, 1.02% and 0.82% for SWE, yeast and chitosan, respectively, as compared to control (1.33%)). The C:N ratio decreased after the application of SWE, yeast and chitosan (6:1, 8:1 and 7:1) as compared to 10:1 for the control, while C:N ratio increased after ASA application (13:1) (Table 3).
| Area | Treatments | Plant analysis | Soil analysis | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N% | P% | K% | pH | Electrical conductivity(dS/m) | Organic matter % | C:N ratio | N mg/100 g | P mg/100 g | K mg/100 g | ||
| Wardana | Seaweed+ NPK | 5.77 | 0.065 | 3.26 | 8.84 | 0.09 | 0.71 | 06:01 | 71 | 4.16 | 10.12 |
| Yeast extract+ NPK | 4.2 | 0.059 | 3.69 | 8.81 | 0.08 | 1.02 | 08:01 | 72 | 3.38 | 8.9 | |
| Chitosan+ NPK | 4.13 | 0.02 | 3.03 | 8.95 | 0.08 | 0.82 | 07:01 | 70 | 2.08 | 8.56 | |
| Ascorbic acid +NPK | 3.53 | 0.018 | 3.09 | 9 | 0.09 | 1.5 | 13:01 | 71 | 2.99 | 9.2 | |
| NPK | 3.3 | 0.081 | 2.83 | 8.83 | 0.15 | 1.33 | 10:01 | 77 | 4.03 | 11.44 | |
| Taliab | Seaweed+ NPK | 5 | 0.07 | 2.91 | 8.07 | 0.45 | 1.22 | 06:01 | 119 | 2.6 | 53.2 |
| Yeast extract+ NPK | 5.14 | 0.095 | 3.25 | 8.09 | 0.54 | 1.7 | 08:01 | 128 | 3.77 | 60 | |
| Chitosan+ NPK | 4.9 | 0.044 | 2.93 | 8.15 | 0.44 | 2.18 | 10:01 | 121 | 3.25 | 38.8 | |
| Ascorbic acid +NPK | 4.1 | 0.093 | 3.12 | 8.16 | 0.46 | 1.9 | 08:01 | 147 | 3.64 | 41.8 | |
| NPK | 4.45 | 0.096 | 4.05 | 8.01 | 0.55 | 1.9 | 09:01 | 130 | 2.34 | 70.2 | |
- aSandy soil area (coarse sand 43.5%, sand 44.2%, silt 8.3% and clay 4%) located in Giza governorate.
- bSilt clay soil area (coarse sand 8%, sand 14.6%, silt 39.5% and clay 37.9%) located in Minufiya governorate.
Area B (Talia village, silty clay soil)
The highest percentage of plant nitrogen was observed after the application of yeast (15% increase) and SWE (12% increase) as compared to control. These trends were accompanied by a decrease in OM percentage in soil (1.22% and 1.7% for SWE and yeast, respectively) as compared to the control (1.9%). The C:N ratio decreased after the application of SWE (6:1) as compared to control (9:1) (Table 3). The electrical conductivity was not affected by the applications, but was higher in soil B than in soil A (more than five times).
3.3.4 Effect of different antioxidants on activity of induced resistance-related enzymes
The activities of CAT and PPO were investigated at plant age of 75 days.
Area A (Wardan village, sandy soil)
The PPO activity was highest after the ASA treatment (approximately three times that of the NPK control, where the disease was most severe). No clear difference was observed after the application of the three other antioxidants.
Slight decreases in CAT activity were observed after the chitosan and ASA treatments (0.38 and 0.43 mg/gm FWt/min, respectively) as compared to that of the control (0.51 mg/gm FWt/min) (Table 4).
| Catalase activity | Polyphenoloxidase | |||
|---|---|---|---|---|
| mg/gm FWt/min | ||||
| Wardan | Talia | Wardan | Talia | |
| NPK | 0.51 | 0.51 | 0.26 | 0.53 |
| NPK+ seaweed extract | 0.49 | 0.41 | 0.26 | 0.22 |
| NPK+ yeast extract | 0.53 | 0.41 | 0.16 | 1.08 |
| NPK+ chitosan | 0.38 | 0.47 | 0.3 | 0.18 |
| NPK+ ascorbic acid | 0.43 | 0.41 | 0.83 | 0.18 |
- Catalase and polyphenoloxidase activities were determined after 75 days of cultivation, following the methods of Aebi (1984) and Benjamin and Montgomery, (1973). NPK fertilizer was added at the rate of 3.3: 1: 4, and total nitrogen set to a final 17 0 kg/ha in soil according to the EU regulations (EC Council Regulation No. 2092/91). Four antioxidants were used in this study, namely seaweed, yeast, chitosan and ascorbic acid. Wardan (Giza, sandy soil) and Talia (Minufiya, silty clay soil) were two naturally infested areas by R. solanacearum pathogen.
Area B (Talia village, silt clay soil)
The activity of PPO decreased to less than half after SWE, chitosan and ASA application as compared to the control. On the other hand, PPO activity increased twice after yeast treatment as compared to the control (Table 4). The most disease suppression for this area was recorded for SWE and yeast.
3.3.5 Effect of different fertilizers on diversity of cultured microorganisms
Wardan Area
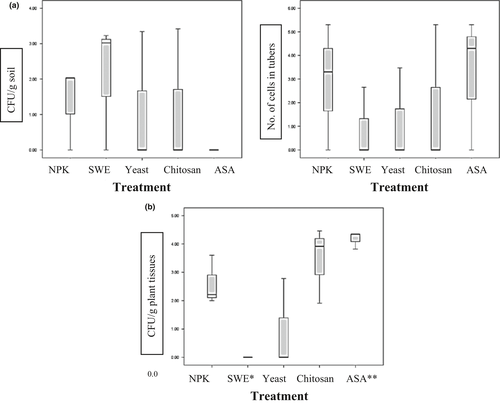
This soil is considered a poor (dry) soil that may explain the high proportion of actinomycetes (33%) and endospores (33%) that were found in the control soil. The count of each group of bacteria was compared to the total count of bacteria developed on different media; that is, the count of actinomycetes on its media was compared to the total count of bacteria developed on actinomycetes media, heterotrophic bacteria media and endospore bacteria media extracted from the same sample. Application of SWE did not significantly affect this proportion much, while a noticeable increase in ratio of heterotrophic bacteria to actinomycetes and endospores was observed after ASA as well as yeast treatment (dominant heterotrophic bacteria for both antioxidants) and to lesser extent for chitosan treatment (1/5 endospores, 1/5 actinomycetes and 3/5 heterotrophic bacteria, data not shown). The ratio of copiotrophic to oligotrophic bacteria in the rhizosphere of potato plants was 1:8 for NPK control, 2:1 for SWE, 25:6 for yeast, 15:6 for chitosan and 11:3 for ASA (data not shown). An antagonistic strain (WS2) that was isolated from chitosan-treated soil (4.2% in relation to the total isolated cultural bacteria grown on copiotrophic and oligotrophic media) showed 99% similarity with the Pseudomonas putida group (P. putida, P. oryzihabitans, P. parafulva, P. fulva, P. cremoricolorata, P. taiwanensis) (Figure 4).

Talia Area
The shift in soil microbial community after different treatments was less in the Talia area than that observed in the Wardan area. The ratio of copiotrophic to oligotrophic bacteria was 44:9 for NPK control, 3:1 for SWE, 51:2 for yeast, 21:2 for chitosan and 44:7 for ASA. ASA and chitosan greatly decreased the CFUs of oligotrophic bacteria in the rhizosphere compared to the control (data not shown). Treatment with chitosan increased the proportion of bacteria with endospores as compared to total bacteria (heterotrophic + endospores) (from 1:5 for control to 5:9 for chitosan), while ASA treatment increased heterotrophic as compared to endospore bacteria (including fluorescent pseudomonads) (data not shown). It should be noted that autotrophs were not tested here.
3.3.6 Effect of different fertilizers on the diversity of non-cultured microorganisms
Wardan Area
ASA increased the microbial biodiversity as expressed by species richness (S) in soil and the Shannon–Wiener index of diversity (H’) in the rhizosphere. Increases of 30% and 44% in soil bacterial and fungal species (S) and of 17% and 13.5% in rhizosphere bacterial and fungal diversity (H’) were detected, respectively, compared to the NPK control (Table 5). All antioxidant-treated soils clustered together and showed 60% similarity for eubacterial and fungal biodiversity, while the NPK controls showed only 5% and 10% difference from the cluster of antioxidant-treated soils for eubacterial and fungal biodiversity, respectively (data not shown). ASA showed the clearest shift in eubacterial diversity in the rhizosphere (10% similarity with the cluster of the other treatments). Chitosan showed the clearest shift in fungal rhizosphere diversity (5% similarity with the cluster of all other treatments) (Figure 5a).
| Treatments | 16S rDNA bacterial diversity | SSU rDNA fungal diversity | ||||||
|---|---|---|---|---|---|---|---|---|
| S | H’ | S | H’ | |||||
| Soil | Rhizosphere | Soil | Rhizosphere | Soil | Rhizosphere | Soil | Rhizosphere | |
| NPK | 23 | 22 ± 1* | 1.24 | 0.95 ± 0.06 | 16 | 27 | 0.4 | 1.03 |
| NPK + seaweed extract | 24 | 17 ± 2 | 0.28 | 0.99 ± 0.05 | 16 | 31 | 0.86 | 1.18 |
| NPK + yeast extract | 19 | 17.5 ± 2.5 | 1.05 | 0.96 ± 0.04 | 14 | 20 | 0.74 | 0.95 |
| NPK + chitosan | 19 | 16.5 ± 3.5 | 0.17 | 1.01 ± 0.06 | 20 | 19 | 0.67 | 0.95 |
| NPK + ascorbic acid | 30 | 21.5 ± 2.5 | 1.01 | 1.11 ± 0.03 | 18 | 39 | 0.94 | 1.19 |
- a *Mean ± standard error.
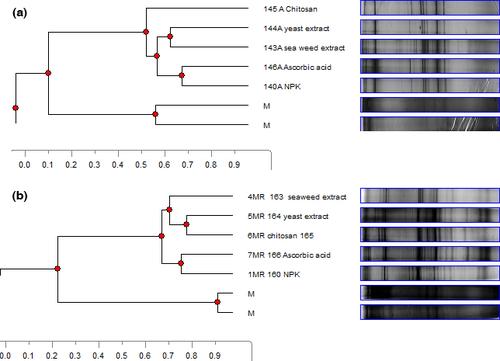
Talia Area
S and H’ of bacteria and fungi decreased after treatment with different antioxidants as compared to the NPK control (data not shown). SWE showed the clearest shift in rhizosphere eubacterial biodiversity (10% similarity with the cluster of all other treatments) (data not shown). The ASA treatment clustered with the NPK control, while the SWE and yeast treatments clustered together but separate from the control, similar to the clustering of rhizosphere fungi in the Wardan area (Figure 5).
4 DISCUSSION
The effect of four antioxidants, namely seaweed extract (SWE), yeast, chitosan and ascorbic acid (ASA), on R. solanacearum race 3 biovar 2 was studied in vitro, under glasshouse conditions as well as under field conditions.
In some cases, the pathogen populations were reduced to undetectable levels in field soil planted with ASA-treated plants and by 69% and 60% in tubers treated with SWE and yeast, respectively. Only minor reductions in pathogen densities in field soil and/or plant were observed with the other antioxidants (Table 2 and Figure 2). The effect of each antioxidant will be discussed separately.
4.1 Seaweed extract
SWE suppressed the disease by 78% under glasshouse conditions. R. solanacearum was under the detection level in plant in the Talia area (clay soil). Zhang et al. (2006) found that the bactericidal properties of SWE are based on its rich content of antioxidant polyphenols. SWE may decrease the harmful pathogenic stress of R. solanacearum by various mechanisms. First, by increasing the non-enzymatic antioxidants, photosynthetic pigment and endogenous ASA were recorded under glasshouse conditions. Endogenous ASA is considered as an indicator of biotic stress reacting with the reactive oxygen species (ROS) (Conklin, 2001). SWE may inhibit the activity of free radicals that cause chlorophyll degradation (Fletcher, Hofstra, & Gao, 1988). SWE caused an increase in enzymatic activity of POD and SOD under glasshouse conditions (data not shown). However, a decrease in PPO activity to half of that in the untreated control was recorded in the Talia area (Table 4). The SWE enhancement of antioxidant enzymes such as SOD, which protects a plant against adverse environmental conditions, was proven by Schmidt (2005). On the other hand, PPO activities were not changed for SWE-treated potato plants in the Wardan area (pH 8.8), while a 50% decrease was obtained in SWE-treated plants in the Talia area (pH 8) when compared with the control plants. The activity of PPO was found to be maximum at pH 6.5-7 (Mizobutsi, Finger, Ribeiro, Puschmann, & Neves Mota, 2010), and the enzyme may have been inactivated as a result of the high soil pH in our experimental fields, exacerbated by the high pH of SWE itself (pH 8-9). Despite the limited effect of SWE on plant physiology and disease suppression, potato production was increased by 38% in the Talia area as a result of increased uptake of nitrogen and potassium. Stimulation of nutrient uptake by the SWE-treated plants and increased growth were also found by Nelson and van Staden (1984). An enhancing effect of SWE on leaf water status, uptake of some plant nutrients, shoot growth and root pull strength was proven by Demir, Günes, Inal, and Alpaslan (2004). In our study, SWE decreased the C: N ratio in soil in both areas. All treated soils had a C: N of less than 20, which is suitable for nitrogen release. In the Talia area where the strongest disease-suppressive effect was observed, a shift in non-cultured rhizosphere eubacterial and fungal diversity (Figure 5b) was recorded for SWE-treated plants. Khan et al. (2009) showed that SWE promoted the beneficial rhizosphere microbial community such as Arbuscular mycorrhizae, but this was not observed by us.
4.2 Yeast
Yeast application also caused disease suppression (91%) under glasshouse conditions. In the Wardan area (sandy soil), R. solanacearum CFUs were slightly reduced (60%, not significant) in potato tubers. The disease reduction in the glasshouse was accompanied by an increase in vegetative growth as expressed by leaf fresh weight and leaf area. Also, an increase in total chlorophyll, decrease in phenols, and accumulation of proline were observed under glasshouse conditions. This was in agreement with a previous research that was performed by Amer (2004), who recorded a significant increase in chlorophyll content after the application of yeast to bean plants. The effect of yeast on chlorophyll content has been ascribed to high levels of vitamin E in yeast (Schmitz & Noga, 1998). In our experiments, yeast increased the SOD and POD activities more than twice (data not shown). A noticeable increase in PPO was recorded only in the Talia area (more than four times that in the control plants), which was accompanied by disease suppression. The induction of SOD, POD and PPO activities by yeast suggests that it might protect plants from different stress factors, including pathogenicity stress. Under field conditions, yeast enhanced nutrient uptake and crop production (similar to the effect of SWE). The increase in nitrogen, potassium and Ca contents of leaves by yeast application was proven earlier by Fathy El-, Farid, and El-Desouky (2000). A 43% increase in potato production was recorded for yeast-treated plants under field conditions. Improving growth and productivity of vegetable crops by the application of active yeast was also addressed by El-Tohamy, El-Abagy, and El-Greadly (2008). Yeast increased the soil heterotrophic bacteria as compared to endosporic bacteria as well as the copiotrophic/oligotrophic ratio in the Wardan area. Copiotrophs increase in response to an increase in easily available carbon and nitrogen sources. In the Talia area (clay soil), the diversity of non-cultured rhizosphere fungi differed significantly in the SWE- and yeast-treated plants from the untreated control and ASA-treated plants. This finding was associated with disease suppression in the SWE- and yeast-treated plants in this area.
4.3 Chitosan
Chitosan showed antibacterial activity in vitro at concentrations >1 g/L and suppressed the disease under glasshouse conditions. Under field conditions, it showed only slight disease suppression in the Wardan area and was not effective in the Talia area. Nutrient uptake was enhanced by chitosan in the Wardan area, but to a lesser extent than by SWE and yeast. Chitosan is an amino polysaccharide biopolymer, and it is a polycation with a high positive charge, reactive hydroxyl and amino groups as well as extensive hydrogen bonding (Raafat & Sahl, 2009). The polycationic characteristics of chitosan were postulated to disturb the negatively charged bacterial and fungal cell wall as well as gram-negative bacterial outer membrane and hence disturb the integrity and permeability of their cells (Je & Kim, 2006). Chitosan treatment increased photosynthetic pigment production and dry weight of the shoot (data not shown). Also, POD activity was the highest in chitosan-treated plants. POD activity was found to be elicited in palm roots injected with chitosan (El Hassni, El Hadrami, Daayf, Barka, & El Hadrami, 2004). Chitosan is an exogenous elicitor of host defence responses, including the accumulation of chitinase, b-1, 3-glucanase and phenolic compounds, induction of lignification, synthesis of phytoalexins by the infected host tissue and inhibition of host tissue maceration enzymes (Reddy, Arul, Angers, & Couture, 1999). However, it had very little effect on R. solanacearum in our experiments.
Nevertheless, the percentage of cultured actinomycetes was highest in soil with chitosan-treated plants (data not shown). Chitosan consists of polysaccharides that are known to stimulate actinomycetes, which may shift the microbial composition into the direction of pathogen suppression (Bell, Hubbard, Liu, Davis, & Subbarao, 1998). Of the total cultured bacterial community in soil treated with chitosan, 4.2% consisted of an antagonistic bacterial strain with 99% similarity to the Pseudomonas putida group. Also, chitosan caused a clear shift in the non-cultured rhizosphere fungal community in the Wardan area, but not in Talia. The dominance of actinomycetes and the P. putida group and the shift in fungal community in the Wardan area may partially explain the disease suppression.
4.4 Ascorbic acid
Although ASA can alleviate the harmful effect of ROS in several ways (Blokhina, Virolainen, & Kurtv, 2002), suppression of R. solanacearum was only observed under glasshouse conditions. The pathogen was under the detection level in ASA-treated Wardan soil (pH 8.9), but was not significantly decreased in plant tissues. On the contrary, ASA enhanced disease conduciveness in the Talia area (pH 8). The decrease in the pathogen density in soil may be correlated with the increase in soil microbial biodiversity. ASA may also decrease the pH inside the plant tissues, which favours the survival of the pathogen (pH 6 for 250 mg/L). The negative correlation between the survival of R. solanacearum and the pH was previously addressed by Messiha et al. (2007). ASA showed the highest induction of endogenous proline content. Several functions such as osmotic adjustment, cell membrane stabilization, C and N reserve and scavenger of free radicals have been proposed for the accumulation of proline in plant tissues under stress (Ozdemir, Melike, Tijen, & Ismail, 2004). Application of ASA increased the shoot dry weight under glasshouse conditions and caused 30% increase in potato production as compared to the untreated control in the Talia area and 25% in the Wardan area. An increase in plant growth after the application of ASA was shown earlier for Artemisia annua (Aftab, Masroor, Khan, Idrees, & Naeem, 2010). ASA regulates cell growth and division (Smirnoff, Conklin, & Loewus, 2001). ASA application was associated with an increase in PPO activity in the Wardan area where potato brown rot was not suppressed, but decreased PPO in the Talia area, where the disease was most severe. An inhibitory effect of ASA on PPO activity was also shown by Pizzocaro and Torreggiani D (1993).
A general increase in S and H’ for soil and rhizosphere bacterial and fungal diversity for ASA-treated plants as compared to untreated control plants was recorded in the Wardan area. However, a trend of decrease in S and H’ diversity was recorded for Talia soil and the rhizosphere of ASA-treated plants as compared to untreated plants. The increase in soil biodiversity was associated with a decrease in pathogen density in soil in the Wardan area, while a decrease in S and H’ diversity was associated with higher disease incidence in the Talia area. It is expected that the higher the microbial diversity, the less chance for the pathogen to dominate.
In conclusion, the antioxidants SWE and yeast extract deserve further field testing for the control of potato brown rot and enhanced nutrient uptake and crop productivity. Additional research on microbial community composition and plant physiological reactions is needed to fully explain the mechanisms underlying disease suppression by these compounds. In addition, the economic aspects of the application of these antioxidants will need to be investigated before it can be recommended for practical use. However, our research results provide an important first step for the application of materials that are relatively easily available and can reduce the incidence of various plants pathogens and increase yield in areas where small-scale potato production has been practised for centuries.
5 ACKNOWLEDGEMENTS
This project was funded by the STDF 2905 entitled “Environmental friendly programme for controlling potato brown rot in Egypt”. The authors appreciate the Potato Brown Rot Project (PBRP) and the Agricultural Research Center (ARC) for hosting the project. The authors are grateful to Dr. Jaap D. Janse (Dutch bacteriology and phytopathology expert) for valuable advice and concise comments on this article.




