Occurrence of Charcoal Rot Caused by Macrophomina phaseolina, an Emerging Disease of Adzuki Bean in China
Abstract
Adzuki bean (Vigna angularis) is an important legume crop in China. Soil-borne charcoal rot caused by Macrophomina phaseolina (Tassi) Goid is an important and devastating disease of many crops including legumes. During late August and early September, 2014, symptoms similar to charcoal rot were observed on adzuki bean plants in Yulin City of Shanxi Province, and Fangshan County of Beijing, China. This study was conducted to determine the causal agent of the emerging disease on adzuki bean. Four fungal isolates were obtained and identified as M. phaseolina based on morphological and molecular characteristics, including species-specific primer detection and sequences of internal transcribed spacer (ITS) of nuclear ribosomal DNA. The resulting sequences showed 99% identity with more than 60 M. phaseolina strains from diverse hosts. The virulence on adzuki bean was verified using pathogenicity tests, producing symptoms similar to those observed in the fields. To our knowledge, this is the first report of M. phaseolina causing charcoal rot on adzuki bean.
Introduction
Adzuki bean (Vigna angularis) is a traditional legume crop that plays a crucial role in the sustainability of agricultural systems and in the food protein supply (Lumpkin and McClary 1994; Zheng 1997). The crop is mainly planted in eastern Asia and northern South Asia including China where has the largest planting area, 2 50 000 ha, and the greatest yield, approximately 3 00 000 t (Tomooka et al. 2002; Zong et al. 2003; Wang et al. 2013). In recent years, soil-borne diseases, including brown stem rot (BSR, Cadophora gregata f. sp. adzukicola), Phytophthora stem rot (PSR, Phytophthora vignae f. sp. adzukicola) and adzuki bean wilt (Fusarium oxysporum f. sp. adzukicola), as well as the emerging soil-borne Rhizoctonia stem rot caused by Rhizoctonia solani AG 4 HGI reported in our previous study, have become primary limiting factors to adzuki bean production (Kondo et al. 2004, 2009; Fujita et al. 2007; Kondo and Tomooka 2012; Sun et al. 2015).
Another important soil-borne disease of legume crops, charcoal rot, caused by the fungus Macrophomina phaseolina (Tassi) Goid, has been increasingly prevalent mainly because of the extreme weather conditions due to climate change (Koenning and Wrather 2010; Radwan et al. 2014). Charcoal rot has seriously reduced soya bean (Glycine max) yields in USA (Wrather 1995; Wrather et al. 2001). The pathogen M. phaseolina has a worldwide distribution and a broad host range that includes more than 500 cultivated and wild plant species (Smith and Wyllie 1999; Su et al. 2001). The diseases it causes have varying names on different hosts, including charcoal rot, damping-off and ashy stem blight (Su et al. 2001; Radwan et al. 2014). Most fungi prefer damp environments and moderate temperatures, but M. phaseolina often occurs in hot and dry conditions (Smith and Wyllie 1999; Radwan et al. 2014). Thus, the high temperatures and drought stress that accompany climate change will provide more favourable conditions for M. phaseolina. Currently, reports of this fungus emerging on diverse crop species are increasing worldwide (Rusuku et al. 1997; Gulya et al. 2002; Yang and Navi 2005; Zveibil and Freeman 2005; Gaetán et al. 2006; Jacob et al. 2013;). Charcoal rot-related diseases caused by M. phaseolina have been documented on legume crops worldwide, such as soya bean (Smith and Wyllie 1999), chickpea (Cicer arietinum) (Nene and Reddy 1987), common bean (Phaseolus vulgaris) (Rusuku et al. 1997), cowpea (Vigna unguiculata) (Ndiaye 2007) and mung bean (V. radiata) (Zhang et al. 2011).
In China, M. phaseolina is emerging as a devastating fungal pathogen of legumes (Zhang et al. 2009). We recently reported it on soya bean and mung bean (Zhang et al. 2009, 2011). Adzuki bean has suffered several diseases and pests (Wang et al. 2000), but thus far, M. phaseolina has not been described on adzuki bean.
During late August and early September of 2014, many wilting plants of adzuki bean with symptoms very similar to charcoal rot were observed in a few fields in China. This study was designed to determine the causal agent of this emerging disease on adzuki bean by observing its morphological and molecular characteristics, including species-specific primer detection and ribosomal DNA (rDNA) sequences of internal transcribed spacer (ITS) region, as well as pathogenicity tests.
Materials and Methods
Sample collection and pathogen isolation
Diseased plants of adzuki bean showing charcoal rot symptoms were collected from fields in Yulin City of Shanxi Province, and Fangshan County of Beijing, China. To isolate the causal agent, sections of stems from diseased plants were surface disinfected in 1% sodium hypochlorite for 3 min and rinsed three times in sterile distilled water, then placed on potato dextrose agar (PDA; AoBoXing; Bio-tech, Beijing, China) supplemented with 50 mg/l streptomycin sulphate (Sigma-Aldrich, St. Louis, MO, USA). Plates were incubated at 28°C with a 12-h light regime for 3–4 days. Isolates were independently transferred to PDA slants for further use and stored long term at −80°C.
Morphological and molecular characteristics
Four isolates (ABMp01 and ABMp02 from Yulin, ABMp03 and ABMp04 from Fangshan) were selected for morphological and molecular identification. The growth rates of colonies and mycelia, as well as the formation of microsclerotia, were observed.
To confirm the morphological identities, genomic DNAs of the four isolates were extracted and used for molecular identification. Next, PCRs were performed using the universal fungal primers ITS4/ITS5 to amplify the ITS1–5.8S–ITS2 region and specific primers for M. phaseolina, MpKF1/MpKR1 (5′-CCGCCAGAGGACTATCAAAC-3′/5′-CGTCCGAAGCGAGGTGTATT-3′) located in ITS1 and ITS2 region, respectively (White et al. 1990; Babu et al. 2007). PCRs were conducted as follows: 5 min at 95°C; followed by 35 cycles of 45 s at 94°C, 45 s at 55 or 60°C (depending on the primer pair), and 1 min at 72°C; and a final extension of 10 min at 72°C. Amplicons were detected by electrophoresis on 1% agarose gels. The purified ITS products were sequenced with a forward or reverse primer. The sequences were analysed by BLAST against the GenBank (National Center for Biotechnology Information) database.
Pathogenicity tests
Pathogenicity tests were performed on 3-week-old seedlings of adzuki bean cv. ‘Tonghong 2’ using a modified hypocotyl puncture inoculation technique with 2-day-old mycelium plugs of the four isolates (Keeling 1982). Plants inoculated with sterile PDA plugs were used as a negative control. The treated plants were incubated in a mist room at 28°C under high humidity (>90%) for 48 h and then moved into a greenhouse at 30°C. The results were observed at 6 and 12 days after inoculation. Pathogens were re-isolated from above or below the infected regions of inoculated plants to verify Koch's rules. All tests were performed twice.
Results and Discussion
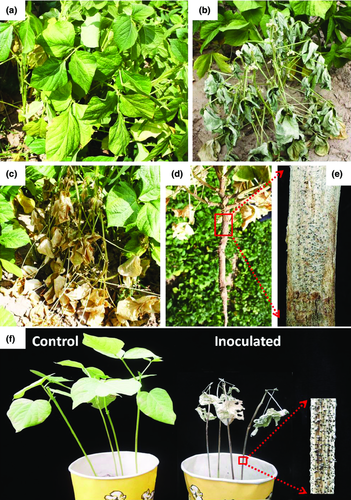
During the late summer in 2014, wilting plants of adzuki bean with symptoms similar to charcoal rot were observed in fields in Yulin City of Shanxi Province and Fangshan County of Beijing, China. Infected plants initially showed leaf chlorosis and wilting (Fig. 1a), followed by stunted growth and withering (Fig. 1b). Finally, the plants died prematurely, with dry leaves and unfilled pods still attached (Fig. 1c). Upon removal of the infected plants from the soil, the epidermal tissues of the taproot and basal stems were rotted (Fig. 1d). Masses of black microsclerotia (41.3–59.5 μm in diameter) formed within and over silvery-grey to dark discoloration of the basal stems and taproots after splitting plants (Fig. 1e).
Diseased plants were collected from the fields, and four fungal isolates were obtained (ABMp01 and ABMp02 from Yulin, ABMp03 and ABMp04 from Fangshan). All four isolates showed rapid mycelial growth on PDA at 28°C with a 12-h light regime. Colonies overgrew the 9-cm Petri dishes after 3–4 days of incubation (Fig. 2a). The mycelium was initially hyaline and turned grey with age, and dark oblong microsclerotia (58–83 × 71–112 μm) formed on the colonies at 2 days after incubation (Fig. 2b). Based on these observed morphological and culture characteristics, the isolates were identified as M. phaseolina (Smith and Wyllie 1999).
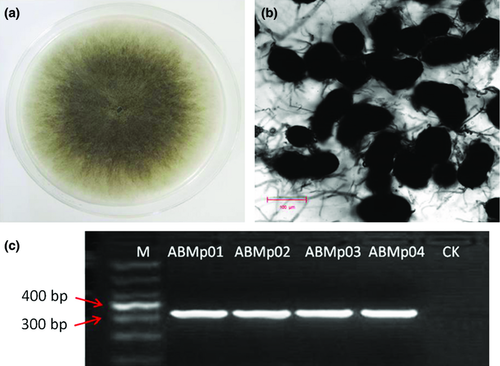
To confirm this morphological identification, the four isolates were further identified molecularly using the primers MpKF1/MpKR1, which are specific for M. phaseolina and yielded a 350-bp PCR fragment (Fig. 2c), which is corresponding to the molecular characteristics of M. phaseolina (Tassi) Goid (Babu et al. 2007). Additionally, the ITS region of the isolates was sequenced using the universal primers ITS4/ITS5. The resulting sequences (Accession Nos. KP859579–KP859582) showed 99% identity with more than 60 M. phaseolina strains by BLAST analysis, including our previously reported strains YLMp1 and YLMp3 isolated from mung bean (HQ660591, HQ660589) in China (Zhang et al. 2011), strains Ezh-1 and IFAPA-CH724 isolated from strawberry (KC822431, AM410964) in China and Spain, strains CK-7 from sweet potato (JX945170) and MAC34 from oil-crop sunflower (AM410964) in Italy and a strain isolated from maize in the USA (GU046903). These results confirmed that the four isolates were M. phaseolina and also indicated that this fungus has a broad host range of crops, consistent with previous reports (Smith and Wyllie 1999; Su et al. 2001). Thus, both morphological and molecular data identified these isolates as M. phaseolina.
Pathogenicity tests were performed on 3-week-old seedlings of adzuki bean cv. ‘Tonghong 2’. Six days after inoculation, all inoculated plants showed wilt and began to produce dark microsclerotia on the stems. By 12 day after inoculation, all plants appeared wilted, with dry leaves still attached and masses of microsclerotia formed on the stems (Fig. 1f). No symptoms were observed on the control plants. M. phaseolina was re-isolated from all inoculated plants, fulfilling Koch's postulates.
Although M. phaseolina has been reported to have a wide host range and was previously described on many other main crops, to our knowledge, this is the first report of M. phaseolina causing charcoal rot on adzuki bean worldwide. Additionally, we also tested the virulence of isolates on mung bean (V. radiata) cv. ‘Jilv 7’, and the results showed strong pathogenicity. Our results suggested that M. phaseolina could be a potential source of inocula causing related diseases on other economically important crops in China.
Acknowledgements
This research was supported by Modern Agro-industry Technology Research System (CARS-09), the Crop Germplasm Conservation and Utilization Program (2014NWB030-14) from the Ministry of Agriculture of China and the Scientific Innovation Program of Chinese Academy of Agricultural Sciences.




