First Report of Bacterial Leaf Spot of Parsley Caused by Pseudomonas syringae pv. apii in Turkey
Abstract
Since March, 2011, typical leaf spot symptoms were observed on parsley in several fields inspected in Hatay and Adana provinces of Turkey. Incidence of the disease was 5–15% in the regions. Symptoms were characterized as angular to irregular, initially water soaked later brown to dark black spots. Spots often limited by veins which were visible from both adaxial and abaxial sides of leaves but were not present on stems. Fluorescent bacterial colonies were consistently isolated from typical leaf spots. Biochemical tests, fatty acid methyl ester (FAME) analysis, molecular, pathogenicity tests and sequence of 16S ribosomal DNA of bacterial isolates were performed to identify possible causal disease agent. The causal disease agent was identified as Pseudomonas syringae pv. apii based on symptoms, biochemical, molecular, pathogenicity tests and sequencing. To our knowledge, this is the first report of bacterial leaf spot on parsley caused by Pseudomonas syringae pv. apii in Turkey.
Introduction
Parsley (Petroselinum crispum (mill.) Nym.ex A.W. Hill) belonging to the family Apiaceae is biennial and glabrous plant, which is originated from Mediterranean region, but today is cultivated all over the world. It is commonly used as a green vegetable and garnish. Parsley has also been used for different medicinal purposes in traditional and folklore medicine in different countries (Farzaei et al. 2013). Flat-leaved parsley (P. crispum var. neapolitanum) is one of the most commercially important vegetable crops, being cultivated on an area of ~50 000 da and yielding ~57.619 tonnes throughout Turkey. Hatay and Adana provinces are the two most important regions of parsley in the eastern Mediterranean region of Turkey with approximately 20.480 da area with the production of 23.575 tonnes in 2013, representing approximately 50% of the country's parsley production (Anonymous 2013). Although fungal diseases such as downy mildew and Septoria blight were reported throughout parsley-growing areas in the region (Kurt 2003; Soylu et al. 2010), there was not any bacterial diseases reported on parsley-growing areas in Turkey. In the early spring of 2011, typical leaf spot disease symptoms were observed on flat-leaved parsley plants in several fields inspected in Arsuz district of Hatay and Seyhan district of Adana provinces with an incidence of disease infection was 5–15% in both regions. Symptoms were characterized as angular to irregular, initially water soaked later brown to dark black spots (Fig. 1). Spots often limited by veins were visible from both adaxial and abaxial sides of leaves but were not present on stems. Leaf spots also lacked the presence of fungal fruiting bodies, such as pycnidia produced by Septoria (Kurt 2003). Thus, the objective of the study was to identify the possible bacterial disease agent causing foliar spotting on parsley cultivated in major growing districts in Hatay and Adana provinces.

Materials and Methods
A total of 25 parsley fields were surveyed to identify possible causal agent of leaf spot disease from main parsley-growing districts of Hatay and Adana provinces, located in the eastern Mediterranean region of Turkey. All fields were visited during March through June 2011 and 2013 growing seasons. A total of 47 plant samples showing leaf spot lesions were wrapped in paper bags and delivered to the laboratory for isolation and identification of possible leaf spot disease agent. Leaves taken from the plants were surface sterilized with 0.5% sodium hypochlorite for 1 min, followed by rinsing in sterile distilled water three times. Small (3-by-3-mm) sections of tissue were excised aseptically from leaf spot margins and macerated in 40 μl of sterile distilled water. The resulting suspensions or a dilution were streaked on plates containing King's medium B (KB) (King et al. 1954) and incubated at 24–26°C. After 3–5 days, single colonies were purified and stored. Pathogenicity was tested with six isolates, representing six different regions, by spraying the bacterial suspension (107 cfu/ml) on the leaves of 5-week-old flat-leaved parsley plants (cv. d'giant Italiana) until runoff. Four plants were used per isolate. Sterile distilled water was applied as a negative control treatment. All plants were kept in a mist room with 100% humidity for 4 h, then transferred to a glasshouse with temperature of 25°C and 80% relative humidity and examined for symptom development over a period of 3 weeks. Selected representative bacterial re-isolates from typical leaf spots on parsley were subjected to biochemical tests for characterization and identification. The biochemical tests such as levan production, oxidase, potato soft rot, arginine dihydrolase and tobacco hypersensitivity (LOPAT) were conducted according to Lelliott et al. 1966;. Isolates were streaked onto trypticase soy broth (TSBA) agar and grown for 24 h at 28°C. Cellular fatty acids were extracted and their fatty acid methyl esters (FAMEs) were derived as described by Janse (1991). FAMEs were separated by the Microbial Identification System (MIDI Microbial ID, Inc., Newark, USA) utilizing an Agilent Technologies 6890N GC with a G2614A autosampler and a 7683 injector. FAME peaks were analysed using MIDI, Sherlock software version RTSBA 6.0 (MIDI Inc., Newark, DE, USA). To confirm the identification based on morphological and biochemical characteristics, genomic DNA was extracted from bacterial suspension of all representative and re-isolates using the genomic DNA Purification Kit (GeneJET; Thermo Scientific Fermentas, Vilnius, Lithuania) according to the manufacturer's instructions. Amplicons for the 16S rDNA sequences were generated using universal primers 27F and 1492R (Lane 1991) using published reaction conditions (Weisburg et al. 1991). The PCR products were sequenced in both forward and reverse directions using an automatic DNA sequencer (ABI 3100, Applied Biosystems). The sequences were compared with those available in the MegaBlast program (http://www.ncbi.nlm.nih.gov/).
Results and Discussion
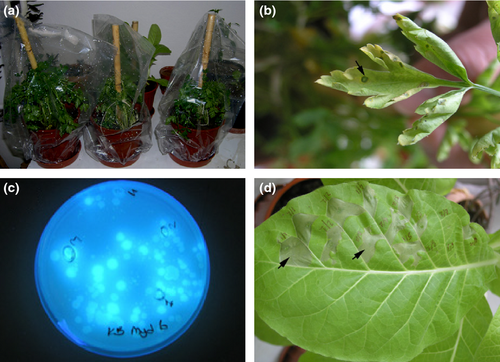
Whitish, opaque, circular fluorescent bacterial colonies were consistently isolated from typical infected parsley leaf samples on KB. Twenty-five representative bacterial isolates from naturally infected leaves were selected and subjected to biochemical tests. These bacterial isolates were characterized as Gram-negative, aerobic, blue-fluorescing on KB medium, positive for levan and tobacco hypersensitive reaction but negative for oxidase, rot of potato slices and arginine dihydrolase. These reactions corresponded to LOPAT group Ia, which includes Pseudomonas syringae pathovars (Lelliott and Stead 1987). Six selected representative isolates from six different regions in Hatay and Adana provinces were used in pathogenicity test on the leaves of 5-week-old flat-leaved parsley plants (cv. d'giant Italiana) (Fig. 2a) and examined for symptom development over a period of 3 weeks. For all isolates, leaf symptoms similar to those on the original plants were observed on the inoculated parsley leaves 10 days after inoculation (Fig. 2b). No lesion developed on the negative control plants. Sixteen blue-fluorescing bacterial isolates (Fig. 2c), obtained from inoculated host, were confirmed to be identical to the original isolates using the LOPAT tests; no target bacteria were isolated from the negative control plants. Both representative and re-isolates were further used to identify by FAME analysis. FAMEs have proven and been recognized as useful biochemical markers for bacterial classification and characterization (Weyent et al. 1996). According to results of FAME analysis, twenty different fatty acids were detected in parsley isolates (Table 1). The fatty acid profiles of parsley isolate revealed that more than 81.77% of the total peak area of all cellular fatty acids occurring in all bacterial isolates was accounted for by three major components: 16:1 w7c, 16:0 and 18:1 w7c. The hydroxy-substituted acids 10:0 3OH, 12:0, 12:0 2OH, 12:0 3OH and 18:0 contributed on average a further 15.0%. All isolates also produced small amount of (0.2%) minor component 17.0 iso, which is produced by only P.s. pv. apii isolates as reported by Bull et al. 2011;. The identity of representative and re-isolates were further confirmed by amplifying the 16S rRNA gene with the universal primers 27F and 1492R (Lane 1991) and sequence analysis (GenBank Accession No. KP284571). The 16S rRNA gene sequences of representative and re-isolates were 99% identical to the corresponding gene sequences of the Pseudomonas syringae pv. apii strains BS426 and BS463 present in the NCBI database (accession numbers HQ584978.1 and HQ584979.1, respectively).
| Retention time (min) | Fatty acid | % |
|---|---|---|
| 1.25 | 10:00 | 0.11 |
| 1.55 | 10:0 3OH | 2.55 |
| 1.68 | 12:00 | 4.25 |
| 1.94 | 13:00 | 0.07 |
| 2.01 | 12:0 2OH | 2.81 |
| 2.09 | 12:0 3OH | 4.37 |
| 2.24 | 14:00 | 0.2 |
| 2.82 | 16:1 w7c/16:1 w6c | 36.51 |
| 2.85 | 16:1 w5c | 0.1 |
| 2.87 | 16:00 | 23.35 |
| 3.07 | 17:0 iso | 0.2 |
| 3.13 | 17:1 w8c | 0.11 |
| 3.19 | 17:00 | 0.26 |
| 3.44 | 18:1 w9c | 0.77 |
| 3.46 | 18:1 w7c | 21.75 |
| 3.48 | 18:1 w5c | 0.09 |
| 3.50 | 18:00 | 1.02 |
| 3.53 | 18:1 w7c 11-methyl | 0.81 |
| 3.77 | 19:0 cyclo w10c/19w6 | 0.62 |
| 4.07 | 20:1 w7c | 0.07 |
Members of P. syringae sensu lato have been previously isolated from parsley plants. Two P. syringae pathovars apii and coriandricola have been reported to cause very similar bacterial leaf spotting diseases on members of the family Apiaceae such as parsley, celery, coriander, carrot and parsnip (Koike et al. 1994; Toben and Rudolph 1996; Bull et al. 2011; Jardini et al. 2012; Popović et al. 2015). Although both bacterial species cannot be easily distinguished by symptoms caused on parsley, P. syringae pv. coriandricola and P. syringae pv. apii can be differentiated in KB media where bacterial colonies of P.s. pv. coriandricola are not fluorescent (Bull et al. 2011; Gupta et al. 2013; Xu and Miller, 2013). Although the bacterial disease agent P. s. pv. apii has been previously reported to cause leaf spot disease to celery, parsley and fennel grown in Greece and California (Elena et al. 2008; Bull et al. 2011; Jardini et al. 2012), there has been no previous report of P. s. pv. apii on parsley or relative plant species such as celery and fennel in Turkey. To our knowledge, this is the first report of bacterial leaf spot disease on parsley caused by P. s. pv. apii in Turkey.
Bacterial leaf spot disease caused by P. s. pv. apii is not the only foliar disease that has been recently reported in Turkey. Although Septoria blight of parsley caused by Septoria petroselini is common and widespread fungal disease in commercial fields in the region (Kurt 2003), disease symptoms are distinctive because of the spherical, brown to black fungal structures pycnidia that form in the angular leaf spots. Bacterial leaf spot disease agent P.s.pv. apii causes angular, tan to brown leaf spots that lack any mycelial growth or fungal structures. Farmers and technical field advisers must now therefore distinguish between these two foliar diseases of parsley. As this pathogen is seedborne disease agent, initial disease outbreaks may be due to seedborne inoculum. Even low level of infested seed could result in problems due to the favourable environment resulting from high-density parsley plantings and splashing of irrigation water; therefore, overhead sprinkler systems should not be used for irrigation of parsley. Upon official report of the disease in the region, the Ministry of Food, Agriculture and Livestock of Republic of Turkey was informed, contamination boundaries were drawn and all necessary measures have been taken in the region by the staff of the Ministry.
Acknowledgements
We thank Prof. Dr. Cigdem Serce for her helps on molecular analyses. This work was financially supported by a Grant (MKÜ-BAP 9660) from the Mustafa Kemal University, Scientific Research Project Unit.




