Efficacy of orofacial myofunctional therapy combined with myofascial release in patients with mild obstructive sleep apnoea: a randomized controlled trial
Abstract
Background
Obstructive sleep apnoea (OSA) is characterized by repetitive narrowing and collapse of pharyngeal airway during sleep, leading to apnoea or hypopnoea. In this context, myofunctional therapy and myofascial release might be effective, despite the literature on the combination of these approaches is still scarce.
Objectives
This randomized controlled trial aimed to assess the efficacy of oro-facial myofunctional therapy combined with myofascial release in terms of functioning in patients with mild OSA.
Methods
Patients aged from 40 to 80 years with diagnosis of mild OSA were randomly allocated into intervention group (oro-facial myofunctional therapy plus myofascial release) and control group (only oro-facial myofunctional therapy). At the baseline (T0), after 4 weeks (T1), and after 8 weeks (T2), the following outcomes were assessed: apnoea/hypopnoea index (AHI), average oxygen saturation (SpO2), sleep time spent with oxygen saturation < 90% (T90), snoring index, and Pittsburgh Sleep Quality Index (PSQI).
Results
Out of the 60 patients enrolled, 28 (aged 61.46 ± 8.74 years) complete the treatment in the intervention group and 24 (aged 60.42 ± 6.61 years) in the control group. There were no significant differences in AHI between groups. A significant difference was reported for ΔT0–T1 SpO2 (p = .01), T90 (p = .030), ΔT0–T1 and ΔT0–T2 snoring index (p = .026 and <.001 respectively), and ΔT0–T1 and ΔT0–T2 Pittsburgh Sleep Quality Index (p = .003 and <.001 respectively).
Conclusion
Taken together, a combination of oro-facial myofunctional therapy and myofascial release showed a potential treatment for sleep quality in patients with mild OSA. Future studies are necessary to better investigate the role of these interventions in OSA patients.
1 INTRODUCTION
Obstructive sleep apnoea (OSA) is characterized by repetitive narrowing and collapse of the pharyngeal airway during sleep, which could lead to serious episodes of apnoea (complete cessation of ventilation) or hypopnoea (insufficient breathing).1 It is considered an emerging public health problem, and its prevalence has increased over the years, ranging from 9% to 38% in the general adult population.2, 3 The pathophysiology of OSA is complex and multifactorial,3 with some predisposing and aggravating factors, including obesity, smoking habit, increased upper airway length, narrow pharynx, particular pharyngeal lumen shapes, and collapsible upper airway.4-6 The intermittent hypoxemia and the sleep fragmentation (with the activation of sympathetic tone) could lead to snoring, unsatisfactory rest, and daytime sleepiness, which considerably affect the patient's quality of life and work performance.7, 8
The first-line treatment is the continuous positive airway pressure (CPAP), which has been shown highly effective in reducing sleep-disordered breathing events; however, between 17% and 85% of patients with OSA have been reported to be not comply with CPAP therapy, mainly due to mask discomfort and cutaneous allergies.9 Thus, alternative approaches have been investigated during the last decades, including the myofunctional therapy of the upper airways.10, 11 Indeed, the upper pharyngeal patency depends on the activity of the surrounding muscles and changes in oropharyngeal muscle activity may occur during sleep, with poor genioglossus muscle responsiveness to negative pharyngeal pressure and an oversensitive ventilatory control system.12, 13 The myofunctional therapy includes several combinations of isotonic and isometric exercises aimed at strengthening the oropharyngeal muscles, and working on functions, such as speaking, breathing, blowing, sucking, chewing, or swallowing.11, 13 In this context, recent systematic reviews showed that the myofunctional therapy (oropharyngeal exercises) could lead to a reduction of apnoea–hypopnoea index (AHI) by approximately 50% in adults and 62% in children, with an improvement of the sleep quality and of the daytime sleepiness, allowing a reinforcement of the upper airway muscle function and a maintenance of the upper airway patency, mainly increasing the strength of the genioglossus that should be considered as the main dilator muscle of the upper airways.10, 14-16
Myofascial release was proposed as adjunct treatment in OSA patients for its effects in removing fibrosis/adhesions and re-establishing optimal texture, resilience, and function of the soft tissues in patients with positive tender points.17-19 Indeed, this approach seems to adapt the neuromuscular tone of the upper airways, with intriguing results in terms of reduction of the AHI in OSA patients, considering that the functional limitations affecting respiratory muscles could create areas of dysfunctional stenosis, thus potentially lowering the threshold for the occurrence of apnoeas and hypopnoeas.19-21
However, to date, the literature on the effects of myofunctional exercises combined with myofascial release is scarce, despite the importance of defining a specific rehabilitation protocol. Indeed, as the genioglossus is considered as the principal upper airway dilator muscle,22 the tongue represents the main anatomical target of the myofunctional therapy, and there is a need of stronger evidence on the effects of this therapy combined to a physiotherapy intervention in patients with mild OSA.
Therefore, this randomized controlled trial (RCT) aimed to evaluate the efficacy of myofunctional exercises combined with myofascial release in terms of AHI, daytime sleepiness, and sleep quality in patients affected by mild OSA.
2 MATERIALS AND METHODS
2.1 Study registration
The protocol followed guidance from the CONSORT Guidelines.23 The Ethics Committee of University “G. D'Annunzio” of Chieti, Chieti-Pescara, Italy approved the research with protocol number: 1837–28.06.2019. Moreover. the present RCT was registered on ClinicalTrials.gov with number: NCT04412941.
2.2 Participants
Patients referred to the Respiratory Medicine Department of “Santo Spirito” Hospital, Pescara, Italy, in collaboration with “G. D'Annunzio” University of Chieti-Pescara, Italy, were recruited from May to November 2020.
The inclusion criteria were as follows: (a) men and women aged from 40 to 80 years; (b) diagnosis at the polysomnography (SOMNOlab 2 PSG, SOMNOmedic, Weinmann, Hamburg, Germany) of mild OSA, consisting of an apnoea/hypopnoea index (AHI) from 5 to 14, considering that the AHI was calculated as number of events per sleep hour1; (c) normal and overweight subjects (with a body mass index, BMI, from 18.5 to 29.9).
We excluded patients with: (a) severe nasal obstructive disease; (b) primary pulmonary pathology; (c) craniofacial malformations; (d) facial traumas; (e) assuming hypno-inducing drugs; (f) hypothyroidism; (g) neuromuscular diseases; (h) recent stroke; (i) systemic infectious diseases; (j) neoplastic diseases; (k) pregnant women.
This study was performed according to the Declaration of Helsinki, with pertinent National and International regulatory requirements. All participants provided written informed consent and were free to withdraw from the study at any time.
2.3 Intervention
A computer-generated algorithm sequence of 1:1 was used for the randomization and concealment of allocation. Therefore, patients who fulfilled the eligibility criteria were enrolled and randomly allocated into two groups as follows: intervention group (myofunctional therapy combined with myofascial release) and control group (only myofunctional therapy).
The only intervention group underwent myofascial release treatment, consisting of 3 sessions per week for 4 weeks; both groups underwent oropharyngeal myofunctional exercises at home for 6 times/day for 8 weeks.
All patients in both groups were advised to follow the rules of sleep hygiene according to the Italian Association of Sleep Medicine,24 which is summarized in Table 1. Indeed, sleep is influenced by our lifestyle and various environmental factors can affect the quality of night sleep.25
| 1 | The room in which you sleep should not accommodate anything other than the essentials for sleeping. It is advisable not to place a television, computer, or desk in the bedroom to avoid establishing links between activities that are not relaxing and the environment in which a condition must instead be established of relaxation that favours the beginning and maintenance of sleep at night |
| 2 | The room in which you sleep must be sufficiently dark, silent and with adequate temperature (avoid excess heat or cold) |
| 3 | Avoid taking caffeine-based beverages and the like (coffee, tea, Coca-Cola, chocolate), especially in the evening |
| 4 | Avoid taking alcoholic beverages (wine, beer, spirits) in the evening or, worse, for hypno-inducing purposes |
| 5 | Avoid high-calorie or high-calorie evening meals with a high protein content (meat, fish). |
| 6 | Avoid tobacco smoke in the evening hours |
| 7 | Avoid daytime naps, except for a short post-prandial nap. Avoid particularly naps after dinner, in the time slot before bedtime |
| 8 | In the hours before bedtime, avoid medium-high intensity physical exercise (e.g. gym). Exercise is instead desirable in the late afternoon |
| 9 | Avoid hot baths or showers in the evening |
| 10 | Avoid, in the hours before bedtime, to engage in activities that are particularly engaging on a mental and / or emotional level (study; computer work; video games, etc.) |
| 11 | Try to lie down in the evening and get up in the morning at regular and constant times and as much as possible in line with your natural tendency to sleep |
| 12 | Do not spend too much time in bed at night, anticipating bedtime and / or postponing the time to get up in the morning |
2.3.1 Myofascial release
The intervention group underwent myofascial release as follows: (i) trigger or tender points treatment (5 min) of the pectoralis major muscle, pectoralis minor muscle, sternocleidomastoid muscle, elevator scapula muscle, and scalene muscles; (ii) myofascial ‘pompage’ techniques on the cervical spine muscles that consists in a rehabilitative mobilization technique that minimizes osteomyoarticular restrictions and promotes muscle relaxation and provides pain relief and increased range of motion, manual stretch of pectoralis major muscle, pectoralis minor muscle, sternocleidomastoid muscle, elevator scapula muscle, scalene muscles, and the upper bundles of the trapezius (15 min); (iii) a third phase of 20 min consisting of: manual therapy on the ribs and sternum, and rib mobilization; (iv) exercises to restore correct diaphragmatic and low costal breathing, consisting of: breathing exercises using only the diaphragm and contracting the abdomen and inhibiting the use of accessory muscles; exercises for strengthening the diaphragm and for the mobility of the rib cage in the different positions (supine, side, and sitting), to inhale by expanding the rib cage against the resistance of the therapist; exercises focusing on expanding the rib cage while breathing in order to strengthen the muscles of the side in contact with the bed and improve the mobility of the ribs on the free side; sitting and learning to manage the abdomen in conjunction with the diaphragm and trying to coordinate movements and contractions; (v) cool-down phase with free breathing (5 min). Therefore, the entire rehabilitative myofascial release protocol consisted of 3 sessions lasting 60 min per week for 4 weeks, thus undergoing a total of 12 sessions (see Figure 1 for further details).
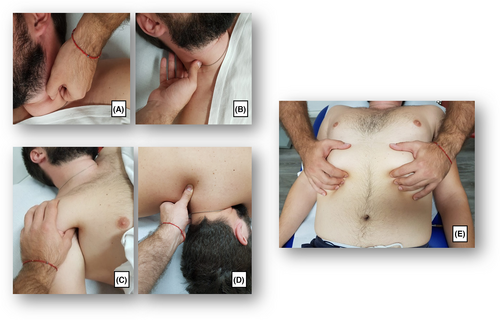
2.3.2 Oro-facial myofunctional therapy
The patients of both groups were instructed to perform oropharyngeal myofunctional exercises during their first rehabilitative session by an experienced physiotherapist. Subsequently, these exercises were assigned to the patients to be performed at home for 6 times/day for 8 weeks.
The patients used a clinical diary to record their compliance and were monitored by a physiotherapist. At the end of each session, time was reserved to verify the correct execution of the oropharyngeal exercises assigned at home (see Figure 2 for further details).

The myofunctional rehabilitative program consisted of the following oral exercises: (i) pronouncing letters ‘A’ and ‘O’, making the tongue adhere to the palate and snapping it off by opening the mouth (A-O); (ii) nose-chin tongue, trying to touch the nose with the tip of the tongue, then trying to touch the chin; (iii) tongue circles, making full circles with the tongue, both inside and outside the mouth; (iv) tongue-checks, pushing the tongue against the cheek, 10 times to the right and 10 times to the left; (v) tongue-gingiva (spot), opening and closing the mouth without detaching the tip of the tongue from the spot. At the end of treatment instructions were given to the patients as follows: “The exercises must be done in a quiet environment and in front of the mirror, so that you can correct yourself. For each exercise, make 3 sets in the morning and 3 in the evening (1 set = 15 repetitions). Breathe for 1 min between one exercise and the next”.
2.4 Outcome measures
At the baseline (T0), at 4 weeks from the baseline (after the end of myofascial release in the intervention group) (T1), and at 8 weeks from the baseline (after the end of myofunctional therapy in both groups) (T2), the following outcomes were assessed through the PSG: (i) the apnoea/hypopnoea index (AHI, measurement of the number of apnoea and hypopnoea events per hour); (ii) total number of obstructive apnoeas and hypopnoeas; (iii) oxygen desaturation index (ODI, the average number of desaturation episodes per hour), (iv) average SpO2; T90, indicating the time in which the peripheral oxygen saturation was less than 90% during the sleep; (v) snoring index (%); (vi) average heart rate, reported as bpm.
Furthermore, at all the time-points the following secondary outcomes were assessed: (i) Epworth Sleepiness Scale (ESS),26 a self-administered test evaluating the changes in daily sleepiness that includes eight items to investigate daytime sleepiness and sleep apnoea risk in different real-life situations during the preceding months, with a final score ranging from 0 to 24; (ii) Pittsburgh Sleep Quality Index (PSQI),27 a self-reported questionnaire to assess sleep quality, consisting of 19 items in seven domains of sleep difficulties (sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping medications, and daytime dysfunction), with an overall score above 5 considered as an indicator of sleep disturbances relevant in at least two components or of moderate difficulty in more than three components.
The author who assessed the outcome measures was blinded regarding the allocation of study participants.
2.5 Statistical analysis
Sample size was calculated using the software GPower 3.1 (University of Dusseldorf). Considering the AHI as primary outcome,11 setting a power of analysis of 95%, an alpha error of .05, and a drop-out of 10%, the resulting sample size for each group was 21 subjects for each group.
The statistical analyses were carried out using the SPSS version 18 package (SPSS Inc). Values are expressed as the median and minimum and maximum for the continuous variables and the proportion for the categorical variables, as appropriate. The demographic and clinical data at baseline included the following parameters: age and BMI (body mass index) expressed as the median and minimum and maximum; gender (female or male); Mallampati Index; and neck circumference. Differences in the baseline characteristics between the 2 groups were calculated by Fisher's exact test or Mann–Whitney U test, as appropriate. The analysis of the time difference between the two groups (treatment group and control group) was performed through Friedmann analysis for repeated measures to determine changes in the different evaluation times in the 2 groups. As a subsequent analysis for each parameter, a pairwise comparison was performed with a Bonferroni correction. The Mann–Whitney U test was used for all parameters to evaluate the time differences between the groups and the variations (Δ) between T0 and T1, T1 and T2, and T2 and T0. All primary and secondary outcomes were analysed according to the principle of intention-to-treat. The p values < .05 were considered significant.
3 RESULTS
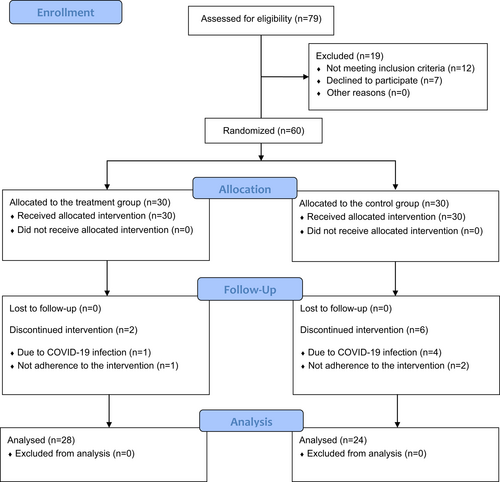
Out of the 79 patients assessed for eligibility, 12 did not meet inclusion criteria and were excluded and 7 subjects declined to participate. Sixty patients were enrolled and so divided into the two groups: 30 in the intervention group and 30 in the control group; however, 8 patients discontinued the intervention (n = 2 in the intervention group; n = 6 in the control group).
Therefore, 28 study participants (aged 61.46 ± 8.74 years) complete the intervention in the intervention group and 24 ones (aged 60.42 ± 6.61 years) in the control group. No adverse events were reported during rehabilitative treatment or the follow-up period. See the CONSORT Flow Diagram depicted in Figure 3 for further details.
The demographic and clinical characteristics are summarized in Table 2. A descriptive analysis was performed, and a comparison between the intervention group and control group was reported for age (p = .645), BMI (p = .927), neck circumference (p = .350), and Mallampati Index (p = .09). Compared to the baseline, the two groups were homogeneous and matched for age and other clinical parameters.
| Intervention group | Control group | p value | |
|---|---|---|---|
| Gender (female/male) | 15/13 | 15/9 | – |
| Age (years) | 61.46 ± 8.74 | 60.42 ± 6.61 | .645 |
| BMI (kg/m2) | 26.51 ± 2.73 | 26.47 ± 3.14 | .927 |
| Neck circumference (cm) | 38.04 ± 2.82 | 36.72 ± 4.41 | .350 |
| Mallampati Index | 3 (2–4) | 3 (2–4) | .09 |
- Note: Continuous variables are expressed as means ± standard deviations; ordinal variables are expressed as median and range (minimum and maximum); ratios are expressed as x/y.
- Abbreviation: BMI, body mass index.
Results of the between-group analysis are shown in Table 3. In particular, no statistically significant differences were reported in terms of AHI, whereas a statistically significant difference was reported for ΔT0–T1 for the following parameters: SpO2 (p = .01); T90 (time in which the peripheral oxygen saturation was less than 90% during sleep; p = .03); Snoring Index (p = .026); Pittsburgh Sleep Quality Index (p = .003). Moreover, a statistically significant difference was reported for ΔT0-T2 for the Snoring index (p < .001) and for the Pittsburgh Sleep Quality Index (p < .001).
| Intervention group | Control group | p value | |
|---|---|---|---|
| Δ T0–T1 AHI (n°/h) | 0.1 (−10.1–20.30) | −0.9 (−7.20–14.5) | .544 |
| Δ T0–T2 AHI (n°/h) | 2.8 (−3.8–11.4) | −0.8 (−6–29.4) | .084 |
| Δ T0–T1 SpO2 (%) | 0 (−4–2) | 0.5 (−3–3) | .010 |
| Δ T0–T2 SpO2 (%) | 0 (−5–4) | 0.25 (−5–1) | .081 |
| Δ T0–T1 ODI (%) | 1 (−6.20–16.8) | −1.5 (−7.7–15.20) | .142 |
| Δ T0–T2 ODI (%) | 2.4 (−2.3–12.8) | 1 (−6–30.3) | .106 |
| Δ T0–T1 T90 (%) | 0.45 (−0.8–26.9) | −0.05 (−57–16.9) | .030 |
| Δ T0–T2 T90 (%) | 0.1 (−12.9–7.9) | 1.5 (−56.8–37.4) | .451 |
| Δ T0–T1 Snoring Index (%) | −1.7 (−30–20.2) | 5.4 (−29.8–51.4) | .026 |
| Δ T0–T2 Snoring Index (%) | −7.1 (−35.4–6) | 8.7 (−25.5–41.20) | <.001 |
| Δ T0–T1 HR (bpm) | −1 (−9–5) | 0 (−11–27) | .782 |
| Δ T0–T2 HR (bpm) | 0.5 (−14–6) | −2 (−9–18) | .312 |
| Δ T0–T1 hypopnoeas (n°) | 0 (−55–91) | −5.5 (−56–82) | .262 |
| Δ T0–T2 hypopnoeas (n°) | 6.5 (−52–90) | 3.5 (−53–95) | .262 |
| Δ T0–T1 apnoeas (n°) | −1 (−24–72) | 3.5 (−11–19) | .146 |
| Δ T0–T2 apnoeas (n°) | 1 (−31–44) | 1 (−10–62) | .321 |
| Δ T0–T1 ESS | −3 (−7–2) | −1 (−6–6) | .063 |
| Δ T0–T2 ESS | −3 (−8–1) | −2.5 (−7–2) | .181 |
| Δ T0–T1 PSQI | −2 (−5–0) | 0 (−3–7) | .003 |
| Δ T0–T2 PSQI | −3 (−7–2) | 1 (−3–5) | <.001 |
- Note: Δ, difference; T0, baseline; T1, after treatment; T2, follow-up; Values are expressed as median and range (minimum and maximum); Significant p values are in bold.
- Abbreviations: AHI, apnoea–hypopnoea index; ESS, Epworth sleepiness scale; HR, heart rate; ODI, oxygen desaturation index; PSQI, Pittsburgh Sleep Quality Index; SpO2, peripheral oxygen saturation; T90, time in which the peripheral oxygen saturation was less than 90% during sleep.
Results of the within-group analysis are shown in Table 4. In both intervention and control groups, no statistically significant differences were reported in terms of primary outcome (AHI). The intervention group results showed a statistically significant improvement at T0-T2 for ODI (p = .001), at T1–T2 and T0-T2 for hypopnoeas (p = .010 and .012 respectively), at T0–T1 and T0–T2 for apnoeas (p < .001 and p = .001 respectively), at T0–T1 and T0–T2 for ESS (p < .001), and at T0–T1 and T0–T2 for PSQI (p < .001).
| T0 | T1 | T2 | p value | p value T0–T1 | p value T1–T2 | p value T0–T2 | |
|---|---|---|---|---|---|---|---|
| Intervention group | |||||||
| AHI (n°/h) | 8.35 (5–14.8) | 8.85 (1.9–28.6) | 13.8 (4.6–17.5) | .069 | – | – | – |
| SpO2 (%) | 94.8 (90.3–97) | 94 (90–97) | 94 (90.6–97) | .091 | – | – | – |
| ODI (%) | 6.3 (3.4–14.6) | 7.05 (1.4–22.4) | 11.2 (3.6–16.5) | .001 | .135 | .326 | .001 |
| T90 (%) | 0.6 (0–28.5) | 0.55 (0–44.8) | 0.95 (0–15.6) | .197 | – | – | – |
| Snoring index (%) | 24.1 (0–75.6) | 15.5 (0–67.7) | 19.1 (0.67.5) | .368 | – | – | – |
| HR (bpm) | 64 (51–77) | 65 (50–77) | 64.5 (53–73) | .406 | – | – | – |
| hypopnoeas (n°) | 28 (11–76) | 23 (3–112) | 27 (17–102) | .002 | 1 | .010 | .012 |
| apnoeas (n°) | 20 (3–52) | 9.5 (2–46) | 10.5 (0–38) | .003 | <.001 | – | .001 |
| ESS | 6.5 (1–15) | 4 (1–13) | 3.5 (1–11) | <.001 | <.001 | 1 | <.001 |
| PSQI | 6 (4–16) | 5 (2–12) | 4 (2–15) | <.001 | <.001 | 1 | <.001 |
| Control group | |||||||
| AHI (n°/h) | 9.25 (6.3–14.6) | 7.9 (2.6–23.9) | 9.9 (3.3–38.8) | .135 | – | – | – |
| SpO2 (%) | 94 (89–96) | 94 (90–97) | 94 (90–95) | .098 | – | – | – |
| ODI (%) | 8.05 (2.1–15.5) | 7.7 (0.4–23.3) | 9.75 (2.5–38.4) | <.001 | .13 | <.001 | .13 |
| T90 (%) | 0.2 (0–62.5) | 0.3 (0–22.7) | 0.5 (0.2–38.9) | .089 | – | – | – |
| Snoring index (%) | 9.2 (0.2–46) | 15.4 (0–64.5) | 21.1 (1.1–54.3) | .011 | .028 | 1 | .028 |
| HR (bpm) | 67.5 (52–76) | 67 (54–84) | 65 (54–75) | .177 | – | – | – |
| hypopnoeas (n°) | 37 (8–84) | 28 (3–129) | 34 (14–142) | <.001 | .028 | <.001 | .250 |
| apnoeas (n°) | 20 (0–40) | 22 (2–37) | 22 (0–89) | .568 | – | – | – |
| ESS | 7.5 (2–14) | 5 (2–11) | 5.5 (1–9) | <.001 | .091 | .250 | <.001 |
| PSQI | 9 (5–14) | 8 (5–13) | 9 (5–15) | .060 | – | – | – |
- Notes: T0, baseline; T1, after treatment; T2, follow-up; Values are expressed as median and rage (minimum and maximum); Significant p values are in bold.
- Abbreviations: AHI, apnoea–hypopnoea index; CG, control group; ESS, Epworth sleepiness scale; HR, heart rate; ODI, oxygen desaturation index; PSQI, Pittsburgh Sleep Quality Index; SpO, peripheral oxygen saturation; T90, time in which the peripheral oxygen saturation was less than 90% during sleep; TG, treatment group.
On the other hand, for the control group, it was reported an improvement only at T1–T2 for ODI (p < .001). Moreover, there was a slight worsening of the Snoring Index at T0–T1 and T0–T2 (p = .028), a slight increase in the number of total hypopnoeas at T0–T1 and T1–T2 (p = .028 and p < .001 respectively), and an improvement only for ESS at T0–T2 (p < .001).
4 DISCUSSION
The present RCT was the first investigation of the clinical effect of oro-facial myofunctional therapy combined to myofascial release, in terms of reducing AHI and daytime sleepiness, improving the quality of sleep in patients affected by mild OSA.
In terms of primary outcome, both the between-group and the within-group analyses did not show a reduction of the sleep-disordered breathing events, and the AHI from T0 and T2 did not improve significantly. These results were not in agreement with a recent systematic review and meta-analysis by Zhang et al.14 that reported a significantly decrease of AHI after therapy (p < .00001). However, the papers included in their systematic review evaluated the effects of the oro-facial myofunctional therapy in both children and adults; on the contrary, in the present RCT we included only mild OSA adults.
On the other hand, our results were in line with a recent RCT by Poncin et al.,22 which assessed the efficacy of a 6-week myofunctional program in patients with moderate OSA. Indeed, the authors reported not significant changes in AHI between groups, but significant improvement in the Epworth Sleepiness Scale, in agreement with our results that showed a significant improvement in daytime sleepiness in both intervention and control group.
Moreover, the results suggested a good trend in the improvement of symptoms in patients in the intervention group who underwent the combined rehabilitative treatment (oro-facial myofunctional exercises plus myofascial release). Specifically, the patients reported a significant overall improvement in Snoring Index and Pittsburgh Sleep Quality Index compared to control group, which suggests an improvement in the indexes attributable to the physiotherapy intervention. It should be taken into consideration that a direct comparison with literature was not possible as it was a new protocol combining myofunctional exercises and physiotherapy intervention.
Furthermore, a better desaturation index was observed in the intervention group, which could be related to the improvement in both the number of total obstructive apnoeas and the number of hypopnoeas. On the other hand, in the control group, patients showed only a slight improvement in daytime sleepiness and a reduction in the number of total hypopnoeas. However, a worsening of the snoring index was reported. Since OSA causes sleep interruption and deterioration of sleep quality, the improvement observed after this novel rehabilitation treatment might be a very important aspect of the rehabilitation goals in these patients.
Considering that about 30%–50% of patients with OSA have comorbid insomnia, all patients were advised to follow the rules of sleep hygiene according to the Italian Association of Sleep Medicine. Indeed, the cognitive-behavioural therapy was recommended as ‘first-line’ approach for insomnia, and a recent meta-analysis suggested the potentially subsequent improvement of the comorbid OSA.28 Indeed, the authors supported that the overall effect of the cognitive-behavioural therapy might induce a consistent improvement in insomnia among patients with comorbid OSA.28
Moreover, about the 51% of OSA patients showed signs and symptoms of TMD and about 30% of TMD patients met the diagnostic criteria for insomnia and OSA.29, 30 The linkage among these conditions may be found in the hyperalgesia that OSA patients might experience, in relation to the fragmented sleep and hypoxemia that might enhance peripheral and central pain sensitization, thus increasing spontaneous pain.29, 31, 32 However, a recent cross-sectional study performed by Bartolucci et al.33 showed that the prevalence of TMDs in adults with OSA was not significantly higher compared to healthy controls in their cohort; therefore, further studies should confirm this potential correlation providing reliable evidence on the role of conservative treatments for TMD in patients affected by OSA.
By the way, it is well known that OSA could cause deterioration in the quality of sleep by interrupting it, which disrupts the organization of cycles during the night.34, 35 Above all, there is a decrease in the duration of ‘deep, slow wave sleep’ (N3), with an increase in ‘light, slow wave sleep’ (N1). N3 is important because it is associated with recovery processes, memory, and neuronal plasticity.34, 35 Together, with the decrease in REM sleep, this is important because it is associated with some processes of memory consolidation. These features might be at the origin of several symptoms disabling for OSA patients in daily life, such as excessive daytime sleepiness and a reduction in cognitive skills, such as memory attention.36-38 Indeed, some studies hypothesized a deterioration of cognitive functions in intermittent hypoxia-related sleep disorders.39-41
The present rehabilitative treatment proposal was based on the re-education aspect and supervised exercise for myofunctional exercises, which the patient performed at home, together with additional manual therapy sessions concentrating the treatment to just 1 month. However, this does not exclude the patient from repeating the rehabilitation treatment cyclically, leading a qualitatively active life from a motor point of view, and strictly respecting the rules of sleep hygiene.
Other authors have proposed a rehabilitation treatment of at least 2 months to observe a significant improvement in OSA. They considered only one therapeutic option, either only myofunctional therapy or only physical exercise, mainly aerobic at low-medium intensity. It is important to highlight that these patients often have a sedentary lifestyle; therefore, the physical exercise might play a key role not only in terms of weight loss but also in reducing the low-grade inflammation and improving function and quality of life, especially in older patients.10, 42-45
Further, it was shown that inspiratory muscles were subjected to potentially fatiguing loads during obstructive sleep apnoea with an increase in gastric pressure and inward abdominal movement during the expiratory phases of the apnoea.46 Thus, respiratory rehabilitation could be an adjunct management for OSA, and diaphragmatic myofascial release techniques could help to improve chest wall mobility and balance the diaphragmatic tensions with a better excursion when breathing.18 Related to visceral fascial mobility, other authors showed that applying myofascial release therapy techniques in the cervical region, such as pompage and diaphragmatic myofascial, could improve the sliding of the organs, such as the larynx and oesophagus, related to these fascial tissues;47 notably, researchers showed that chest and neck mobilization soft techniques improved spirometry parameters in young and healthy subjects.48
Overall, these results are encouraging compared to the scientific literature, also the potential correlation between palatal morphology and OSA.48-51 Considering the statistically significant results in the comparison between the groups, the intervention group showed an improvement in terms of reduction of daytime sleepiness, an improvement of the snoring index together with a better oxygen saturation (T-90) versus the control group. Thus, it is possible to hypothesize that oropharyngeal exercises and mobilization exercises of the inspiratory musculature could improve respiratory dynamics during sleep reducing snoring.49
To the best of our knowledge, this is the first study assessing the efficacy of this novel rehabilitative approach consisting of the combination of myofunctional therapy and myofascial release in mild OSA patients. This rehabilitation protocol required close collaboration among physical and rehabilitative medicine physicians, pulmonology physicians, dentists, and physical therapists.
However, this study presents some limitations: first, the inclusion of patients with a BMI <30 kg/m might limit the generalizability of the findings, considering that a very large OSA population affect by obesity; second, muscle tension and trigger point assessments were done manually by the physiotherapist, without any specific instrumentation; lastly, the improvement in pain of the cervical spine, which some patients complained of before starting the rehabilitation treatment, was not recorded.
5 CONCLUSIONS
Taken together, the findings of this RCT showed that the add-on of myofascial release to the oro-facial myofunctional therapy did not significantly change the AHI in patients with mild OSA. However, the combination of oro-facial myofunctional therapy and myofascial release showed to be effective in terms of sleep quality. Future studies with larger samples and longer follow-ups are warranted to investigate the role of these rehabilitative interventions also combined with other therapies in the management of patients with OSA.
AUTHOR CONTRIBUTIONS
TP, AS and AdS were involved in conceptualization. TP, MF, AS and AdS were involved in methodology. TP, LP and AS were involved in formal analysis. MF, LP and ADM were involved in investigation. TP, PP and ADM were involved in data curation. TP and MF were involved in writing—original draft preparation. AS and AdS were involved in writing—review and editing. LP, FA, ADM, PP, MM and AB were involved in visualization. TP and AdS were involved in supervision. All authors read and agreed to the published version of the manuscript.
ACKNOWLEDGEMENTS
We thank Sean Kim for his support in this work. Open Access Funding provided by Universita degli Studi Magna Graecia di Catanzaro within the CRUI-CARE Agreement.
CONFLICT OF INTEREST STATEMENT
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
ETHICS STATEMENT
The Ethical Committee of the ‘Gabriele D'Annunzio’ University of Chieti approved the research protocol (No 1837–28.06.2019). The study was registered in ClinicalTrials.gov (No NCT04412941).
INFORMED CONSENT
Informed consent was obtained from all subjects involved in the study.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




