The Effect of Endogenous Adenosine on Neuronal Activity in Rats: An FDG PET Study
ABSTRACT
2–18F-fluorodeoxy-D-glucose (FDG) is a glucose analog that is taken up by cells and phosphorylated. The amount of FDG accumulated by cells is a measure of the rate of glycolysis, which reflects cellular activity. As the levels and actions of the neuromodulator adenosine are dynamically regulated by neuronal activity, this study was designed to test whether endogenous adenosine affects tissue accumulation of FDG as assessed by positron emission tomography (PET) or by postmortem analysis of tissue radioactivity. Rats were given an intraperitoneal injection of the adenosine A1 receptor antagonist 8-cyclopentyl-1,3-dipropyl-xanthine (DPCPX, 3 mg/kg), the adenosine kinase inhibitor ABT-702 (3 mg/kg), or vehicle 10 minutes prior to an intravenous injection of FDG (15.4 ± 0.7 MBq per rat). Rats were then subjected to a 15 minute static PET scan. Reconstructed images were normalized to FDG PET template for rats and standard uptake values (SUVs) were calculated. To examine the regional effect of active treatment compared to vehicle, statistical parametric mapping analysis was performed. Whole-brain FDG uptake was not affected by drug treatment. Significant regional hypometabolism was detected, particularly in cerebellum, of DPCPX- and ABT-702 treated rats, relative to vehicle-treated rats. Thus, endogenous adenosine can affect FDG accumulation although this effect is modest in quiescent rats.
Introduction
Positron emission tomography (PET) is a noninvasive imaging methodology that is used both in research and in clinical medicine to assess brain neurochemistry and metabolism. PET utilizes radiotracers that emit positrons as they undergo radioactive decay. The most widely used radiotracer for PET is 2–18F-fluorodeoxy-D-glucose (FDG), a glucose analog that is taken up by cells and phosphorylated.1 The amount of FDG accumulated by cells is a measure of the rate of glucose uptake, which is dictated by the rate of glucose consumption (ie, glycolysis) by the cells.
Adenosine is a signaling molecule that acts, via adenosine A1 and A2A receptors, to reduce excitatory and/or facilitate inhibitory neurotransmission.2 Adenosine levels increase during pathological conditions, such as stroke and seizure, and during physiological conditions, such as neuronal activity and prolonged wakefulness. Basal adenosine levels are sufficient to provide an inhibitory tone and adenosine levels increase with neuronal activation. The purpose of this study was to test whether FDG accumulation in brain of healthy rats is affected by endogenous adenosine acting at adenosine A1 receptors. The study made use of a potent and selective adenosine A1 antagonist, 8-cyclopentyl-1,3-dipropropyl xanthine (DPCPX), which crosses the blood–brain barrier3 and an adenosine kinase inhibitor, ABT-702, that can reduce adenosine metabolism and enhance adenosine signaling in brain.4, 5
Methods
All procedures with animals were in accordance with guidelines set by the Canadian Council on Animal Care and approved by the University of Manitoba Animal Protocol Management and Review Committee.
All rats were fasted for 16 hours prior to use. At the beginning of the experiment, each rat was weighed, and then anesthetized using 5% isoflurane for induction and 2.5% for maintenance. A blood sample from tail vein was collected for a fasting blood glucose determination using a standard glucometer (OneTouch Ultra2, LifeScan Inc). Rats were then given an intraperitoneal (i.p.) injection of the adenosine A1 receptor antagonist DPCPX (3 mg/kg, n = 4), the adenosine kinase inhibitor ABT-702 (3 mg/kg, n = 4), or an equivalent volume of vehicle (15% dimethyl sulfoxide, 15% cremophor EL, 70% saline, n = 4) to manipulate the effect of endogenous adenosine on neuronal activities. Ten minutes after i.p. injection, rats were administered FDG (15.4 ± 0.7 MBq) in 0.3-0.5 ml saline by intravenous (i.v.) tail vein injection. Rats were allowed to recover from anesthesia after the FDG injection but were reanesthetized for 15-minute-static PET scan (P4 microPET scanner; Siemens) with the head in the center of the field of view. All images were reconstructed using the Acquisition Sinogram and Image Processing Software (Siemens). Attenuation was corrected using CT scans (SkyScan). Reconstructed PET images were spatially normalized to FDG PET template for rats6 and standard uptake values (SUVs) were calculated. using Small Animal Molecular Imaging Toolbox (http://mic-umcg.github.io/samit/). Normalized images were smoothed with 0.8 × 0.8 × 0.8 mm Gaussian kernel. Differences in global FDG SUV in the whole brain across the groups were assessed using one-way ANOVA (IBM SPSS Statistics v22). To examine the regional effect of active treatment compared to the vehicle, statistical parametric mapping (SPM) analysis was performed using SPM8 (http://www.fil.ion.ucl.ac.uk/spm/software/spm8/) with proportional scaling to mean of the whole brain signal to adjust interindividual differences in global uptake. The clusters identified by unpaired t-test was considered significant at P < 0.05 for cluster-level (family-wise error) and P < 0.002 for voxel level (uncorrected).
Results
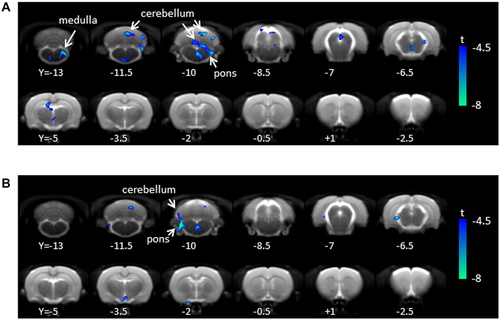
Body weight (316.8 ± 28.4 g; mean ± SD) and blood glucose (5.5 ± 1.7 mM) were not significantly different among three groups. Whole-brain PET SUV values were 1.6 ± 0.4, 1.6 ± 0.6, and 1.8 ± 0.6 for vehicle, ABT-702, and DPCPX-treated rats, respectively (F(2,9) = 0.298, P = 0.75). SPM analysis revealed significant regional hypometabolism in the cerebellum, mesencephalic region, and medulla in the ABT-702-treated rats compared to the vehicle-treated rats (Table 1; Fig 1A). Similarly, DPCPX-treated rats showed decreased FDG uptake in the cerebellum (Table 1, Fig 1B). No significant increase of FDG uptake was observed compared to the vehicle-treated group in either treatment group.
| Peak Voxel | |||||||
|---|---|---|---|---|---|---|---|
| Regions | kE | P (FWE) | T | P | X | y | z |
| ABT-702 versus vehicle | |||||||
| *Cerebellum | 1,472 | 0.002 | −16.15 | <0.001 | 0.9 | −10.8 | −3 |
| 327 | 0.313 | −8.24 | <0.001 | 4.5 | −11.4 | −4.4 | |
| *Cerebellum/Pons/Mesencephalic Region | 1,385 | 0.003 | −9.34 | <0.001 | 1.5 | −7.8 | −6.6 |
| Hippocampus (Antero-Dorsal) | 590 | 0.087 | −6.74 | <0.001 | −1.5 | −3.8 | −3.2 |
| Geniculate (Medial) | 259 | 0.435 | −7.55 | <0.001 | 3.5 | −5.4 | −5.4 |
| Hypothalamus (Medial) | 422 | 0.196 | −6.7 | <0.001 | 0.3 | −5.4 | −7 |
| *Medulla | 1,344 | 0.004 | −9.71 | <0.001 | 2.5 | −13.4 | −8.2 |
| DPCPX versus vehicle | |||||||
| *Cerebellum/Pons | 1,129 | 0.004 | −16.18 | <0.001 | −4.5 | −10.8 | −6.4 |
| 299 | 0.42 | −8.17 | <0.001 | 2.3 | −11 | −3 | |
| Geniculate (Medial) / Hippocampus (Ventral) | 268 | 0.473 | −13.33 | <0.001 | −3.9 | -6.2 | −6.2 |
| Hypothalamus (Medial) | 247 | 0.513 | −8.79 | <0.001 | −1.1 | −2.2 | −9.6 |
| Pons | 365 | 0.324 | −7.57 | <0.001 | 0.3 | −9.8 | −8.8 |
- This table summarizes the clusters (kE) > 200 with voxels that are significant at P < 0.002 (uncorrected at voxel level). *P < 0.05 (FWE at cluster-level). PET = positron emission tomography; FDG = 2–18F-fluorodeoxy-D-glucose; DPCPX = 8-cyclopentyl-1,3-dipropropyl xanthine; ABT-702 = 5-(3-Bromophenyl)-7-[6-(4-morpholinyl)-3-pyrido[2,3-d]byrimidin-4-amine dihydrochloride; SUV = standard uptake value; FWE = family-wise error.
Discussion
The main finding of this study was that DPCPX, an adenosine A1 receptor antagonist, and ABT-702, an adenosine kinase inhibitor, had no global effects on neuronal activity measured with whole-brain FDG SUV with PET. Interestingly, we did observe localized effects of treatments to inhibit FDG accumulation in rat brain using voxel-based analysis of PET SUV.
Our finding that ABT-702 treatment decreased regional FDG uptake is consistent with its effects on endogenous adenosine acting at A1 receptors. A1 receptors on presynaptic neurons reduce calcium influx and reduce neurotransmitter release; postsynaptically, these receptors enhance potassium efflux and suppress neuronal activity. Therefore, increased endogenous adenosine levels induced by ABT-702 may have decreased neuronal activity and decreased energy consumption in the A1 receptor-abundant regions. The most significantly affected region was the cerebellum, which is in line with previous studies that demonstrated the highest abundance of adenosine A1 receptors in rat cerebellum and hippocampus.7, 8 Although not significant at corrected level, a sizable reduction of FDG uptake was also observed in the hippocampus (P = 0.087). A significant decrease in FDG uptake was also observed in the mesencephalic regions and medulla, brain regions where adenosine has an important role in cardiovascular control.9 The lack of global effect on brain glucose metabolism (other than focal effects in cerebellum and brainstem) is in line with a previous report that adenosine kinase inhibitors have little effect on adenosine levels in the absence of a stimulus to increase neuronal activity (eg, pain, seizure).10 In our study, no particular sensory stimulus was administered although rats were awake during FDG accumulation until PET acquisition. Thus, the lack of effect of ABT-702 in many brain regions may have been due to low levels of adenosine kinase activity at baseline.
In the voxel-based analysis, DPCPX decreased FDG uptake in cerebellum. This inhibition was unexpected, since A1 receptor antagonism is predicted to increase neuronal activity. It is possible that the stimulatory effect of DPCPX was counteracted by a further increase in endogenous adenosine release11 or by a shift in relative A1 to A2A receptor activity. Further, a potential interaction between DPCPX and anesthesia cannot be ruled out.
In conclusion, this study indicates that endogenous adenosine acting at A1 receptors can affect FDG accumulation in brain regions that express this receptor although this effect is modest in awake unstimulated rats. Further studies with stimuli that evoke localized increases in adenosine levels are warranted.
Acknowledgments
This research was supported by the Natural Sciences and Engineering Research Council of Canada, the Canadian Institutes of Health Research, and the University of Manitoba. We thank Drs. Andrew Goertzen (Radiology, University of Manitoba) and Michael Jackson (Director, Small Animal Materials and Imaging Core Facility, University of Manitoba) for their assistance.