Longitudinal decline of plasma neurofilament light levels after antiretroviral initiation in people living with HIV
Enrico Ripamonti and Arvid Edén share first co-authorship.
Abstract
Background
This retrospective follow-up study aims to investigate the dynamic longitudinal change of plasma neurofilament light (NfL) levels after antiretroviral therapy (ART) initiation in a cohort of people living with human immunodeficiency virus (HIV) (PWH).
Methods
We tested a convenience sample of 116 patients from the NORTHIV study. Plasma NfL levels—measured using Single molecule array (Simoa) technology—as well as other laboratory parameters were collected at baseline, weeks 4, 48, 96, and 144. Linear mixed-effects models were estimated to evaluate longitudinal change over time. Baseline CD4+ T-cell levels, CDC classification, and HIV RNA levels were considered. Models were adjusted by age, sex, treatment regimen, and baseline serum creatinine levels.
Results
Plasma NfL levels were higher at baseline and also declined faster during the follow-up for participants with CD4+ count <100 cells/µl compared with >100 cells/µl. No significant difference was found between the CD4+ strata 100–199 and 200–499/µl. Participants with CDC classification stages B and C had higher levels of plasma NfL at baseline, as well as faster decline compared with participants with stage A. No significant main effects or change over time was found in baseline HIV RNA levels, treatment regimen, or sex.
Conclusion
Plasma NfL is a sensitive biomarker to assess ongoing central nervous system injury in PWH. Plasma NfL concentrations decline relatively fast following ART initiation and then stabilize after 48 weeks. Plasma NfL concentrations are associated with CD4+ count and stage of HIV disease. No correlations were seen with different ART regimens.
Graphical Abstract
Introduction
The human immunodeficiency virus type 1 (HIV-1) enters the central nervous system (CNS) as a very early event [1, 2]. HIV RNA can be detected in the cerebrospinal fluid (CSF) of virtually all infected individuals with plasma viremia, starting in the acute infection [3, 4]. In untreated chronic disease, as immune function deteriorates, neurological signs and neuropsychological impairment can be observed as a consequence of CNS infection and inflammation [5-7].
CSF biomarkers of neuronal injury have been extensively studied in people living with HIV (PWH). Of particular interest is the role of the light subunit of the neurofilament light (NfL) chain protein [8]. Neurofilaments comprise about 85% of the cytoskeleton proteins and consist of three subunits with different molecular weights: light (68 kDa), medium (150 kDa), and heavy (190–210 kDa). NfL is the most soluble and abundant component and is a major structural constituent of myelinated axons that maintains axonal caliber and facilitates effective nerve conduction [9].
NfL levels in CSF rise with age as a result of biological senescence, with an estimated annual increase of about 3.1% [8]. The triggers and biochemical pathways of these processes are still relatively unknown. Abnormally elevated NfL levels in CSF, at all ages, provide a sensitive but not disease-specific marker of CNS injury, including neural injury related to HIV infection [10, 11]. In patients undergoing antiretroviral therapy (ART), CSF NfL levels generally decline and stabilize in the weeks following treatment initiation [12, 13]. However, even PWH successfully treated with ART with durable viral suppression may still have slightly higher levels of CSF NfL than HIV-negative controls [12, 14]. Moreover, CSF NfL concentrations increase after treatment interruptions [15].
NfL can also be measured in plasma, although concentrations are 50–100 times lower than in CSF [16, 17]. Plasma NfL concentration was measured cross-sectionally in a sample of 19 HIV controls [16]. The estimated median concentration was 9.3 nmol/L (IQR = 5.9–13.1) and was significantly correlated to age. CSF and plasma NfL are usually interrelated in PWH and show a similar age-related increasing pattern [16, 18]. However, plasma NfL levels may be influenced by other factors, including peripheral neuropathies [19], and, in varying degree, serum creatinine concentration [20-23]. The CSF:serum albumin ratio is an indicator of the blood–brain barrier permeability, and increased levels are often found in HIV-infected patients with severe immunodeficiency and HIV-associated dementia [24]. In PWH, this has been found to be correlated (coefficient about 0.30) both with CSF and plasma NfL concentrations [16]. Additionally, some antiretroviral drugs may have inherent neurotoxic properties [25, 26], which could potentially influence plasma NfL levels. These caveats notwithstanding, plasma NfL may potentially be used as an accessible biomarker of neurodegeneration [23], with the advantage of not requiring lumbar puncture. However, in the current state of research, the pattern and the specific trajectory of plasma NfL levels after ART initiation are still relatively unknown. This constitutes a major limitation for the application of plasma NfL measurements in clinical routine. Consequently, this study aimed to investigate the longitudinal kinetics of plasma NfL after ART initiation in a well-defined cohort of PWH. We focused on (i) how long it takes for plasma NfL levels to stabilize and (ii) possible differences in plasma NfL trajectories across treatment regimes.
Methods
Study design and participants
We used data from the NORTHIV study [27, 28], a phase 4, open-label, randomized multicenter trial, conducted between 2004 and 2007 at 29 sites in Sweden and Norway. A total of 227 treatment-naïve HIV-infected patients were randomized to receive lopinavir/ritonavir, atazanavir/ritonavir, or efavirenz in combination with 2 nucleoside reverse transcriptase inhibitors. Inclusion criteria for the NORTHIV study were as follows: (i) adult (>18 years), HIV-1 infected and ART naïve; (ii) indication to start treatment according to contemporary guidelines [29, 30]; (iii) being able to understand and provide informed consent. Pregnant women or women not practicing birth control during the study period, as well as PWH with known resistance to any of the study drugs, were excluded. Additionally, for the current analyses, patients who were non-adherent to or interrupted treatment during follow-up were excluded.
Clinical and laboratory parameters were collected at all study visits, including baseline and weeks 4, 48, 96, and 144. All patients provided written informed consent to participate. The study protocol was approved by the Research Ethics Committee of the University of Gothenburg, the Regional Committees for Medical Research Ethics in Norway, and the Swedish Medical Products Agency.
Blood sampling and laboratory methods
Blood was obtained according to standard protocols [31] and collected in EDTA tubes, and plasma was aliquoted and stored for later batch analysis. Plasma NfL concentration was measured using the commercially available NfL Advantage Kit on a Single molecule array (Simoa) HD-X instrument as described by the manufacturer (Quanterix, Billerica, MA, USA). All plasma samples were analyzed in a single run in the Clinical Neurochemistry Laboratory at the University of Gothenburg by board-certified laboratory technicians blinded to clinical data using a single batch of reagents for each assay. Intra-assay coefficients of variation were below 10% for all analyses.
Clinical evaluation
At all visits, participants underwent routine clinical screening for symptoms or signs of neurological disorders, CNS opportunistic infections, or other conditions that could impact plasma NfL concentration.
Measures
We considered as an outcome log10-transformed plasma NfL levels along the entire follow-up period. As covariates, we took into consideration age as a time-varying variable. Other variables measured at baseline and considered time-fixed variables were sex; treatment regimen; CD4+ levels (categorized as: 0: ≥500/µl; 1: 200–499/µl; 2: 100–199/µl; 3: <100 µl); stage of HIV disease (CDC classification, A: asymptomatic, acute HIV disease or persistent generalized lymphadenopathy; B: symptomatic non-A and non-C; C: AIDS-indicator condition); plasma HIV RNA levels (0: <50k; 1: 50–100k; 2: >100k); serum creatinine levels (as a continuous variable).
Statistical methods
Linear mixed-effects models were estimated to evaluate longitudinal change over time. Variables separately considered in interaction with time were baseline CD4+ levels, stage of HIV disease, and baseline HIV RNA levels. Each analysis was adjusted by age (time-varying) and the other variables (time-fixed), also including baseline serum creatinine. Supplementary analyses were run considering treatment regimen or sex in interaction with time. A value of p < 0.05 was considered statistically significant. Data analysis was performed with R software (v. 4.0.5).
Results
Baseline characteristics
Plasma NfL was measured on a convenience sample of N = 116 participants (81 males, median age = 39 (1q. = 32, 3q. = 47) of the NORTHIV study. Participants’ demographics and baseline characteristics are shown in Table 1. Out of 116 participants, 41 were randomized to receive lopinavir/ritonavir, 44 to receive atazanavir/ritonavir, and 31 to receive efavirenz. Follow-up assessment was available for 111 participants (95.7%) at week 4, for 85 (73.3%) at week 48, for 76 (65.5%) at week 96, and for 60 (51.7%) at week 144. At baseline, 41 participants (35.3%) had CD4+ levels in the range 200–499/µl, 48 participants (41.1%) in the range 100–199/µl, and 27 participants (23.3%) below 100/µl. As to diseases reported at baseline evaluation, the three most prevalent conditions were pneumocystis jiroveci pneumonia (N = 11), varicella zoster (N = 9), and oral candidiasis (N = 5). Three participants had symptomatic primary HIV infection.
| Characteristic | Lopinavir/ritonavir (N = 41) | Atazanavir/ritonavir (N = 44) | Efavirenz (N = 31) | Total (N = 116) |
|---|---|---|---|---|
| Sex, N (%) | ||||
| Male | 28 (68.3) | 31 (70.5) | 22 (71.0) | 81 (69.8) |
| Female | 13 (31.7) | 13 (29.5) | 9 (29.0) | 35 (30.2) |
| Age, year | ||||
| Median (IQR) | 39 (12) | 39 (16.8) | 37 (14) | 39 (15) |
| Follow-up available, N (%) | ||||
| Week 4 | 39 (95) | 42 (95.5) | 30 (96.7) | 111 (95.7) |
| Week 48 | 27 (65.8) | 32 (72.7) | 26 (83.9) | 85 (73.3) |
| Week 96 | 23 (56.1) | 27 (61.4) | 26 (83.9) | 76 (65.5) |
| Week 144 | 17 (41.5) | 22 (50) | 21 (67.7) | 60 (51.7) |
| CDC classification, N (%) | ||||
| A | 23 (56.1) | 24 (54.5) | 21 (67.8) | 68 (58.6) |
| B | 7 (17.1) | 7 (15.9) | 5 (16.1) | 19 (16.4) |
| C | 10 (24.4) | 8 (18.2) | 5 (16.1) | 23 (19.8) |
| NA | 1 (2.4) | 5 (11.4) | 0 (0) | 6 (5.2) |
| CD4+ levels, N (%) | ||||
| ≥500/µl | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 200–499/µl | 11 (26.8) | 19 (43.2) | 11 (35.5) | 41 (35.3) |
| 100–199/µl | 21 (51.2) | 15 (34.1) | 12 (38.7) | 48 (41.4) |
| <100/µl | 9 (22.0) | 10 (22.7) | 8 (25.8) | 27 (23.3) |
| HIV RNA levels, N (%) | ||||
| <50k | 8 (19.5) | 6 (13.6) | 8 (25.8) | 22 (19.0) |
| 50–100k | 3 (7.3) | 11 (25.0) | 4 (12.9) | 18 (15.5) |
| >100k | 30 (73.2) | 27 (61.4) | 19 (61.3) | 76 (65.5) |
| Creatinine, mmol/L | ||||
| Median (IQR) | 72.5 (14) | 72.5 (17.5) | 77.5 (33) | 73.3 (18.8) |
| Diseases or other conditions | ||||
| Primary symptomatic HIV infection | 0 | 3 | 0 | 3 |
| Persistent generalized lymphadenopathy | 0 | 1 | 1 | 2 |
| Oral candidiasis | 1 | 2 | 2 | 5 |
| Candida esophagitis | 0 | 2 | 0 | 2 |
| Herpes simplex genitalis | 0 | 2 | 0 | 2 |
| Herpes simplex mouth, recurrent | 1 | 0 | 1 | 2 |
| Varicella zoster | 5 | 3 | 1 | 9 |
| Kaposi's sarcoma | 1 | 1 | 0 | 2 |
| Pneumocystis jiroveci pneumonia | 3 | 7 | 1 | 11 |
| Tuberculosis, pulmonary | 2 | 0 | 2 | 4 |
| Tuberculosis, pleuritis | 0 | 0 | 1 | 1 |
| Tuberculosis, glandular | 0 | 0 | 1 | 1 |
| Weight loss | 1 | 2 | 2 | 5 |
| Wasting syndrome | 2 | 1 | 0 | 3 |
- Abbreviations: HIV, human immunodeficiency virus; IQR, interquartile range; NA, not available.
Multivariable analyses
A first mixed-effects model was estimated (N = 116) using plasma NfL as dependent variable, and only age (time-varying) and time as predictors. As expected, the effect of age was significant (estimated 5% annual increase, p < 0.00005). The effect of time was negative, but not statistically significant at week 4 (9% decline from baseline, p = 0.19), but significant at week 48 (29% decline from baseline, p < 0.00005), week 96 (17% decline from baseline, p = 0.01), and week 144 (27% decline from baseline, p = 0.0003) (Table 2).
| Model | N | Estimate (CI) | p-Value |
|---|---|---|---|
| Model 1 | 116 | ||
| Time | |||
| Week 1 | Ref. | ||
| Week 4 | −0.038 (−0.097 to 0.019) | 0.19 | |
| Week 48 | −0.148 (−0.212 to −0.084) | <0.00005*** | |
| Week 96 | −0.083 (−0.150 to −0.016) | 0.015* | |
| Week 144 | −0.137 (−0.211 to −0.064) | 0.0003*** | |
| Age | 0.021 (0.017–0.025) | <0.00005*** | |
| Model 2 | 114 | ||
| Time | |||
| Week 1 | Ref. | ||
| Week 4 | −0.039 (−0.097 to 0.019) | 0.191 | |
| Week 48 | −0.148 (−0.213 to −0.094) | <0.00005*** | |
| Week 96 | −0.083 (−0.149 to −0.016) | 0.015* | |
| Week 144 | −0.138 (−0.211 to −0.064) | 0.0003*** | |
| Age | 0.021 (0.017–0.025) | <0.00005*** | |
| ART regimen | |||
| Efavirenz | Ref. | ||
| Lopinavir | 0.062 (−0.038 to 0.161) | 0.222 | |
| Atazanavir | 0.021 (−0.085 to 0.128) | 0.693 | |
| Gender | |||
| Male | Ref. | ||
| Female | 0.032 (−0.061 to 0.125) | 0.496 | |
| Model 3a | 114 | ||
| CD4+ (200–499/µl) | Ref. | ||
| CD4+ (100–199/µl) | 0.039 (−0.088 to 0.167) | 0.541 | |
| CD4+ (<100/µl) | 0.266 (0.115–0.417) | 0.0008*** | |
| Week 4* CD4+ (100–199/µl) | 0.041 (−0.091 to 0.173) | 0.538 | |
| Week 48* CD4+ (100–199/µl) | −0.093 (−0.238 to 0.053) | 0.210 | |
| Week 96* CD4+ (100–199/µl) | −0.076 (−0.226 to 0.074) | 0.321 | |
| Week 144* CD4+ (100–199/µl) | −0.104 (−0.271 to 0.063) | 0.221 | |
| Week 4* CD4+ (<100/µl) | −0.135 (−0.288 to 0.017) | 0.081 | |
| Week 48* CD4+ (<100/µl)) | −0.291 (−0.388 to −0.051) | 0.011* | |
| Week 96* CD4+ (<100/µl) | −0.371 (−0.552 to −0.190) | 0.0001*** | |
| Week 144* CD4+ (<100/µl) | −0.295 (−0.483 to −0.108) | 0.002** | |
| Model 4b | 108 | ||
| CDC A | Ref. | ||
| CDC B | 0.183 (0.026–0.341) | 0.024* | |
| CDC C | 0.169 (0.011–0.328) | 0.036* | |
| Week 4* CDC B | −0.050 (−0.188 to 0.135) | 0.561 | |
| Week 48* CDC B | −0.176 (−0.354 to −0.004) | 0.039* | |
| Week 96* CDC B | −0.261 (−0.431 to −0.078) | 0.005** | |
| Week 144* CDC B | −0.295 (−0.486 to −0.089) | 0.005** | |
| Week 4* CDC C | −0.0006 (−0.161 to 0.135) | 0.993 | |
| Week 48* CDC C | −0.198 (−0.366 to −0.029) | 0.025* | |
| Week 96* CDC C | −0.310 (−0.474 to −0.134) | 0.0006*** | |
| Week 144* CDC C | −0.236 (−0.428 to −0.048) | 0.016* | |
| Model 5c | 114 | ||
| HIV RNA (<50k) | Ref. | ||
| HIV RNA (50–100k) | 0.072 (−0.119 to 0.263) | 0.456 | |
| HIV RNA (>100k) | 0.137 (−0.011 to 0.285) | 0.07 | |
| Week 4* HIV RNA (50–100k) | −0.136 (−0.236 to 0.164) | 0.725 | |
| Week 48* HIV RNA (50–100k) | 0.116 (−0.102 to 0.333) | 0.295 | |
| Week 96* HIV RNA (50–100k) | 0.135 (−0.089 to 0.360) | 0.237 | |
| Week 144* HIV RNA (50–100k) | −0.044 (−0.274 to 0.186) | 0.707 | |
| Week 4* HIV RNA (>100k) | −0.132 (−0.287 to 0.023) | 0.095 | |
| Week 48* HIV RNA (>100k) | −0.063 (−0.224 to 0.098) | 0.442 | |
| Week 96* HIV RNA (>100k) | −0.080 (−0.253 to 0.092) | 0.359 | |
| Week 144* HIV RNA (>100k) | −0.142 (−0.326 to 0.042) | 0.129 |
- Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus.
- a Adjusted by sex, age, ART regimen, baseline RNA HIV levels (categorical), and baseline serum creatinine levels.
- b Adjusted by sex, age, ART regimen, baseline CD4+ levels (categorical), baseline serum creatinine levels.
- c Adjusted by sex, age, ART regimen, baseline CD4+ levels (categorical), baseline serum creatinine levels.
- Significance codes: * 0.05; ** 0.01; *** 0.001.
We estimated a second mixed-effects model (N = 114) inserting plasma NfL levels as a dependent variable, and time, ART regime, age (time-varying), and sex as predictors. At all available follow-up time points, the effect of time was negative, indicating a longitudinal decline of plasma NfL levels. The effect was not significant (n.s.) at the first very early follow-up at week 4 (9% decline from baseline, p = 0.19, n.s. due to much shorter time, but still representing a faster decline) but at later time points, a significant decline was found—at week 48 (29% decline from baseline, p < 0.00005), week 96 (17% decline from baseline, p = 0.01), and week 144 (28% decline from baseline, p = 0.0003). As expected, the effect of age was positive (5% annual increase, p < 0.00005), whereas neither ART regimen nor sex had a significant effect.
A third mixed-effects model (N = 114) was estimated, and we inserted as predictors baseline CD4+ levels (categorical) in interaction with time, adjusting by sex, age, ART regimen, baseline RNA HIV levels (categorical), and baseline serum creatinine levels. At baseline, plasma NfL of participants with CD4+ at the intermediate level in the sample (100–199/µl) was not significantly different from that of participants with CD4+ at the highest level in the sample (200–499/µl), whereas we found significantly higher plasma NfL for the lowest CD4+ level in the sample (<100/µl) as compared with the highest level of CD4+ (84% higher for the lowest level of CD4+, p = 0.0008). Change over time was n.s. considering the intermediate level of CD4+ versus the highest level of CD4+, but a trend toward significance was observed considering the lowest level of CD4+ versus the highest level of CD4+ (Fig. 1a) at week 4 (β = 27% decline from baseline, p = 0.08), and significant for the other time points (week 48, 49% decline from baseline, p = 0.01; week 96, 58% decline from baseline, p = 0.0001; week 144, 49% decline from baseline, p = 0.002). The effect of age was significant (5% annual increase, p < 0.00005), but not that of other covariates.
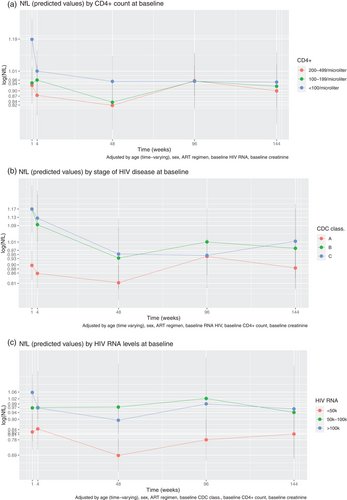
In a fourth mixed-effects model (N = 108), we inserted as predictors the baseline stage of HIV disease (categorical) in interaction with time, adjusting by sex, age, ART regimen, baseline CD4+ levels (categorical), and baseline serum creatinine levels. Plasma NfL levels were higher for baseline CDC stage B than for stage A (52% higher for stage B, p = 0.02); the same was true for stage C as compared with stage A (48% higher for stage C, p = 0.04). The effect of age was also significant (5% annual increase, p < 0.00005). Compared with stage A, we found significant change over time for stage B at week 48 (33% decrease from baseline, p = 0.04), at week 96 (45% decrease from baseline, p = 0.005), and week 144 (49% decrease from baseline, p = 0.005). The same was true for stage C, at week 48 (36% decrease from baseline, p = 0.02), week 96 (51% decrease from baseline, p = 0.0006), and week 144 (42% decrease from baseline, p = 0.02, see Fig. 1b).
Fifth, we estimated the mixed-effects model (N = 114), inserting the baseline HIV RNA levels (categorical) as predictors in interaction with time, and adjusting by sex, age, ART regimen, baseline CD4+ levels (categorical), and baseline serum creatinine levels. Baseline RNA HIV levels did not emerge as significant, either as a main effect or in interaction with time (see Fig. 1c). We also had available follow-up HIV RNA assessments, and we reestimated the model inserting HIV RNA levels as a time-varying effect instead of time-fixed. However, also in this case, no significant change over time was found of plasma NfL values related to HIV RNA levels.
Supplementary analyses
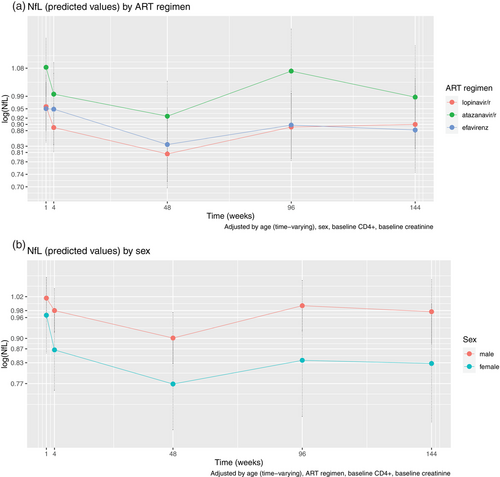
We estimated a mixed-effects model, inserting as predictor ART regimen in interaction with time, and adjusting by sex, age, baseline CD4+, and baseline serum creatinine levels. The effect of ART regimen was n.s. as a main effect, nor in interaction with time (Fig. 2). Finally, we inserted sex in interaction with time, adjusting by ART regimen, baseline CD4+, age, and baseline serum creatinine levels. As expected, sex did not prove significant as a main effect, nor did the rate of decline differ between sexes.
Discussion
In the present study, we addressed an unsolved issue in the literature—namely, the determination of the longitudinal trajectory of plasma NfL in PWH. For this purpose, we measured plasma NfL levels at baseline and at four follow-up assessments on a sample of 116 PWH who initiated ART and were previously naïve to treatment. The highest baseline plasma NfL levels were found in patients with the most advanced disease with CD4+ cell counts (<100/µl) compared with patients with higher baseline CD4+ levels (100–199 or 200–499/µl). This provides an indirect indicator of ongoing neuronal injury for PWH not taking ART and with poor immunologic function, as compared with PWH with higher CD4+ count. This result is in agreement with other findings in the neurological and neuropsychological literature [32, 33]. In patients with baseline CD4+ count <100/µl, we also observed a relatively fast initial decline in plasma NfL from ART initiation to week 4 regardless of treatment regimen, whereas levels subsequently stabilized at week 48. We found a longitudinal decay of plasma NfL values at week 48 for PWH in the CD4+ count strata (100–199 and 200–499/µl). Values at week 96 or 144 seem to be stable or slightly increased as compared to week 48. However, this result should be considered with caution and addressed by future research, in light of the non-negligible loss at follow-up of this study (34.5% at weeks 96 and 48.3% at week 144).
Despite effective ART and viral suppression, many PWH suffer from a systematic inflammatory state [34, 35], including residual CNS immune activation [36-38]. Persistent immune activation may potentially contribute to age-related morbidity and mortality in PWH [39]. Although most PWH on suppressive ART do not suffer from apparent CNS symptoms, the prevalence of subclinical or mild neurocognitive impairment in PWH on ART has been considerable in some previous studies [40, 41]. However, it has remained a challenge to differentiate a possible ongoing CNS injury despite apparent viral suppression from sequelae of previous insults or other causes [42]. NfL has the potential of providing an objective measure of active ongoing neuronal injury. Analysis of CSF NfL has demonstrated that a substantial number of untreated asymptomatic HIV-infected patients, mainly those with low CD4+ cell counts, have biomarker signs of subclinical CNS injury [8]. The recent availability of techniques to quantify NfL in plasma [16] has opened up new possibilities for monitoring ongoing neuronal injury in PWH using a less invasive method. In addition to residual CNS immune activation, some antiretroviral drugs may have neurotoxic effects [43], which could be objectively assessed using a biomarker. Plasma NfL may also be useful for detecting neuronal injury caused by other non-HIV-related events or co-morbidities (e.g., substance use or other CNS diseases, such as Alzheimer's and dementia) [44-46]. Moreover, symptomatic CSF escape, although a rare condition, can potentially lead to neuronal injury and is important to identify [47, 48]. The presence of viral RNA in CSF, even at very low levels, has been associated with increased CSF neopterin concentrations in asymptomatic CSF escape, indicating that loss of strict viral control can result in an immune response [49, 50]. However, the potential impact of this immune activation on CNS injury is less clear [51].
We also found an association of plasma NfL levels with stage of HIV disease, both at baseline and in terms of change over time. At baseline, both patients at CDC stages B and C had higher levels of plasma NfL than those at stage A. In terms of change over time, for those at stages B and C plasma NfL levels declined fast at weeks 4 and 48, but at week 48, levels were still relatively higher than those of participants at stage A. For all stages, we observed a weak increase of estimates at weeks 96 and 144 of uncertain significance, but not as a consequence of increase due to normal ageing, as age was included as an adjuster in the model. It could indicate that ART reduces CNS damage by lowering the CNS inflammation with a magnitude that is related to pretreatment immune status. In later phases, residual immune activation may generate some CNS injury despite ART. Alternatively, we cannot rule out that results could be influenced by loss to follow up and the reduced sample size at weeks 96 and 144. Confirmation of these results, preferably from larger cohorts, is of importance.
No significant differences between levels of plasma NfL or change over time were found in comparing the three ART groups. Although levels of plasma NfL at baseline were slightly higher for males (predicted mean: 1.02 [0.96, 1.08]) than for females (predicted mean: 0.97 [0.86, 1.08]), there was no significant difference at baseline, and the trajectory of decline was comparable across groups—similar to what has been reported previously [8].
It is known that, cross-sectionally, age is correlated with plasma NfL (coefficient about 0.79, [16]). In addition, plasma NfL age-related reference values for HIV controls have been recently published [52]. Such values have been obtained by pooling data from eight cohorts covering the age range 5–90. To our knowledge, data on repeated samples of HIV controls are still not available. This study found that NfL increases with age at a rate of about 5%/year in PWH. We may assume that this reflects the physiologic effect of aging, which is well demonstrated for CSF NfL and has been recently shown cross-sectionally also for plasma NfL.
Limitations of this study include the use of a convenience sample with relatively small size and the availability of some important covariates’ measurement only at baseline. In addition, because of the non-negligible dropout rate during follow-up and the consequent loss of statistical power, the estimation of change over time effects at weeks 96 and 144 should be interpreted with caution. Serum creatinine levels were available only at baseline, prohibiting estimation of the effect of changes in kidney function over time. Of note, plasma NfL concentrations can increase as a consequence of peripheral neuropathies [19]. Although none of the participants had known current or previous peripheral neuropathy, we cannot rule out a potential impact of undiagnosed conditions. Antiretroviral regimens used in the NORTHIV study reflect treatment guidelines at the time of recruitment and are no longer considered first-line regimens. Of note, efavirenz has been associated with neurological and neurocognitive symptoms [53]. All subjects were evaluated by clinical neurological examination; however, formal neuropsychiatric testing was not available for patients of the NORTHIV study.
In conclusion, in this study, we have shown the longitudinal trajectory of plasma NfL levels in a sample of PWH who initiated ART and the relationship with baseline CD4+ levels, HIV RNA levels, and HIV disease stage. We provided evidence that NfL decreases until week 48 and thereafter stabilizes—that is, according to our data, NfL levels would take at least 48 weeks from ART initiation to normalize. Although plasma NfL has the potential to constitute an objective, sensitive, and reliable biomarker to assess CNS damage in PWH, additional studies are needed to establish the validity in a clinical setting.
Funding
This study was supported by the Swedish state under an agreement between the Swedish government and the county councils (ALF agreement ALFGBG-965885). HZ is a Wallenberg Scholar supported by grants from the Swedish Research Council (#2018-02532), the European Research Council (#681712), Swedish State Support for Clinical Research (#ALFGBG-720931), the Alzheimer Drug Discovery Foundation (ADDF), USA (#201809-2016862), the AD Strategic Fund and the Alzheimer's Association (#ADSF-21-831376-C, #ADSF-21-831381-C, and #ADSF-21-831377-C), the Olav Thon Foundation, the Erling-Persson Family Foundation, Stiftelsen för Gamla Tjänarinnor, Hjärnfonden, Sweden (#FO2019-0228), the European Union's Horizon 2020 research and innovation program under the Marie Skłodowska-Curie grant agreement no 860197 (MIRIADE), European Union Joint Program for Neurodegenerative Disorders (JPND2021-00694), and the UK Dementia Research Institute at UCL.
Conflicts of interest
HZ has served at scientific advisory boards and/or as a consultant for Abbvie, Alector, Annexon, Artery Therapeutics, AZTherapies, CogRx, Denali, Eisai, Nervgen, Pinteon Therapeutics, Red Abbey Labs, Passage Bio, Roche, Samumed, Siemens Healthineers, Triplet Therapeutics, and Wave has given lectures in symposia sponsored by Cellectricon, Fujirebio, Alzecure, Biogen, and Roche and is a cofounder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program (outside submitted work). MG has received research grants from Gilead Sciences and Janssen-Cilag and honoraria as speaker and/or scientific advisor from Amgen, Biogen, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline/ViiV, Janssen-Cilag, MSD, Novocure, Novo Nordic, and Sanofi. AS has received research grants from Gilead Sciences and honoraria as speaker and/or scientific advisor from Abbvie, AstraZeneca, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline/ViiV, Janssen-Cilag, MSD, and Immune System Regulation.