Humoral response to two doses of BNT162b2 vaccination in people with HIV
Abstract
Background
People with HIV (PWH) are at increased risk of severe COVID-19. We aimed to determine humoral responses in PWH and controls who received two doses of BNT162b2.
Methods
In 269 PWH and 538 age-matched controls, we measured IgG and neutralizing antibodies specific for the receptor-binding domain of SARS-CoV-2 at baseline, 3 weeks and 2 months after the first dose of BNT162b2.
Results
IgG antibodies increased from baseline to 3 weeks and from 3 weeks to 2 months in both groups, but the concentrations of IgG antibodies were lower in PWH than that in controls at 3 weeks and 2 months (p = 0.025 and <0.001), respectively. The IgG titres in PWH with a humoral response at 2 months were 77.9% (95% confidence interval [62.5%–97.0%], age- and sex-adjusted p = 0.027) of controls.
Conclusions
Reduced IgG antibody response to vaccination with BNT162b2 was found in PWH, and thus increased awareness of breakthrough infections in PWH is needed.
Introduction
People with immunodeficiencies are at increased risk of severe COVID-19 [1, 2]. In untreated people with HIV (PWH), the immune dysfunction is characterized by loss of CD4+ T cells, but initiation of antiretroviral therapy (ART) often leads to immune recovery with normalization of CD4+ T-cell counts and undetectable viral replication [3]. However, immune recovery is not universal, and some PWH remain immunological nonresponders for many years despite ART [4]. Thus, PWH have been prioritized for early vaccination against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in many countries. However, previous studies on vaccine response to other vaccines reported suboptimal immunogenicity in PWH [5], and published evidence regarding efficacy and efficiency of the new mRNA-based vaccines against SARS-CoV-2 in PWH is limited [6, 7]. In the phase 2/3 study of BNT162b2, 196 PWH were enrolled. However, data on PWH were excluded from the published analyses [8, 9]. The present study investigates humoral response after two doses of BNT162b2 SARS-CoV-2 mRNA vaccine in PWH and controls. Our primary outcome was IgG antibody titres and secondary outcome was neutralizing capacity. The relative risks of nonresponse as well as associations with HIV-related risk factors were also assessed as secondary outcomes. We hypothesized that PWH would have an impaired humoral immune response to BNT162b2.
Methods
From December 2020 through April 2021, PWH followed at Copenhagen University Hospital, Rigshospitalet and health care professionals (controls) [10] at Rigshospitalet and Herlev-Gentofte Hospital aged 18 years or older were invited to participate in this prospective observational study. COVID-19 vaccines were offered as part of the Danish vaccination programme. Participation in the study was voluntary and did not interfere with the vaccination strategy. All participants received the SARS-CoV-2 mRNA vaccine BNT162b2.
To compare antibody responses in PWH and controls, participants without evidence of prior SARS-CoV-2 infection (nucleocapside (N-protein) antibody negative) were matched on age (1:2). Only participants with an available blood sample collected 2 months after receiving the first dose of BNT162b2 vaccine were included in this study. A baseline sample was defined as a sample collected either before or up to 13 days after the first dose of BNT162b2 vaccine. A 3-week sample was collected from 2 weeks after the first dose of BNT162b2 vaccine until a maximum of 2 days after the second dose, and a 2-month sample was collected from 1 week after the second dose and up to 12 weeks after the first dose.
In PWH, information on current and nadir CD4+ T-cell count, HIV RNA and current ART was collected from medical records. In all participants, data regarding vaccination status were collected from the Danish Vaccination Register.
IgG antibodies specific for the receptor-binding domain (RBD) of the spike (S) protein were determined using an in-house ELISA [11]. Briefly, purified recombinant RBD was coated onto Nunc Maxisorp 384-well plates (Thermo Fisher Scientific, MA, USA) overnight in phosphate-buffered saline (PBS) (Rigshospitalet, Copenhagen, Denmark). Before adding the buffer-diluted patient serum, the wells were blocked for 1 hour in PBS and Tween 20 (PBS-T; Merck, Darmstadt, Germany). Horseradish peroxidase (HRP) conjugated polyclonal rabbit-anti-human IgG (Agilent Technologies, Santa Clara, CA, USA) was added. Plates were washed in PBS-T four times between each step. Tetramethylbenzidine (TMB) One substrate (Kem-En-Tec, Taastrup, Denmark) was added and the reaction was stopped using H2SO4. A Synergy HT absorbance reader was used to measure the optical density at 450–630 nm (BioTek Instruments, Winooski, VT, USA). IgG levels were given in arbitrary units (AU)/ml. A value of >1 AU/ml was considered detectable, and the threshold of a positive IgG antibody response was 150 AU/ml. Samples with a value below 1 AU/ml was set to 1 AU/ml [10].
SARS-CoV-2 N-protein antibodies were determined by electrochemiluminescence (Anti-SARS-CoV-2 Elecsys assay, Roche Diagnostics, GmbH, Germany) and a COBAS analyser system (Roche Diagnostics) according to the manufacturers’ instructions.
We used an in-house ELISA to estimate inhibition of the ACE-2 host receptor/RBD interaction as a proxy for neutralizing capacity. This pseudo-neutralizing assay correlates well with the gold standard plaque reduction neutralization test (r = 0.9231) [12]. Recombinant ACE-2 ectodomain was coated onto Nunc Maxisorp 96-well plates in PBS overnight. For 1 hour, a solution of patient serum, Pierce High Sensitivity Streptavidin-HRP (Thermo Fisher Scientific) and biotinylated recombinant RBD were incubated in nonbinding 96-well plates. The mixtures of biotinylated RBD/Streptavidin-HRP and patient serum were transferred to the ACE-2 ectodomain-coated wells for 35 minutes. Between each step, the wells were washed three times with PBS-T. Plates were developed as described above.
A positive humoral response was defined as having the combination of SARS-CoV-2 RBD IgG ≥150 AU/ml and ≥25% inhibition in the neutralizing assay after vaccination. The 25% threshold was based on a receiver operating characteristic (ROC) curve analysis to estimate the optimal cutoff between naturally infected convalescent sera and sera from individuals obtained before 2020 [11].
Statistics
Continuous data were reported as medians with interquartile range (IQR), and differences were assessed by Mann–Whitney U test or t-test, as appropriate. Categorical data were reported as frequency counts and percentages, and differences were assessed using chi-square (χ2) test or Fisher's exact test, as appropriate.
Antibody levels were reported as geometric mean concentrations (GMCs) with 95% confidence intervals (CIs). To compare changes in antibody levels from baseline to 3 weeks post vaccination and 3 weeks to 2 months post vaccination, Wilcoxon matched-pairs signed-rank test was used. To compare antibody levels of PWH and controls at 3 weeks and at 2 months, Mann–Whitney U test and log-linear regression were used for unadjusted and adjusted analyses, respectively. Poisson regression with robust standard errors was used to test associations between humoral response to the BNT162b2 vaccine and independent variables. In the multivariable models, we adjusted for sex and age. p-Values <0.05 were considered significant. Statistical analyses were performed using R Version 3.6.0 (R Foundation for Statistical Computing, Vienna, Austria).
Results
We included 269 PWH and 538 age-matched controls. Median [IQR] ages were 56 years [49–64] and 56 years [49–63] in PWH and controls, respectively (p = 0.165). The proportion of men was higher in PWH than in controls (90% vs. 13.6%, p < 0.001). ART was used by 99.6% of PWH, and 96.7% had undetectable viral replication (<50 copies/ml). The median [IQR] CD4 count was 640 cells/mm3 [500–780] with 72.9%, 16.7%, 8.9% and 1.5% of PWH having current CD4 counts >500, 350–500, 200–349 and <200 cells/mm3, respectively. CD4 nadir was <200 cells/mm3 in 32.3% of PWH. Baseline samples were accepted up to 2 weeks after the first vaccination, 21/258 (8.1%) PWH and nine of 408 (2.2%) of controls had a baseline sample collected 1 or more days after administration of their first dose (Table 1).
| PWH | Controls | p-Value | |
|---|---|---|---|
| N | 269 | 538 | N/A |
| Median age, years (IQR) | 56.0 (49–64) | 56 (49–63) | 0.165 |
| Male sex, n (%) | 242 (90.0) | 73 (13.6) | <0.001 |
| BMI, mean (SD) | 25.0 (3.7) | 26.1 (7.2) | 0.009 |
| BMI grouped, n (%): | <0.001 | ||
| ≤18.5 | 5 | 9 | |
| 18.6–29.9 | 225 | 386 | |
| ≥30 | 19 | 90 | |
| N/A | 20 | 53 | |
| Nadir CD4 count (cells/mm3) | |||
|
Median (IQR) Groups: >500 350–500 200–349 <200 N/A |
246 (150–375) 25 44 75 87 38 |
N/A | N/A |
| Current CD4 count (cells/mm3) | |||
|
Median (IQR) Groups: >500 350–500 200–349 <200 |
640 (500–780) 196 (72.9) 45 (16.7) 24 (8.9) 4 (1.5) |
N/A | N/A |
| Current viral load (copies/ml) | |||
|
Median (IQR) <50 ≥50 |
19 (19–19) 260 (96.7) 9 (3.3) |
N/A | N/A |
| Current cART use, n (%) | 268 (99.6%) | N/A | N/A |
| Days between first and second dose, median (IQR) | 23 (22–25) | 30 (29–32) | <0.001 |
| IgG GMC AU [95% CI] | |||
|
–Baseline –Three weeks –Two months |
4.83 [3.67–6.36] 1079.22 [897.85–1299.85] 20,442.13 [18,033.74–23,155.79] |
2.17 [1.82–2.59] 1326.43 [1130.03–1556.20] 35,171.05 [31,571.18–38,948.67] |
<0.001 0.025 <0.001 |
| IgG GMC ≥150 AU/ml, n (%) | |||
|
–Three weeks –Two months |
218 (92%) 269 (100%) |
355 (95%) 536 (99.6%) |
0.162 0.555 |
| Neutralizing Index ≥25%, n (%) | |||
|
–Three weeks –Two months |
142 (60%) 263 (98%) |
256 (69%) 530 (99%) |
0.036 0.568 |
| Humoral response, n (%) | |||
|
–Three weeks –Two months |
142 (60%) 263 (98%) |
255 (68%) 530 (99%) |
0.048 0.568 |
At baseline, the GMCs of SARS-CoV-2 RBD IgG antibodies were 4.8 AU/ml (95% CI [3.7–6.4]) and 2.2 AU/ml (95% CI [1.8–2.6]) in PWH and controls, respectively (p < 0.001, Fig. 1a). The GMC of SARS-CoV-2 RBD IgG antibodies increased significantly from baseline to 3 weeks, and from 3 weeks to 2 months after first dose of BNT162b2 in both PWH and controls (p < 0.001 for both groups at both time points, Fig. 1a). However, GMCs 3 weeks after vaccination were significantly lower in PWH (1079.2 AU/ml, 95% CI [897.8–1299.8]) than in controls (1326.4 AU/ml, 95% CI [1130.0–1556.2], p = 0.025; Fig. 1a). This was also the case after 2 months, where GMCs were 20,442.1 AU/ml (95% CI [18,033.7–23,155.8]) and 35,171.1 AU/ml (95% CI [31,571.2–38,948.7]) for PWH and controls, respectively (p < 0.001).
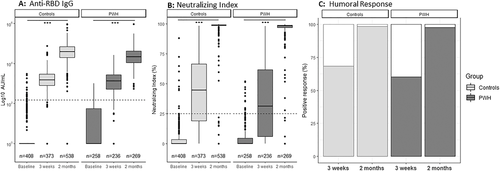
After 3 weeks, the SARS-CoV-2 RBD IgG titres in PWH with a humoral response were 97.0% (95% CI [81.9%–116.2%], p = 0.759) and 118.5% (95% CI [88.7%–158.4%], p = 0.245) of titres in controls in unadjusted analysis and after adjusting for age and sex, respectively. After 2 months, the IgG titres in PWH with a humoral response were 55.4% (95% CI [47.7–65.4], p < 0.001) and 77.9% (95% CI [62.5–97.0], p = 0.027) of titres in controls in unadjusted and adjusted analyses, respectively.
Neutralizing index ≥25% was found in 142/236 (60%) of PWH and 256/373 (69%) of controls after 3 weeks (p = 0.036, Fig. 1b) and in 263/269 (98%) of PWH and 530/538 (99%) of controls after 2 months (p = 0.568, Fig. 1b). A positive humoral response was present in 142/236 (60%) of PWH and 255/373 (68%) of controls after 3 weeks (p = 0.048, Fig. 1c). After 2 months, a positive humoral response was present in 263/269 (98%) of PWH and 530/538 (99%) of controls (p = 0.568, Fig. 1c). The unadjusted risk ratio (RR) of humoral nonresponse in PWH 3 weeks after first vaccination was 1.26 (95% CI [1.01–1.56], p = 0.037) and adjusted RR of humoral nonresponse in PWH was 0.95 (95% CI [0.69–1.30], p = 0.748). After 2 months, the unadjusted RR of humoral nonresponse in PWH was 1.50 (95% CI [0.53–4.28], p = 0.448) and adjusted RR of humoral nonresponse in PWH after 2 months was 1.12 (95% CI [0.18–6.79], p = 0.905). In PWH, CD4 count <350 (p = 0.17 and 0.23) and CD4 nadir <200 (p = 0.20 and 0.54) were not significantly associated with IgG levels in age- and sex-adjusted models after 3 weeks and 2 months, respectively. However, CD4 nadir <200 was significantly associated with humoral response in a sex- and age-adjusted model after 3 weeks, but not after 2 months; RR of humoral response at 3 weeks: 0.62 (95% CI [0.48–0.88], p = 0.005).
Discussion
In this study of humoral response to SARS-CoV-2 vaccination with BNT162b2 in 269 PWH and 538 controls, SARS-CoV-2 RBD IgG antibodies increased significantly after vaccination with BNT162b2 in both PWH and controls. However, IgG antibody concentrations were lower in PWH than in controls after both the first and second dose of BNT162b2, although the neutralizing capacity was not reduced. Nadir CD4 count was associated with impaired humoral response after the first vaccine dose; however, after the second dose, this association was no longer seen in this cohort of well-treated PWH.
Only very few studies have shown data after two doses of mRNA-based SARS-CoV-2 vaccination in PWH [6, 7]. A recently published study showed impaired but adequate immunogenicity after two doses of BNT162b2 in 143 PWH [6]. These results are concordant with our findings. We further expand existing knowledge by the inclusion of age-matched controls. The importance of age matching has been stressed in our recent study showing an almost linear decline in humoral response with age [11]. GMC of IgG was slightly higher in PWH than controls at baseline, and a few individuals had elevated neutralizing index at baseline despite exclusion of individuals with N-protein antibodies. This was most likely attributable to a higher proportion of samples collected in the days after the first vaccination in the group of PWH. However, minor uncertainties in the RBD S-ELISA and neutralizing assays cannot be ruled out.
PWH with low CD4 counts are known to have decreased humoral responses to multiple vaccines [5]. We found a tendency towards decreased IgG titres after vaccination with BNT162b2 in PWH, but, importantly, the neutralizing capacity was not reduced. This is likely because of a well-treated cohort where >96% were virally suppressed and >89% had CD4 counts >350 cells/mm3. Furthermore, although the proportion of PWH with humoral response was lower than in controls in unadjusted analysis after 3 weeks, this was not significant after adjusting for age and sex, and after 2 months this association was no longer observed. PWH were mainly males, whereas controls were mainly females. Females often develop more pronounced antibody responses to vaccines [10, 13], which may, in part, explain these findings.
Limitations to our study include the sex difference between groups and that the analysis of T-cell immunity was not available. Furthermore, this study was not powered to assess vaccine efficacy. Strengths include a large well-defined cohort and analysis of neutralizing capacity.
This study of humoral response to SARS-CoV-2 vaccination in PWH found reduced antibody response to vaccination with BNT162b2 in PWH. Thus, increased attention towards protection after vaccination is warranted in PWH. However, no study has yet been powered to find a robust correlation between SARS-CoV-2 antibody levels and COVID-19 vaccine efficacy, and in this study, we found comparable neutralizing capacity in both groups despite lower antibody concentrations in PWH. The relative risk of nonresponse was not significantly elevated in PWH. However, nadir CD4 count was associated with impaired humoral response after the first vaccine dose, but not after the second dose of BNT162b2 in PWH.
Acknowledgements
The authors would like to cordially thank all patients and health care professionals who participated in the study. They would also like to thank Fie Andreasen, Ann Kristine Thorsteinsson, Lisbeth Andreasen, Annie Mørk, Camilla Xenia Holtermann Jahn, Mads Engelhardt Knudsen and Sif Kaas Nielsen for their expert technical assistance. This work was financially supported by grants from the Carlsberg Foundation (grant number: CF20-476 0045) and the Novo Nordisk Foundation (grant numbers: NFF205A0063505 and NNF20SA0064201). The funding sources were not involved in any part of the study design, data collection, data analysis, interpretation of the data or the writing of this manuscript.
Conflict of interest
The authors declare that there is no conflict of interest.




