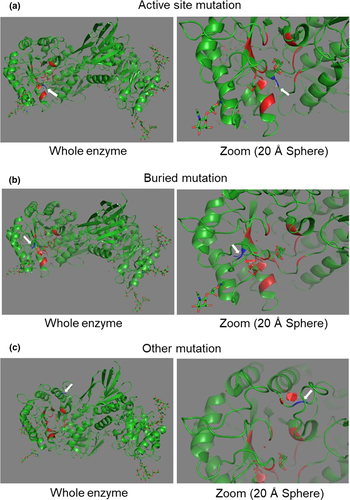
Stratification of Fabry mutations in clinical practice: a closer look at α-galactosidase A-3D structure
Abstract
Background
Fabry disease (FD) is an X-linked lysosomal storage and multi-system disorder due to mutations in the α-galactosidase A (α-GalA) gene. We investigated the impact of individual amino acid exchanges in the α-GalA 3D-structure on the clinical phenotype of FD patients.
Patients and methods
We enrolled 80 adult FD patients with α-GalA missense mutations and stratified them into three groups based on the amino acid exchange location in the α-GalA 3D-structure: patients with active site mutations, buried mutations and other mutations. Patient subgroups were deep phenotyped for clinical and laboratory parameters and FD-specific treatment.
Results
Patients with active site or buried mutations showed a severe phenotype with multi-organ involvement and early disease manifestation. Patients with other mutations had a milder phenotype with less organ impairment and later disease onset. α-GalA activity was lower in patients with active site or buried mutations than in those with other mutations (P < 0.01 in men; P < 0.05 in women) whilst lyso-Gb3 levels were higher (P < 0.01 in men; <0.05 in women).
Conclusions
The type of amino acid exchange location in the α-GalA 3D-structure determines disease severity and temporal course of symptom onset. Patient stratification using this parameter may become a useful tool in the management of FD patients.
Graphical Abstract
Introduction
Fabry disease (FD) is an X-linked congenital disorder due to mutations in the gene encoding the lysosomal enzyme α-galactosidase A (α-GalA). α-GalA malfunction leads to progressive accumulation of sphingolipids, such as globotriaosylceramide (Gb3) and its deacylated form globotriaosylsphingosine (lyso-Gb3) in various tissues [1]. Consecutive cellular dysfunction causes multi-organ impairment [2, 3].
Several hundreds of different mutations leading to FD have been described including nonsense, missense and splice-site mutations, and gene rearrangements such as deletions affecting multiple exons [4, 5]. Currently, FD mutations are stratified according to whether they lead to a 'classical' or 'nonclassical' clinical phenotype. The classical FD phenotype is associated with low to no residual α-GalA activity and high lyso-Gb3 levels resulting in early disease manifestation and higher disease impact with multi-organ involvement. Patients with classical FD usually present with neuropathic pain, nephropathy, cardiomyopathy and gastrointestinal symptoms [6-8]. The nonclassical FD phenotype, also referred to as 'late-onset' FD, is associated with residual but low α-GalA activity and lower lyso-Gb3 levels, usually resulting in single- or oligo-organ involvement starting in adulthood [9-11].
The major drawback of this classification is that stratification into 'classic' and 'nonclassic' is based on long-term clinical assessment of patients carrying the same mutation. Hence, novel mutations cannot be stratified per se, leaving prognosis and need for treatment obscure. Also, this classification ignores the underlying pathophysiology of why some mutations lead to a 'classic' and others to a 'nonclassic' phenotype.
We set out to evaluate an alternative classification system of α-GalA missense mutations based on the exact location of consecutive amino acid exchange within the 3D-structure of the enzyme: active site mutations locally alter the composition of the active site of α-GalA, buried mutations disrupt the α-GalA core and induce protein misfolding with consecutive degradation, and other mutations modify, for example the protein surface with less explicit functional impairment [12, 13]. In the latter two, α-GalA function is deficient although the active site is intact. Except for few reports [14, 15], this stratification system has not been investigated for its clinical suitability.
We analysed a large group of FD patients based on the exact location of the amino acid exchange within the 3D-structure of α-GalA caused by individual mutations and report on an association with FD severity. We advocate the inclusion of this stratification system in the management of FD patients.
Patients and methods
Our study was approved by the Würzburg Medical Faculty Ethics Committee (#135/15), and all participants gave written informed consent before inclusion. Between August 2015 and July 2019, 80 adult patients with genetically confirmed FD, who were seen at the Fabry Center for Interdisciplinary Therapy (FAZIT) at the University Hospital of Würzburg, Germany, were included. The cohort consisted of 32 men (mean age 47 [21–68] years) and 48 women (mean age 53 [20–77] years).
Clinical phenotyping
All patients underwent complete neurological and general medical examination and filled in the Würzburg Fabry Pain Questionnaire (FPQ) [16]. All patients also received nerve conduction studies to exclude polyneuropathy. Small fibre neuropathy was diagnosed according to published criteria [17], and additionally, patients were asked for hypohidrosis or anhidrosis. Further, patients were interviewed for past cerebral stroke and respective medical reports were screened. Cardiomyopathy was assessed by signs of left ventricular hypertrophy in echocardiography and late gadolinium enhancement (LGE) in magnetic resonance imaging (MRI) [18, 19]. To distinguish between mild and severe cardiomyopathy, we calculated a score based on the degree of left ventricular hypertrophy and LGE. Left ventricular hypertrophy was categorized as none–mild–severe (i.e. 0–1–3 points) based on septal diameter and weight. Mild (severe) hypertrophy was defined as septal wall thickness >12 (>14) mm and/or left ventricular mass index >95 (>120) g m−2 in women and >115 (>135 g m−2) in men. LGE was categorized as none–mild–severe (i.e. 0–1–3 points), based on the amount and localization of LGE (mild: any intramural LGE; severe: transmural or >2 segments with LGE). Carrying a cardiac device (e.g. pacemaker or implantable cardioverter–defibrillator) was scored 3 points. Mild cardiomyopathy was defined at a sum score of ≤3, severe cardiomyopathy at a sum score of ≥4. Nephropathy was diagnosed by estimating glomerular filtration rate and measuring urine albumin to creatinine ratio in morning spot urine. Albuminuria was categorized distinguishing between incipient nephropathy (>30 mg g−1 category A2) and overt nephropathy (>300 mg g−1, category A3) according to Kidney Disease: Improving Global Outcomes, KDIGO) [20].
Laboratory phenotyping
α-GalA activity was determined in leucocytes (reference: 0.4–1.0 nmol min−1 mg−1 protein; Podskarbi Laboratory, Munich, Germany). Lyso-Gb3 was measured in plasma samples (reference: <0.9 ng mL−1; Centogene, Rostock, Germany) using liquid chromatography and mass spectrometry.
Localization of mutations in the α-GalA gene
The 3D-structure of α-GalA was downloaded from the Protein Data Bank (PDB) in Europe [21] into PyMOL 1.8 graphics system [22]. Each mutation was individually entered, and the consecutive amino acid exchange was evaluated in the 3D-structure of the enzyme. Mutations were stratified into three categories based on the location of the amino acid exchange as previously described [12, 13]: (i) mutations altering the active site of α-GalA (‘active’); (ii) mutations disrupting the α-GalA core, leading to enzyme misfolding (‘buried’); and (iii) mutations altering the protein surface (‘other’), distant from the active site.
Statistical analysis
SPSS 25 (for statistics; IBM, Ehningen, Germany) was used. Data distribution was determined with a Kolmogorov–Smirnov test. Since data were non-normally-distributed, we performed the nonparametric Mann–Whitney U-test to compare different groups. Significance was assumed at P < 0.05.
Results
Classification of individual mutations
All 80 patients carried a missense mutation. A total of 17/32 men (53%) and 27/48 (56%) women carried a mutation leading to a classical FD phenotype. A total of 15/32 (47%) men and 21/48 (44%) women carried a mutation predictably leading to a nonclassical phenotype [23].
Active site mutations are rare in FD patients
Examples of the three mutation types are shown in Fig. 1. As demonstrated in Table 1, 4/80 (5%) patients (1/32, 3% men; 3/48, 6% woman) carried an active site mutation, 35/80 (44%) patients (13/32, 41% men; 22/48, 46% women) carried a buried mutation, and 41/80 (51%) patients (18/32, 56% men; 23/48, 48% women) carried other mutations. As seen in Table 2, the group of patients with an active site mutation carried the same mutation, and the group of patients with buried mutations showed 16 different mutations. The group of patients with other mutations consisted of eight different mutations. Since patients with active site and buried mutations presented with similar clinical phenotypes which was very different from those patients carrying other mutations, the two groups 'active site' and 'buried' were combined for analysis.
| N | M | F |
|---|---|---|
| Total cohort | 32 | 48 |
| Active site mutations | 1 (3%) | 3 (6%) |
| Buried mutations | 13 (41%) | 22 (46%) |
| Other mutations | 18 (56%) | 23 (48%) |
- F, female; M, male; N, number.
| Location of amino acid exchange in α-GalA-3D structure | Mutation | Number of patients |
|---|---|---|
| Active site | c.515G>A (p.Cys172Tyr) C172Y | n = 4 (m = 1; f = 3) |
| Buried | c.202C>T(p.Leu68Phe) L68F | n = 1 (m) |
| c.334C>T (p.Arg112Cys) R112C | n = 1 (f) | |
| c.386T>C (p.Leu129Pro) L129P | n = 4 (m = 2; f = 2) | |
| c.404C>T (p.Ala135Val) A135V | n = 7 (m = 2; f = 5) | |
| c.408T>A (p.Asp136Glu) D136E | n = 3 (m = 1; f = 2) | |
| c.471T>G (p.Gln157His) Q157H | n = 1 (f) | |
| c.484T>G (p.Trp162Gly) W162G | n = 2 (m = 1; f = 1) | |
| c.486G>T (p.Trp162Cys) W162C | n = 1 (m) | |
| c.559A>G (p.Met187Val) M187V | n = 1 (f) | |
| c.708G>C (p.Trp236Cys) W236C | n = 3 (m = 1; f = 2) | |
| c.784T>C (p.Trp262Arg) W262R | n = 1 (m) | |
| c.806T>G (p.Val269Gly) V269G | n = 1 (f) | |
| c.860G>C (p.Trp287Ser) W287S | n = 1 (f) | |
| c.1021G>A (p.Glu341Lys) E341K | n = 1 (m) | |
| c.1025G>T (p.Arg342Leu) R342L | n = 5 (m = 2; f = 3) | |
| c.1250T>G (p.Leu417Arg) L417P | n = 2 (f) | |
| Other | c.155G>C (p.Cys52Ser) C52S | n = 1 (f) |
| c.188G>A (p.Cys63Tyr) C63Y | n = 2 (m = 1; f = 1) | |
| c.416A>G (p.Asn139Ser) N139S | n = 5 (m = 3; f = 2) | |
| c.427G>A (p.Ala143Thr) A143T | n = 4 (f) | |
| c.644A>G (p.Asn215Ser) N215S | n = 23 (m = 13; f = 10) | |
| c.902G>A (p.Arg301Gln) R301Q | n = 3 (f) | |
| c.963G>C (p.Gln321His) Q321H | n = 2 (m = 1; f = 1) | |
| c.973G>A (p.Gly325Ser) G325S | n = 1 (f) |
- α-GalA, α-galactosidase A; f, female; m, male; n, number.
A classical FD phenotype is mainly caused by active site and buried mutations
In the group of patients with active site or buried mutations, 16/17 (94%) were predicted to lead to a classical FD phenotype, whilst only 1/17 (6%) to lead to a nonclassical FD phenotype. Within the group of patients with other mutations, only 3/8 (37%) mutations were predicted to cause a classical FD phenotype, and the remaining 5/8 (63%) mutations to a nonclassical phenotype were an apathogenic FD genotype [23].
Clinical phenotype depends on the type of mutation
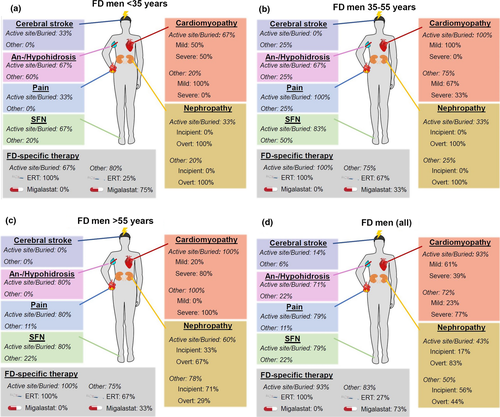
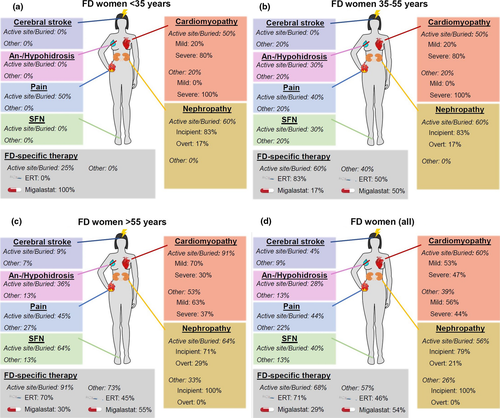
Figures 2 and 3 and Tables 3 and 4 give a synopsis of male and female patient characteristics for the different mutation types, grouped by sex and age: male and female patients ≤ 35 years (Figs 2a and 3a), 35–55 years (Figs 2b and 3b), and >55 years (Figs 2c and 3c). Figures 2d and 3d depict patient characteristics of the whole study cohort.
| Active site/buried mutations | Other mutations | |||||||
|---|---|---|---|---|---|---|---|---|
| All | <35 year | 35–55 year | >55 year | All | <35 year | 35–55 year | >55y | |
| N of patients | 14 | 3 | 6 | 5 | 18 | 5 | 4 | 9 |
| Cardiomyopathy | 13/14 (93%) | 2/3 (67%) | 6/6 (100%) | 5/5 (100%) | 13/18 (72%) | 1/5 (20%) | 3/4 (75%) | 9/9 (100%) |
| Mild | 8/13 (61%) | 1/2 (50%) | 6/6 (100%) | 1/5 (20%) | 3/13 (23%) | 1/1 (100%) | 2/3 (67%) | 0/9 (0%) |
| Severe | 5/13 (39%) | 1/2 (50%) | 0/6 (0%) | 4/5 (80%) | 10/13 77%) | 0/1 (0%) | 1/3 (33%) | 9/9 (100%) |
| Nephropathy | 6/14 (43%) | 1/3 (33%) | 2/6 (33%) | 3/5 (60%) | 9/18 (50%) | 1/5 (20%) | 1/4 (25%) | 7/9 (78%) |
| Inicipient | 1/6 (17%) | 0/1 (0%) | 0/2 (0%) | 1/3 (33%) | 5/9 (56%) | 0/1 (0%) | 0/1 (0%) | 5/7 (71%) |
| Overt | 5/6 (83%) | 1/1 (100%) | 2/2 (100%) | 2/3 (67%) | 4/9 (44%) | 1/1 (100%) | 1/1 (100%) | 2/7 29%) |
| FD-associated pain | 11/14 (79%) | 1/3 (33%) | 6/6 (100%) | 4/5 (80%) | 2/18 (11%) | 0/5 (0%) | 1/4 (25%) | 1/9 (11%) |
| Av. pain intensity (range) [NRS] | 4 (2–6) | 3 | 4 (3–8) | 4 (3–5) | 3 (2–4) | 2 | 4 | |
| Max. pain intensity (range) [NRS] | 7 (3–10) | 7 | 8 (3–8) | 8 (7–10) | 6 (5–7) | 5 | 7 | |
| SFN | 11/14 (79%) | 2/3 (67%) | 5/6 (83%) | 4/5 (80%) | 4/18 (22%) | 1/5 (20%) | 2/4 (50%) | 2/9 (22%) |
| Anhidrosis/hypohidrosis | 10/14 (71%) | 2/3 (67%) | 4/6 (67%) | 4/5 (80%) | 4/18 (22%) | 3/5 (60%) | 1/4 (25%) | 0/9 (0%) |
| Cerebral stroke | 2/14 (14%) | 1/3 (33%) | 0/6 (0%) | 1/5 (20%) | 1/18 (6%) | 0/5 (0%) | 1/4 (25% | 0/9 (0%) |
| FD-specific therapy | 13/14 (93%) | 2/3 (67%) | 6/6 (100%) | 5/5 (100%) | 15/18 (83%) | 4/5 (80%) | 3/4 (75%) | 8/9 (89%) |
| ERT | 13/13 (100%) | 2/2 (100%) | 6/6 (100%) | 5/5 (100%) | 4/15 (27%) | 1/4 (25%) | 2/3 (67%) | 1/8 (12%) |
| Migalastat | 0/13 (0%) | 0/2 (0%) | 0/6 (0%) | 0/5 (0%) | 11/15 (73%) | 3/4 (75%) | 1/3 (33%) | 7/8 (88%) |
- av, average; ERT, enzyme replacement therapy; FD, Fabry disease; max., maximum; N, number; SFN, small fibre neuropathy; y, years.
| Active site/buried mutations | Other mutations | |||||||
|---|---|---|---|---|---|---|---|---|
| All | <35 year | 35–55 year | >55 year | All | <35 year | 35–55 year | >55 year | |
| N of patients | 25 | 4 | 10 | 11 | 23 | 3 | 5 | 15 |
| Cardiomyopathy | 15/25 (60%) | 0/4 (0%) | 5/10 (50%) | 10/11 (91%) | 9/23 (39%) | 0/3 (0%) | 1/5 (20%) | 8/15 (53%) |
| Mild | 8/15 (53%) | 1/5 (20%) | 7/10 (70%) | 5/9 (56%) | 0/1 (0%) | 5/8 (63%) | ||
| Severe | 7/15 (47%) | 4/5 (80%) | 3/10 (30%) | 4/9 (44%) | 1/1 (100%) | 3/8 (37%) | ||
| Nephropathy | 14/25 (56%) | 1/4 (25%) | 6/10 (60%) | 7/11 (64%) | 6/23 (26%) | 1/3 (33%) | 0/5 | 5/15 (33%) |
| Inicipient | 11/14 (79%) | 0/1 (0%) | 5/6 (83%) | 5/7 (71%) | 6/6 (100%) | 1/1 (100%) | 5/5 (100%) | |
| Overt | 3/14 (21%) | 1/1 (100%) | 1/6 (17%) | 2/7 (29%) | 0/6 (0%) | 0/1 (0%) | 0/5 (0%) | |
| FD-associated pain | 11/25 (44%) | 2/4 (50%) | 4/10 (40%) | 5/11 (45%) | 5/23 (22%) | 0/5 (0%) | 1/5 (20%) | 4/15 (27%) |
| Av. pain intensity (range) [NRS] | 5 (2–8) | 4 (3–5) | 5 (2–7) | 5 (2–8) | 4 (3–5) | 3 | 5 (4–6) | |
| Max. pain intensity (range) [NRS] | 8 (4–10) | 8 (7–9) | 8 (4–10) | 7 (5–10) | 7 (4–8) | 8 | 6 (5–7) | |
| SFN | 10/25 (40%) | 0/4 (0%) | 3/10 (30%) | 7/11 (64%) | 3/23 (13%) | 0/3 (0%) | 1/5 (20%) | 2/15 (13%) |
| Anhidrosis/hypohidrosis | 7/25 (28%) | 0/4 (0%) | 3/10 (30%) | 4/11 (36%) | 3/23 (13%) | 0/3 (0%) | 1/5 (20%) | 2/15 (13%) |
| Cerebral stroke | 1/25 (4%) | 0/4 (0%) | 0/10 (0%) | 1/11 (9%) | 2/23 (9%) | 0/3 (0%) | 1/5 (20%) | 1/15 (7%) |
| FD-specific therapy | 17/25 (68%) | 1/4 (25%) | 6/10 (60%) | 10/11 (91%) | 13/23 (57%) | 0/3 (0%) | 2/5 (40%) | 11/15 (73%) |
| ERT | 12/17 (71%) | 0/1 (0%) | 5/6 (83%) | 7/10 (70%) | 6/13 (46%) | 1/2 (50%) | 5/11 (45%) | |
| Migalastat | 5/17 (29%) | 1/1 (100%) | 1/6 (17%) | 3/10 (30%) | 7/13 (54%) | 1/2 (50%) | 6/11 (55%) | |
- av, average; ERT, enzyme replacement therapy; FD, Fabry disease; max., maximum; N, number; SFN, small fibre neuropathy; y, years.
Cardiomyopathy
Amongst young male FD patients, cardiomyopathy was observed in more patients with active site/buried mutations than in those with other mutations. In patients with higher age, severe cardiomyopathy was present in almost all cases in both groups. FD women developed cardiomyopathy less frequently and later compared to men. Almost all women >55 years carrying active site/buried mutations were diagnosed with cardiomyopathy, whilst only half of those carrying other mutations developed cardiomyopathy.
Nephropathy
Amongst male FD patients, nephropathy developed at young age in patients with active site/buried mutations and other mutations. Especially in patients >55 years, more patients carrying active site/buried mutations than those with other mutations were diagnosed with overt nephropathy. In FD women, patients with active site/buried mutations were affected to a higher percentage and more severely than those with other mutations. Overt nephropathy was observed in no woman carrying an other mutation.
FD-associated pain
FD-associated pain was mainly present in patients with active site/buried mutations, whilst those carrying other mutations reported FD-associated pain only in few cases. Also, FD-associated pain was more severe in patients with active site/buried mutations than in those with other mutations.
SFN
In both, men and women, SFN was often found in patients with active site/buried mutations, but only in few patients carrying other mutations. FD women developed SFN later than FD men.
Anhidrosis/hypohidrosis
Anhidrosis/hypohidrosis was mainly reported by male patients with active site/buried mutations, and by only few patients carrying other mutations. FD women described anhidrosis/hypohidrosis later and in fewer cases than FD men. More women with active site/buried mutation reported anhidrosis/hypohidrosis when compared to those carrying other mutations.
Central nervous system
Only few patients showed residual signs of central nervous system impairment in terms of hemi-hyperreflexia, hemi-dysesthesia or impaired speech in the neurological examination after documented cerebral stroke. In other individual cases (n = 3), transient central neurological symptoms (e.g. short-lasting hemiplegia or speech impairment) had been interpreted as transient ischaemic attacks.
FD-specific therapy
Patients carrying active site/buried mutations mainly received enzyme replacement therapy (ERT), especially men. Patients with other mutations often took the oral chaperone migalastat. Untreated patients did not receive FD-specific therapy due to side effects, therapy refusal or low disease severity such that no treatment was necessary.
Enzyme activity is lowest in patients with active site or buried mutations
Figure 4a and c shows median α-GalA activity in FD patients. Amongst FD men (Fig. 4a), median enzyme activity was higher in patients with other mutations when compared to patients with active site or buried mutations (P < 0.01).
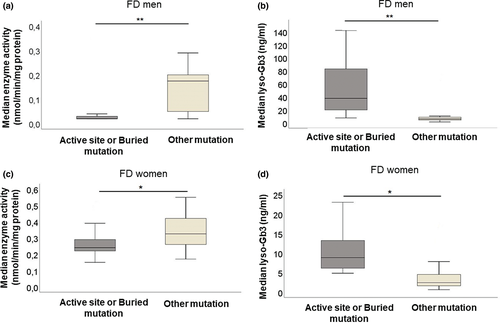
In FD women (Fig. 4c), median α-GalA activity was higher in patients with other mutations when compared to those with active site or buried mutations (P < 0.05).
Lyso-Gb3 is highest amongst patients with active site or buried mutations
Figure 4b and d shows median lyso-Gb3 levels in FD patients. In FD men (Fig. 4b), median lyso-Gb3 was lower in patients with other mutations when compared to patients with active site or buried mutations (P < 0.01). Amongst FD women (Fig. 4d), patients with other mutations showed lower lyso-Gb3 than those with active site or buried mutations (P < 0.05).
Discussion
We investigated a large group of adult FD patients carrying missense mutations and correlated the individual amino acid exchange within the 3D-structure of α-GalA with disease burden. We report on a severe clinical phenotype in patients with active site/buried mutations, which is paralleled by multi-organ manifestations, frequently reported and severe FD-associated pain, and low α-GalA activity as well as high lyso-Gb3 levels. Patients with other mutations were less likely to develop multi-organ manifestations, reported pain less frequently and had higher α-GalA activity as well as lower lyso-Gb3 levels. Organ involvement occurred earlier, and disease severity was higher in patients with active site/buried mutations compared to those carrying other mutations with men being affected even earlier than women. Whilst patients with active site/buried mutation were characterized by multi-organ disease manifestation including severe neurological symptoms, organ involvement of those patients with other mutations was limited mainly to inner organs, especially the heart. Our findings support the notion that the location of the amino acid exchange in the α-GalA 3D-structure defines disease severity. Hence, stratification of FD missense mutations hereupon may serve as an alternative classification system and a reliable determinant of patients’ need for treatment and prognosis.
Keeping to the stratification system of 'classic' and 'nonclassic' FD mutations, new variants in the α-GalA gene cannot be categorized until a clinical phenotype is described. This assessment can be troublesome and may even be conflicting. For instance, variant c.352C>T (R118C) was categorized 'apathogenic' [24] and 'possibly disease triggering' [25, 26]. The same applies to variant c.937G>T (D313Y) [27-29]. Since classification into 'classical' and 'nonclassical' mutations relies on long-term clinical observation and laboratory findings only, new individual variants cannot be categorized immediately upon detection. However, determining the localization of amino acid exchange may serve as a stratification method that can be applied at an earlier time-point, predicting the potential impact of the mutation on enzyme function.
In FD patients, lyso-Gb3 levels are higher than in the healthy population [30]. Lyso-Gb3 is involved in cardiac and renal dysfunction [31, 32], for example by promoting the release of pro-inflammatory cytokines [1]. Hence, lyso-Gb3 may serve as a biomarker to distinguish FD phenotypes from subjects without FD [33, 34], monitor disease progression [30], and may be used as a factor indicating prognosis and disease burden [35, 36]. Lyso-Gb3 was found higher in men with FD when compared to women and in patients with classical FD phenotype than in those with nonclassical phenotype [37]. We detected higher lyso-Gb3 levels in patients with active site and buried mutations, supporting the concept of a more severe phenotype in these patients.
It is of note that the c.644A>G (N215S) mutation (Table 2), which belongs to the group of other mutations [21], is known to be a severe and primarily cardiac variant [29, 38]. Interestingly, also those patients carrying one of the remaining other seven mutations in the group of other mutations presented with mono- to oligo-organ manifestations, in most cases sparing the nervous system. Hence, other mutations seem to induce impairment of fewer organs with later disease onset than the group of active site/buried mutations. However, as exemplified for the N215S genotype, some may lead to a fatal phenotype and disease burden of a single organ may become severe and even life-threatening with age and particularly in men [39]. Women carrying other mutations are less affected, also with regard to inner organ involvement. Hence, identifying the individual FD mutation remains crucial.
As for clinical practice, we believe that classification of FD mutations based on the individual amino acid exchange within the 3D-structure of α-GalA is well feasible. The list of published FD mutations characterized in this particular way is growing, which enables the assessment of more FD patients and facilitates clinical usage particularly at Fabry centres. Also, application in clinical trials would be of great impact adding a crucial stratification parameter which may help better distinguishing the effects of novel FD-modulating compounds.
One limitation of our study is that the method cannot be used for mutations other than missense mutations, such as nonsense mutations or deletions and insertions, since the latter prevent the formation of the complete enzyme, hindering the localization of the impaired residue. Further, we did not analyse patients with active site mutations separately due to their relatively low number, which we ascribe to the fact that only 15 of 397 residues form the active site of α-GalA [12, 13].
The major strength of our study is the protein-based classification of FD mutations linking the underlying pathophysiology with clinical and laboratory parameters of FD patients. We extend the existing knowledge on the impact of alterations in α-GalA 3D-structure by analysis of objective disease manifestations in FD patients, connecting protein pathology with clinical practice. Our findings may lay the basis for studies on the pathophysiological role of alterations in α-GalA 3D-structure, or future clinical trials, to underscore this classification system as a reliable determinant of disease severity and prognosis of FD patients such that it may serve as an additional stratification and evaluation tool in clinical practice.
Acknowledgements
Expert technical help by Barbara Broll (Department of Neurology, University of Würzburg, Germany), and Irina Schumacher (Würzburg Fabry Center for Interdisciplinary Therapy, FAZIT, University of Würzburg, Germany) is gratefully acknowledged.
Conflicts of interest
The authors declare the following conflicts of interest: V.R., L.W. and S.R. report no conflicts of interest. C.S. has received honoraria for lectures from Sanofi Genzyme and Takeda. P.N. has received honoraria for lectures and advisory board participation from Amicus, Greenovation, Idorsia, Sanofi Genzyme and Takeda. C.W. has received honoraria for advisory board participation and lectures from Idorsia, Sanofi Genzyme and Takeda. N.Ü. has received travel grants and honoraria for lectures from Sanofi Genzyme and Shire Corp., and research funds from Sanofi Genzyme, Takeda and Idorsia.
Author Contribution
Vanessa Rickert: Conceptualization (equal); Data curation (equal); Formal analysis (equal); Investigation (equal); Methodology (equal); Writing-original draft (equal). Laura Wagenhäuser: Data curation (equal); Formal analysis (equal); Writing-review & editing (equal). Peter Nordbeck: Data curation (equal); Writing-review & editing (equal). Christoph Wanner: Data curation (equal); Writing-review & editing (equal). Claudia Sommer: Data curation (equal); Writing-review & editing (equal). Simone Rost: Data curation (equal); Formal analysis (equal); Methodology (equal); Software (equal); Supervision (equal); Writing-review & editing (equal). Nurcan Üçeyler: Conceptualization (equal); Data curation (equal); Formal analysis (equal); Methodology (equal); Supervision (equal); Writing-original draft (equal).
Funding
Our study was supported by the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG: UE171-6/1). N.Ü. was supported by DFG: UE171-5/1.