Familial chylomicronemia syndrome: an under-recognized cause of severe hypertriglyceridaemia
Abstract
Familial chylomicronemia syndrome (FCS) is a rare autosomal recessive disorder of chylomicron metabolism causing severe elevation of triglyceride (TG) levels (>10 mmol L−1). This condition is associated with a significant risk of recurrent acute pancreatitis (AP). AP caused by hypertriglyceridaemia (HTG) has been associated with a worse prognosis and higher mortality rates compared to pancreatitis of other aetiology. Despite its association with poor quality of life and increased lifelong risk of HTG-AP, few healthcare providers are familiar with FCS. Because this condition is under-recognized, the majority of FCS patients are diagnosed after age 20 often after consulting several physicians. Although other forms of severe HTG such as multifactorial chylomicronemia have been associated with high atherosclerotic cardiovascular disease (ASCVD) risk and metabolic abnormalities, ASCVD and metabolic syndrome are not usually observed in FCS patients. Because FCS is a genetic condition, the optimal diagnosis strategy remains genetic testing. The presence of bi-allelic pathogenic mutations in LPL, APOC2, GPIHBP1, APOA5 or LMF1 genes confirms the diagnosis. However, some cases of FCS caused by autoantibodies against LPL or GPIHBP1 proteins have also been reported. Furthermore, a clinical score for the diagnosis of FCS has been proposed but needs further validation. Available treatment options to lower triglycerides such as fibrates or omega-3 fatty acids are not efficacious in FCS patients. Currently, the cornerstone of treatment remains a lifelong very low-fat diet, which prevents the formation of chylomicrons. Finally, inhibitors of apo C-III and ANGPTL3 are in development and may eventually constitute additional treatment options for FCS patients.
Graphical Abstract
Introduction
Familial chylomicronemia syndrome (FCS) (OMIM # 238600) is also known as type I hyperlipoproteinemia or lipoprotein lipase (LPL) deficiency (LPLD). FCS (which here we use interchangeably with monogenic chylomicronemia) is an autosomal recessive disease of chylomicron metabolism associated with severe elevation in circulating triglyceride (TG) concentration as well as a higher incidence of pancreatitis, recurrent pancreatitis and mortality associated with acute pancreatitis. Because of impairment in the clearance of postprandial lipids, these patients respond poorly to standard triglyceride-lowering medications including fibrates and require a very severe diet focusing on restricted fat intake. Unfortunately, this disease is often unknown by many healthcare providers leading to delays in diagnosis and inappropriate treatment 1. The objectives of this review are to increase awareness of this disease and to provide an up-to-date overview of diagnosis and treatment of FCS.
Evidence used in this review
We identified all relevant articles in PubMed using search terms such as "familial chylomicronemia syndrome", "familial hyperchylomicronemia", "lipoprotein lipase deficiency", "type I hyperlipoproteinemia", "type I hyperlipidemia" and related terms not directly referring to FCS including "severe hypertriglyceridaemia" and "HTG-induced acute pancreatitis". We also reviewed the reference lists of each selected publication. We reviewed all the available literature, but focused mainly on recent publications (i.e. emphasis on the 5 last years), reviews or highly cited articles (i.e. articles published in the highest impact factor journals). We excluded articles that were not written in English and also abstracts.
What is the clinical presentation of FCS?
Monogenic chylomicronemia or FCS is an autosomal recessive disorder whose clinical signs and symptoms frequently manifest early in life. However, because this disease is unfamiliar to most healthcare providers, diagnosis is often delayed, occurring later in life and often in adulthood. Indeed, according to the baseline data of the international APPROACH study, the median age at diagnosis was 24 years, with more than half of them diagnosed after the age 20 2. Furthermore, individuals with FCS were found to have consulted on average with five physicians before the diagnosis was made 3. FCS is characterized by persistent chylomicronemia even after fasting up to 14 h, leading to extremely high fasting TG levels (>10 mmol L−1). Because chylomicron clearance is severely impaired in FCS, the severity of the symptoms is primarily related to the fat content of the diet.
The presence of chylomicrons in a fasting plasma sample is easily observable. Immediately after blood sampling and centrifugation, lactescent (milky) plasma will be observed when TG ≥ 10 mmol L−1, indicating the presence of chylomicrons and/or other large TG-rich lipoproteins 4. However, after overnight storage at 4 °C, the presence of chylomicrons can be confirmed, as only this lipoprotein is capable of forming a creamy supernatant layer on the top of the tube (Fig. 1). If the sample does not contain very low-density lipoprotein (VLDL), which is typical in FCS, the lower layer of the plasma will be clear.

The manifestations associated with FCS are quite heterogeneous and nonspecific. The classical and most frequent findings include eruptive xanthomas occurring on the trunk, buttocks and upper limbs, lipaemia retinalis, hepatosplenomegaly or splenomegaly, nausea, vomiting, failure to thrive, transient confusion or ‘brain fog’ and recurrent abdominal pain. FCS patients have a high risk of acute pancreatitis episodes, which are potentially life-threatening and can be recurrent if TG is not optimally controlled. Relapsing acute pancreatitis episodes can lead to chronic pancreatitis, as well as both exocrine and endocrine pancreatic insufficiency 5. The prevalence of FCS-related manifestations or complications from cohorts is listed in Table 1.
| Manifestations or complications of FCS | Prevalence | Reference |
|---|---|---|
| Eruptive xanthomas | 17–23% | 2, 6, 7 |
| Lipaemia retinalis | 4–36% | 2, 6, 7 |
| Hepatosplenomegaly or splenomegaly | 12–25% | 2, 6, 7 |
| Abdominal pain | 26–63% | 2, 3, 6 |
| Pancreatitis | 60–88% | 2, 6, 7 |
| Multiple pancreatitis | 17–48% | 2, 6 |
- FCS, familial chylomicronemia syndrome.
Although the precise mechanism by which chylomicronemia or hypertriglyceridemia (HTG) leads to acute pancreatitis is unknown, some hypotheses have been proposed 8-10. Indeed, the proposed mechanisms include excessive formation of oxidized radicals from free fatty acids aberrantly catabolized by pancreatic lipase or inflammation elicited by mitochondrial overload, as well as the hyperviscosity of blood from excessive circulating chylomicrons leading to local ischaemia and acidosis within the pancreas. A detailed review of risk, presentation or management of HTG-induced acute pancreatitis is beyond the scope of the present review, but other recent reviews addressed this subject 8-11. In brief, after gallstones and alcohol consumption, the third most frequent cause of pancreatitis in some surveys is severe hypertriglyceridaemia, which accounts for 2–10% of acute pancreatitis episodes 12-14. The threshold to claim that acute pancreatitis is caused by hypertriglyceridaemia has been set at TG> 1000 mg dL−1 (>11.2 mmol L−1) 8, which corresponds to definitions of severe (1000–1999 mg dL−1) and very severe (≥2000 mg dL−1) hypertriglyceridaemia 15. For the majority of FCS patients, TG values at presentation exceeded 20 mmol L−1 1. However, in a prospective cohort study from Copenhagen of> 116 000 individuals, even nonfasting mild-to-moderate hypertriglyceridaemia (defined as 2–9.9 mmol L−1) was associated with significantly increased risk of acute pancreatitis 16. It is worth mentioning that pancreatitis by itself can also be associated with reactive hypertriglyceridaemia. Therefore, some hypertriglyceridaemia during pancreatitis would be a consequence of the pancreatitis rather than a cause. Acute pancreatitis can be fatal with an overall mortality rate of ~ 6% 17, but can reach as high as 10–30% if severe complications are present 18. Although there is no correlation between the absolute maximal TG value and more severe pancreatitis episodes 19, a recent systematic review and meta-analysis of observational studies found that HTG-induced acute pancreatitis is associated with a worse prognosis compared to pancreatitis of other aetiology 20. This include higher occurrence of renal failure, respiratory failure, shock, systemic inflammatory response syndrome and higher Acute Physiology and Chronic Health Evaluation (APACHE-II) scores 20. Higher mortality rates are also reported for acute pancreatitis secondary to severe HTG 20, 21, suggesting that HTG aggravates pancreatitis episodes. On the other hand, atherosclerotic cardiovascular disease (ASCVD) is considered to be a rare complication of FCS. Whilst occasional instances of ASCVD have been reported in FCS patients 22, 23, these are considered exceptions that prove the rule. In contrast, other forms of severe HTG, such as multifactorial chylomicronemia or dysbetalipoproteinemia, appear to be associated with ASCVD 4, 6, 24.
Elevated circulating triglyceride levels, which indicate the presence of triglyceride-rich lipoprotein particles, are a strong and independent predictor of ASCVD 25. However, the association between triglycerides and atherosclerosis is not caused by the TG content itself, but is rather related to the presence of remnant lipoproteins, which are smaller than chylomicrons and VLDL and can therefore enter in the arterial intima 26, 27. In FCS patients, the LPL-mediated lipolysis that generates remnant lipoproteins is defective, leading to an accumulation of circulating chylomicrons rather than smaller atherogenic chylomicron remnants. Finally, the presence of severe hypertriglyceridaaemia seen in FCS patients is not an indicator of the presence of cardiometabolic risk factors (such as metabolic syndrome, poor lifestyle or type 2 diabetes) but rather results from an inherited monogenic condition.
Previous studies aimed at assessing the burden of FCS have focussed on both symptoms and complications. More recent studies have also investigated the impact of FCS on the quality of life as well as other psychosocial or cognitive aspects 3, 28-32. Importantly, FCS patients experience constant fear and anxiety related to the ongoing risk of potentially fatal acute pancreatitis and difficulty adhering to ultra-strict low-fat diet necessary to prevent these episodes. Patients often feel powerless, and the majority reported experiencing abdominal pain even when adhering to a low-fat diet 29, 30. The anxiety related to the diet is even worse with eating out. Eating disorders are present in 23% of FCS patients 29.
The IN-FOCUS study, a web-based research survey of 166 FCS patients in 10 countries, also described decreased quality of life. Most patients reported that their disease influenced their career choice and employment status 3, 29; 92% of patients reported impaired ability to fulfil responsibilities at school or work 3. Because the average length of hospitalization for HTG-associated acute pancreatitis is 6.5 days 30, these patients reported missing an average of 30 workdays per year, compared to 3.5 workdays missed per year for the average American in the same period 3. Fewer than 60% of FCS patients had either full- or part-time employment, compared to the age-matched general rate of 80% in the USA 3. In addition, FCS patients reported more depression (22%) than the general adult US population (6.7%) 3.
Familial chylomicronemia syndrome is also associated with impaired social interactions, as was reported by up to 82% of patients 29. Friends and family members may not fully understand the seriousness of the disease and such implications as the need to strictly adhere to the low-fat diet. Furthermore, the disease can be misunderstood by medical professionals; about half of FCS patients reported feeling more knowledgeable than their physicians about their condition 29.
How is the diagnosis of FCS made?
Familial chylomicronemia syndrome should be suspected in patients with fasting severe HTG, that is TG ≥ 10 mmol L−1, in the absence of secondary causes such as obesity, alcohol, insulin resistance, specific medications, diabetes or other diseases 33. FCS or monogenic chylomicronemia represents ~ 1–2% of all patients referred to lipid clinics with TG levels this high. Most of the remaining patients with severe HTG have multifactorial or polygenic chylomicronemia.
Genetic diagnosis
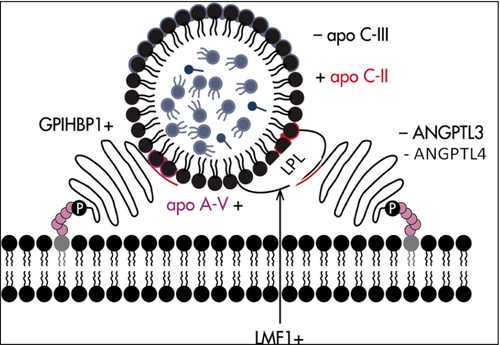
Familial chylomicronemia syndrome is an autosomal recessive genetic condition. Next-generation DNA sequencing of five FCS-causing genes is currently the gold standard for diagnosis. The presence of a pathogenic mutation on both alleles (i.e. bi-allelic) is required to establish the genetic diagnosis. About 80-90% of patients with monogenic chylomicronemia have bi-allelic mutations in the LPL gene. LPL is secreted by adipocytes and myocytes, and reaches the capillary endothelium where it mediates lipolysis of triglycerides from chylomicrons, VLDL, intermediate-density lipoprotein (IDL) and remnant particles. The liberated-free fatty acids (FFA) are internalized to be used for energy or are stored. The complete loss of LPL activity is associated with massive accumulation of chylomicrons in the plasma (Illustrated in Fig. 2). The remaining 10–20% of cases have bi-allelic mutations in one of four other causal genes that encode cofactors and proteins that interact with LPL, including APOC2, GPIHBP1, APOA5 and LMF1. Relatively few families worldwide with bi-allelic mutations in these minor genes have been reported to date 7, 24, 35. Genetically defined LPL-FCS and non-LPL-FCS patients were recently shown to have very similar baseline untreated clinical and biochemical phenotypes 36.
Classical diagnosis by LPL activity
Before genetic testing became available, a traditional test to diagnose LPL deficiency was measurement of LPL activity in plasma obtained postinjection of heparin. A marked reduction or absence of LPL activity (i.e. ≤20% of normal) was often used in the past to document LPL deficiency 2, 36. However, this assay is no longer commonly available in clinical setting and is relatively unreliable, as the performance varies widely depending on the experience of the diagnostic laboratory.
Cases of autoantibodies against LPL or GPIHBP1
Interestingly, a rare subset of FCS is immunologic rather than monogenic in nature. A patient with autoantibodies against LPL was been reported in 1989 37, with a few other cases reported subsequently 38. More recently, autoantibodies against glycosylphosphatidylinositol high-density lipoprotein-binding protein type 1 (GPIHBP1) were reported in six patients with chylomicronemia 39; this would represent about 3% of patients who appear to have FCS clinically, but who have tested negative for mutations in the five canonical genes for monogenic chylomicronemia 40. Autoimmune chylomicronemia is thus a rare possibility when DNA testing does not show either monogenic or polygenic chylomicronemia; however, the anti-GPIHBP1 antibody test is not yet widely available for clinical use.
Comparison with other causes of hypertriglyceridaemia
Familial chylomicronemia syndrome is a relatively infrequent cause of TG ≥ 10 mmol L−1, which is seen in patients with multifactorial chylomicronemia (MCM), but also in patients with severe primary hypertriglyceridaemia due to excessive VLDL levels or dysbetalipoproteinemia due to the pathogenic presence of IDLs [reviewed in 4]. However, chylomicrons are only observed in the fasting plasma of MCM and FCS patients. Whilst it is possibly challenging for some clinicians to differentiate between these disorders, a proper diagnosis could guide appropriate treatment. MCM is an archetypal polygenic disorder that is frequently exacerbated by the presence of secondary factors, such as metabolic syndrome, obesity or diabetes. MCM, with a prevalence of 1:600, is much more frequent than FCS, which prevalence has been estimated at 1 in 1 million people 24. Next-generation sequencing and bioinformatics in a cohort of 563 patients with TG> 10 mmol L−1 showed that the vast majority had polygenic HTG, whilst only 1% had a bi-allelic monogenic mutation 41. Approximately 1–3% of patients with TG levels >11.3 mmol L−1 (>1000 mg dL−1) would have FCS 42. Lipoprotein electrophoresis and ultracentrifugation can differentiate between these two disorders: the serum of MCM patients contains both VLDL and chylomicrons, whereas the serum of FCS patients contains only chylomicrons; however, this biochemical method is rarely available. Furthermore, clinical profiles of MCM and FCS patients are quite different. Indeed, a higher prevalence of metabolic abnormalities (elevated body mass index, high fasting glucose or high blood pressure) and ASCVD (prevalence of 17% in MCM vs 0% in FCS) were observed in the MCM group, whereas the FCS group had higher frequency of pancreatitis (prevalence of 60% in FCS vs 6% in MCM), multiple pancreatitis (prevalence of 48% in FCS vs 3% in MCM) and abdominal pain 6. However, the mean triglycerides levels were similar between groups 6. Whilst lifetime risk of pancreatitis is lower in MCM compared to FCS, the higher prevalence of MCM means that it underlies most cases of HTG-associated pancreatitis. Indeed, clinical experience has shown that patients admitted to hospital with HTG-associated acute pancreatitis (mean TG levels 45.5 mmol L−1) were all assumed by their providers to have FCS, and subsequent DNA analysis showed that none had FCS and most had MCM 43.
Finally, it worth mentioning that in rare cases, patients can have a clear plasma sample even if the triglycerides are higher than 10 mmol L−1. If it is the case, the even rarer condition of glycerol kinase deficiency (GKD) should be suspected 4. A synthesized description of each hyper-TG conditions and their respective estimated prevalence is presented in Table 2.
| HTG conditions | OMIM number | Prevalence | Lipoprotein elevation | Plasma appearance |
|---|---|---|---|---|
| Familial chylomicronemia syndrome (formerly type I HLP) | 238 600 | ~1: one million | Chylomicrons | Lactescent |
| Familial combined hyperlipidemia (formerly type IIB HLP) | 144 250 | ~1: 20 | VLDL + LDL | Clear |
| Dysbetalipoproteinemia (formerly type III HLP) | 617 347 | ~1: 250–833 | Chylomicron remnants + IDL | Lactescent if TG ≥ 10 mmol L−1 |
| Familial HTG (formerly type IV HLP) |
144 600 145 750 |
~1: 10–20 | VLDL | Lactescent if TG ≥ 10 mmol L−1 |
| Multifactorial chylomicronemia (formerly type V) | 144 650 | ~1: 600 | Chylomicrons + VLDL | Lactescent |
| Glycerol kinase deficiency | 307 030 | Very rare/unknown | None; glycerolaemia leads to artefactual HTG | Clear |
Clinical diagnosis algorithms
Because FCS is a rare condition, the diagnosis might be missed in some patients. From a clinical point of view, some have proposed that it may be useful to have a diagnostic algorithm. Also, despite decreasing costs, use of next-generation sequencing for diagnosis is still not widely accessible. Recently, an expert panel proposed the first diagnostic scoring system for the differential diagnosis of FCS. In this score, weighting is given to each clinical variables: fasting TG values, the presence of secondary factors, previous history of pancreatitis, unexplained recurrent abdominal pain, history of combined hyperlipidemia, response to lipid-lowering therapy and the age at onset of symptoms in order to determine whether the patient has ‘very likely FCS’, ‘unlikely FCS’ or ‘very unlikely FCS’ 47. However, this type of score remains to be validated in larger and more heterogeneous patients with severe HTG.
What are the treatments for FCS?
Treatment of FCS can be broken down into management of an acute crisis related to pancreatitis and chronic management of hypertriglyceridaemia to reduce the risk of future episodes. During an episode of acute pancreatitis, complete fasting with parenteral fluid support and analgesia if required is usually very effective: TG levels fall by 50% within 30 h 43. In patients with diabetes and uncontrolled hyperglycaemia, intravenous insulin therapy may also be of benefit. There is no evidence that plasma exchange positively affects short- or long-term outcomes any more than conservative management; furthermore without ongoing metabolic control, TG levels rapidly rebound. Therefore, with the possible exception of controlling severe HTG due to monogenic chylomicronaemia during pregnancy, the use of plasmapheresis is not recommended.
Long-term management involves patient education and support centred on maintaining a low-fat diet, defined as < 10% of calories from fat. In the real world, adherence to such a regimen is challenging and is almost never consistently achieved by most patients. Medium chain TGs can supplement calories and essential fatty acids whilst avoiding increases in plasma TG levels, although their use is not well established. Fibrates and high dose omega-3 fatty acids are typically not helpful in monogenic chylomicronemia. Investigational treatments have included LPL gene therapy intended for patients who specifically have LPL deficiency, although development of this treatment has recently been abandoned. Also in development are biological inhibitors of apo C-III (antisense RNA therapy) and of ANGPTL3 (both monoclonal antibodies and antisense RNA) 48.
Conclusion
Familial chylomicronemia syndrome or monogenic chylomicronemia patients are a small subset of an already relatively uncommon patient population with severe HTG (fasting TG> 10 mmol L−1) and chylomicronemia. FCS patients represent 1–3% of the total patient cohort with chylomicronemia, which is most commonly polygenic or multifactorial in nature. Whilst certain features such as young age of onset, a low level of apolipoprotein B and lack of secondary conditions suggest FCS, definitive diagnosis is through DNA sequencing. Chylomicronemia of any aetiology is associated with increased risk of life-threatening acute pancreatitis; the relative risk is higher with FCS compared with MCM, although the absolute number of cases is much greater with MCM compared to FCS. MCM patients are also at increased risk of ASCVD, due to species of additional diverse atherogenic lipoproteins. Because of its autosomal recessive nature (complete loss of LPL activity), FCS is challenging to treat, whilst MCM may be more readily amenable to long-term lifestyle modifications and traditional pharmacotherapy for HTG. Future biological treatments may prove to be helpful additions to the therapeutic armamentarium for FCS, although they may also be useful to avert acute pancreatitis episodes in severe MCM cases.
Unanswered questions
- Which is more important for diagnosis and guiding interventions in FCS: the biochemical abnormality (i.e. degree of TG elevation) or the precise molecular diagnosis?
- For future biological therapies to reduce severe hypertriglyceridaemia and pancreatitis risk, does knowing the genetic basis of the chylomicronemia (i.e. monogenic versus polygenic) really matter?
- What is the mechanism of hypertriglyceridaemia-associated acute pancreatitis?
- Will reducing severely elevated triglycerides in polygenic or multifactorial chylomicronemia also reduce future risk of atherosclerotic cardiovascular disease?
Conflict of interest statement
A.B. received research grants from Akcea, Amgen, Astra Zeneca, the Fondation Leducq, Merck Frosst and Sanofi. He has participated in clinical research protocols from Acasti Pharma Inc., Akcea, Amgen, Astra Zeneca, Ionis Pharmaceuticals, Inc., The Medicines Company, Merck Frosst, Novartis, Pfizer, Regeneron Pharmaceuticals Inc. and Sanofi. He has served on advisory boards and received honoraria for symposia from Akcea, Amgen and Sanofi. S.B. has participated in clinical research protocols from Akcea, Amgen, The Medicines Company and Sanofi. She has served on advisory boards for Akcea, Amgen, Eli Lilly, Merck Frosst, Novo Nordisk, Sanofi and Valeant Pharmaceuticals, and received honoraria for symposia from Akcea, Amgen, Boehringer Ingelheim, Merck Frosst, Novo Nordisk and Sanofi-Aventis. R.A.H. has received honoraria for membership on advisory boards and speakers' bureaus for Aegerion, Akcea/Ionis, Amgen, Gemphire, Lilly, Merck, Pfizer, Regeneron, and Sanofi. M.P. has nothing to declare.
Funding
The authors received no external funding for this review.
Contribution
All of the authors contributed substantially to the writing of the manuscript and revised it for important intellectual content. All of the authors approved the final version and agreed to act as guarantors of the work.





