Prediction of no-reflow and major adverse cardiovascular events with a new scoring system in STEMI patients
Abstract
Background
No-reflow is associated with a poor prognosis in STEMI patients. There are many factors and mechanisms that contribute to the development of no-reflow, including age, reperfusion time, a high thrombus burden, Killip class, long stent use, ejection fraction ≤40, and a high Syntax score. In this study, we aimed to evaluate the parameters associated with no-reflow prediction by creating a new scoring system.
Methods
The study included 515 consecutive STEMI patients who underwent PCI; 632 STEMI patients who had undergone PCI in another center were included in the external validation of the scoring system. The correlations between 1-year major adverse cardiac events and low/high risk score were assessed.
Results
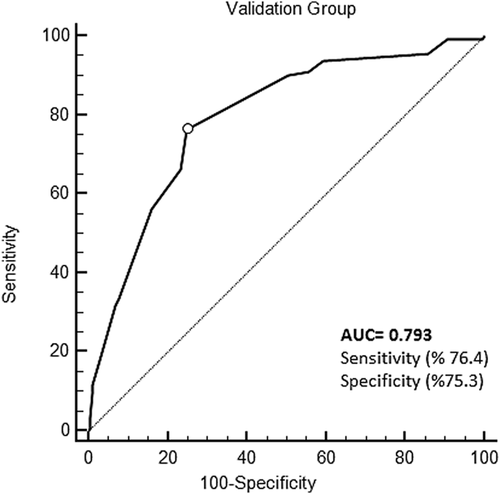
In this study, seven independent variables were used to build a risk score for predicting no-reflow. The predictors of no-reflow are age, EF ≤40, SS ≥22, stent length ≥20, thrombus grade ≥4, Killip class ≥3, and pain-balloon time ≥4 h. In the derivation group, the optimal threshold score for predicting no-reflow was >10, with a 75% sensitivity and 77.7% specificity (Area under the curve (AUC) = 0.809, 95%CI: 0.772-0.842, P < 0.001). In the validation group, AUC was 0.793 (95%CI: 0.760-0.824, P < 0.001).
Conclusion
This new score, which can be calculated in STEMI patients before PCI and used to predict no-reflow in STEMI patients, may help physicians to estimate the development of no-reflow in the pre-PCI period.
1 INTRODUCTION
ST segment elevation myocardial infarction (STEMI) remains one of the leading causes of death worldwide.1 The main goal in the treatment of acute myocardial infarction is to recanalize the culprit artery occlusion at an early stage. Primary percutaneous coronary intervention (PCI) has been shown to be the most effective reperfusion strategy in the treatment of acute myocardial infarction.2 No-reflow is defined as insufficient myocardial perfusion throughout a specified portion of the coronary circulation, without angiographic evidence of mechanical vessel occlusion.3 No-reflow is thought to result from a combination of different pathogenic compounds, such as distal embolization, ischemic damage, reperfusion injury, and the susceptibility of coronary microcirculation to injury.4 This phenomenon may develop in 5-50% of acute myocardial infarction patients after thrombolysis or primary percutaneous intervention.5
Patients with no-reflow may develop in-hospital major adverse cardiac events (MACE) and congestive heart failure in the early periods after myocardial infarction, and they may present with progressive left ventricular dilatation during recovery from infarction.3 The development of no-reflow is multifactorial, and the etiology is not fully understood.6 Although age ≥65 years, reperfusion time >4 h, a high thrombus burden, Killip class ≥3, long stent use, ejection fraction ≤40 and a high syntax score (SS) have been shown as predictors, new scales may guide interventions prior to a procedure when no-reflow is likely to develop.7, 8
In this study, we aimed to evaluate the parameters associated with no-reflow in previous studies in terms of no-reflow and MACE prediction by creating a new scoring system.
2 METHODS
The study included 515 consecutive STEMI patients who underwent PCI between July 2015 and July 2016. In addition, 632 STEMI patients who had undergone PCI in another centre between July 2015 and July 2016 were included in the external validation of the scoring system.
Written or verbal informed consent was received from all patients, and the study protocol was approved by the hospital's local ethics committee in accordance with the Helsinki Declaration and Good Clinical Practice Guidelines. The estimated score was produced using a stepwise logistic regression, with demographic and angiographic variables that have previously been defined as predictors (age ≥65 years, reperfusion time >4 h, a high thrombus burden, Killip class ≥3, long stent use, and SS). Patients with end-stage renal failure, liver failure, coagulopathy, malignancy, unstable angina pectoris, no ST elevation myocardial infarction, and elective PCI patients were excluded from the study. The correlations between 1-year MACE and low-high risk score were assessed in STEMI patients. A 12-lead electrocardiogram was acquired at the time of presentation. STEMI was defined as chest pain or angina equivalent over 30 min in the last 15 h, which suggests myocardial ischemia. This was accompanied by a 1 mm (0.1-mV) ST-segment elevation in two or more adjacent derivations and then an increase in creatine kinase (CK) and CK-MB-troponin after PCI. Clinical correspondence of heart failure was evaluated by Killip classification. Echocardiographic images were recorded before PCI, and then EF was calculated using the Simpson method. Transthoracic echocardiographic images were recorded without PCI delay. Anterograde coronary flow in the responsible vessel was graded according to the thrombolysis in myocardial infarction (TIMI) scale.9 The thrombosis score was assessed as previously defined by the TIMI Study Group.10 Angiography no-reflow was defined as TIMI grade <3 or myocardial blush grade <2.11
Any major coronary vessels with a diameter narrowed by 50% or more were defined as significant stenosis. If a stenosis of >50% diameter contained more than two epicardial coronary arteries, it was defined as multivessel disease. Providing TIMI III flow with <20% residual stenosis in major epicardial coronary arteries was defined as complete revascularization.
The SS was calculated using an online calculator (http://www.syntaxscore.com). Two experienced interventional cardiologists who were blinded to the study calculated the SS. In the case of contradictory results, the two cardiologists decided based on consensus.
All patients underwent coronary angiography with a transfemoral approach. Before PCI, patients were given 600 mg of clopidogrel, 300 mg of acetylsalicylic acid and 70 IU/kg of intravenous heparin. MACE is defined as stent thrombosis, target vessel revascularization, myocardial infarction, and death.
2.1 Statistical analysis
The data analysis was conducted using SPSS (version 20.0, SPSS Inc., Chicago, IL) and MedCalc statistical software (trial version 12.7.8, Mariakerke, Belgium). Continuous variables data are expressed as the mean ± standard deviation. Categorical variables were compared using Chi-square or Fisher's exact tests and summarized as percentages. The Kolmogorov-Smirnov test was used to evaluate the distribution of the continuous variables. To predict no-reflow and MACE, age, gender, diabetes mellitus (DM), hypertension (HT), hyperlipidemia (HL), smoking, preinfarct angina, time interval from pain to PCI, systolic blood pressure (SBP), diastolic blood pressure (DBP), heart rate (HR), EF, thrombus score ≥ and Killip ≥3, SS, stent length, stent diameter, and pre-MI medication were included in the univariate analysis. The parameters with P < 0.05 were included in the multiple logistic analysis.
Receiver operating characteristic (ROC) curves were used to predict the future incidence of no-reflow and MACE. Age (≥65 years), EF (% 40), SS (≥22), time interval from pain (240 min), thrombus score (≥4), Killip class (≥3) and stent length (>20 mm) values were used for the categorization, and the score was created according to the beta values from the multiple logistic regression analysis. The area under curve independent values obtained for the calculated score for derivation and validation groups were further compared. The Hosmer-Lemeshow goodness of fit test was used for the models.
3 RESULTS
Among the STEMI patients, 15 of 530 patients who were accepted based on compliance with primary PCI were excluded from the study since they were not eligible between July 2015 and July 2016. Following the primary PCI procedure, no-reflow was observed in 72 (14%) of 515 patients. In the validation arm, no-reflow was detected in 110 (17.4%) of the 632 patients. Age, pain-balloon time, stent length, Killip class, thrombus grade, SS, and EF were statistically different in the group that developed no-reflow compared to the reflow group. In addition, after these parameters were categorized, a statistically significant difference was observed between the two groups. The clinical characteristics as well as the angiographic and PCI features of the findings are listed in Table 1.
| Derivation group (n = 515) | Validation group (n = 632) | |||||
|---|---|---|---|---|---|---|
| Reflow (n = 443) | No-reflow (n = 72) | P | Reflow (522) | No-reflow (n = 110) | P | |
| Age (years) | 59.5 ± 12.3 | 63.0 ± 12.6 | 0.028 | 56 ± 11 | 62 ± 10 | <0.001 |
| Age ≥65 (n, %) | 139 (31.4) | 39 (54.2) | <0.001 | 125 (23.9) | 55 (50) | <0.001 |
| Sex (%, male) | 358 (79.5) | 58 (80.6) | 0.830 | 436 (83.5) | 94 (85.5) | 0.617 |
| Diabetes mellitus (n, %) | 104 (23.5) | 22 (30.6) | 0.195 | 106 (20.3) | 24 (21.8) | 0.669 |
| Hypertension (n, %) | 201 (45.4) | 34 (47.2) | 0.770 | 232 (44.4) | 42 (38.2) | 0.228 |
| Hyperlipidemia (n, %) | 199 (44.9) | 30 (41.7) | 0.606 | 220 (42.1) | 52 (47.3) | 0.324 |
| Smoking (n, %) | 181 (40.9) | 34 (47.2) | 0.502 | 306 (58.6) | 69 (62.7) | 0.426 |
| SBP | 129.1 ± 30.9 | 129.6 ± 37.2 | 0.884 | 133 ± 29 | 134 ± 30 | 0.591 |
| DBP | 76.7 ± 19.0 | 76.0 ± 22.2 | 0.776 | 79 ± 17 | 79 ± 18 | 0.649 |
| Heart rate (p/min) | 77 ± 16 | 75 ± 19 | 0.240 | 73 ± 15 | 74 ± 12 | 0.673 |
| Ejection fraction | 46.7 ± 9.1 | 42.5 ± 6.9 | <0.001 | 47.8 ± 6.3 | 42.6 ± 5.5 | <0.001 |
| Ejection fraction ≤40 (%) | 82 (18.5) | 32 (44.4) | <0.001 | 68 (13) | 40 (36.4) | <0.001 |
| Pain to-PCI time | 198 ± 106 | 286 ± 140 | <0.001 | 190 ± 104 | 252 ± 103 | <0.001 |
| Pain to −pcitime ≥4 h (%) | 242 (54.6) | 56 (77.8) | <0.001 | 207 (39.7) | 67 (60.9) | <0.001 |
| Thrombus score ≥4 (%) | 318 (71.8) | 67 (93.1) | <0.001 | 346 (66.3) | 96 (87.3) | <0.001 |
| Killip ≥3 (%) | 41 (9.3) | 28 (38.9) | <0.001 | 38 (7.3) | 27 (24.5) | <0.001 |
| Syntax score | 16.0 ± 7.1 | 20.5 ± 10.1 | <0.001 | 14.7 ± 6.5 | 17.9 ± 8.4 | <0.001 |
| Syntax score ≥22 (%) | 79 (17.8) | 43 (89.7) | <0.001 | 70 (13.4) | 52 (47.3) | <0.001 |
| Stent length mm | 21.8 ± 8.4 | 29.6 ± 15.2 | <0.001 | 21.0 ± 0.7 | 21.3 ± 0.6 | 0.731 |
| Stent length ≥20 mm (%) | 145 (32.7) | 51 (70.8) | <0.001 | 196 (37.5) | 69 (62.7) | <0.001 |
| Stent diameter (mm) | 3.0 ± 0.3 | 3.1 ± 0.4 | 0.070 | 3.07 ± 0.3 | 3.09 ± 03 | 0.571 |
| Thrombus aspiration (n, %) | 115 (25.9) | 20 (27.7) | 0.644 | 150 (28.7) | 28 (25.4) | 0.486 |
| Direct stenting (n, %) | 116 (26.2) | 15 (20.8) | 0.333 | 142 (27.2) | 35 (31.8) | 0.327 |
| Β-blocker (n, %) | 61 (13.8) | 9 (12.5) | 0.771 | 81 (15.5) | 11 (10) | 0.136 |
| CCB (n, %) | 38 (8.6) | 3 (4.2) | 0.199 | 28 (5.4) | 2 (1.8) | 0.112 |
| Statin (n, %) | 93 (21) | 12 (16.6) | 0.398 | 115 (22) | 26 (23.6) | 0.713 |
| Aspirin (n, %) | 60 (13.5) | 12 (16.6) | 0.487 | 62 (11.9) | 16 (14.5) | 0.439 |
| Pre-infarction angina (n, %) | 101 (22.8) | 18 (25) | 0.681 | 117 (22.4) | 19 (17.3) | 0.233 |
| PAD (n, %) | 41 (9.3) | 10 (13.9) | 0.220 | 42 (8) | 9 (8.2) | 0.962 |
| Prior MI (n, %) | 60 (13.5) | 9 (12.5) | 0.809 | 53 (10.2) | 5 (5.4) | 0.064 |
| Prior PCI (n, %) | 57 (12.9) | 6 (8.3) | 0.276 | 52 (10) | 13 (11.8) | 0.560 |
| IRA | ||||||
| LM | - | - | 0.710 | 1 (0.2) | - | 0.486 |
| LAD | 225 (50.8) | 33 (45.8) | 233 (44.6) | 55 (50) | ||
| CX | 64 (14.4) | 13 (18.1) | 76 (14.6) | 16 (14.5) | ||
| RCA | 147 (33.2) | 26 (36.1) | 195 (37.4) | 39 (35.5) | ||
| Other | 5 (1.1) | - | 11 (1.7) | - | ||
- CCB, calcium channel blocker; CX, circumflex artery; DBP, diastolic blood pressure; IRA, infarct related artery; LM, left main; LAD, left ascending artery; MI, myocardial infarction; PAD, peripheral artery disease; PCI, percutaneous coronary intervention; RVA, right coronary artery; SBP, systolic blood pressure.
Among the significant parameters in the univariate analysis, those that were also found to be significant in the multiple regression analysis included age (≥65; odds ratio [OR] = 2.111, 95% confidence interval [CI]: 1.109-4.018, P = 0.023), EF (≤40; [OR] = 2.185, [CI]: 0.1.147-4.162, P = 0.017), SS (≥22; [OR] = 3.676, [CI]: 1.980-6.826, P < 0.001), stent length (≥20; [OR] = 3.607, [CI]: 1.932-6,734, P < 0.001), thrombus grade (≥4; [OR] = 3.139, [CI]: 1.081-9.113, P = 0.035), Killip class (≥3; [OR] = 3.530, [CI]: 1.700-7.329, P < 0.001) and pain-balloon time (≥4 h; [OR] = 3.240, [CI]: 1.630-6.439, P < 0.001). These identified predictors were then used to establish a clinical scoring system for estimating post-procedural no-reflow. The score ranged from 0 to 19 (Table 2).
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| OR 95%CI | P-value | OR | 95%CI | P-value | Point assigned | |
| Age | 2.585 | <0.001 | 2.111 | 1.109-4.018 | 0.023 | 2 |
| Sex | 1.071 | 0.830 | ||||
| Smoking | 0.653 | 0.502 | ||||
| Diabetes mellitus | 1.434 | 0.195 | ||||
| Syntax score ≥22 | 6.832 | <0.001 | 3.676 | 1.980-6.826 | <0.001 | 3 |
| Thrombus grade ≥4 | 6.655 | <0.001 | 3.139 | 1.081-9.113 | 0.035 | 3 |
| Pain to −pcı time ≥4 h | 4.214 | <0.001 | 3.240 | 1.630-6.439 | 0.001 | 3 |
| Kilip ≥3 | 6.259 | <0.001 | 3.530 | 1.700-7.329 | 0.001 | 3 |
| Pre-infarct angina | 0.886 | 0.681 | ||||
| SBP | 1.001 | 0.634 | ||||
| DBP | 0.998 | 0.847 | ||||
| Ejection fraction ≤40 | 3.630 | <0.001 | 2.185 | 1.147-4.162 | 0.017 | 2 |
| Stent length ≥20 (mm) | 4.218 | <0.001 | 3.607 | 1.932-6.734 | <0.001 | 3 |
- DBP, diastolic blood pressure; SBP, systolic blood pressure.
For the derivation group, the optimal threshold score for predicting no-reflow was >10, with a 75% sensitivity and 77.7% specificity (area under the curve [AUC]: 0.809, 95% CI: 0.772-0.842, P < 0.001) (Figure 1). For the validation group, AUC was 0.793 (95% CI: 0.760-0.824, P < 0.001) (Figure 2). (derivation vs. validation sample, difference: 0.016, P = 0.644). The Hosmer-Lemeshow test produced a value of χ2 = 7.63 (P = 0.470).

In the derivation and validation groups and in the total patient population, 1-year mortality (P = 0.012, P < 0.001, P < 0.001, respectively) and 1-year MACE, which becomes meaningful with the mortality rate (P = 0.002, P < 0.001, P < 0.001, respectively), were significantly higher in the high-risk group (score >10; Table 3).
| Derivation group | Validation group | Total population | |||||||
|---|---|---|---|---|---|---|---|---|---|
| High ≥10 | Low <10 | P | High ≥10 | Low <10 | P | High ≥10 | Low <10 | P | |
| MACE | 44 (28.8) | 62 (17.1) | 0.003 | 39 (26.7) | 72 (14.8) | 0.001 | 83 (27.7) | 134 (15.8) | <0.001 |
| MI | 6 (4.4) | 12 (3.4) | 0.605 | 8 (5.9) | 20 (4.2) | 0.408 | 14 (4.6) | 33 (3.9) | 0.558 |
| Stent thrombosis | 3 (2.2) | 3 (0.8) | 0.201 | 3 (2.1) | 7 (1.4) | 0.353 | 6 (2) | 10 (1.1) | 0.296 |
| TVR | 15 (9.8) | 24 (6.6) | 0.213 | 12 (8.2) | 28 (5.8) | 0.285 | 27 (7.3) | 52 (6.1) | 0.098 |
| Death | 20 (13.1) | 23 (6.4) | 0.012 | 16 (11) | 17 (3.5) | <0.001 | 36 (12) | 37 (4.3) | <0.001 |
- MACE, stent thrombosis; TVR, target vessel revascularization; MI, myocardial infarction; death; High-risk score ≥10; Low-risk score <10.
4 DISCUSSION
In our study, seven different parameters were found to predict the development of no-reflow in STEMI patients. The new score developed from these parameters was sensitive in predicting the development of no-reflow (AUC: 0.809). When this score was tested in the validation arm, its predictive power remained high (AUC 0.793; difference = 0.016, P = 0.644). It was observed that a high-risk value of this score may help for estimating 1-year MACE and mortality rates.
No-reflow is associated with a poor prognosis in STEMI patients treated with PCI. There are a number of factors that contribute to the development of no-reflow.12-15 An important pathophysiological mechanism is thought to be distal embolisation.16, 17 In our study, age, EF, thrombus grade, Killip class, pain-PCI time, stent length, and SS were found to be predictors of no-reflow. T. Liang and coworkers observed that the development of no-reflow was higher in advanced age patients.18 A higher incidence of diffuse atherosclerosis, severe calcification, distal microvessel embolization, and microvascular diseases in elderly patients are thought to cause the development of no-reflow.19 Consistent with this, we showed that advanced age was an independent risk factor in the development of no-reflow.
Previous studies have shown that the incidence of no-reflow is higher in patients with low EFs, and that low EF is an independent predictor in the development of no-reflow. In addition, in a study by Wang et al., high Killip class (≥3) was demonstrated to be associated with the development of no-reflow.20, 21 Similar to those studies, in the present study low EF and high Killip class were observed to be independent predictors in the development of no-reflow.
Yip et al. found that the presence of a high thrombus in STEMI was an independent predictor of post-procedural no-reflow in patients undergoing PCI.22 In addition, Sabin et al. found that high thrombus load was an independent predictor of the development of no-reflow.6 The results of our study support this finding. Several studies have shown that the length of the target lesion is important in the development of no-reflow.10 In our study, the length of the stent used in the culprit lesion predicted the development of no-reflow. Several studies have shown that pain-balloon time predicts the development of no-reflow. Although this time differs among various studies, 4h, the closest to our median value of 215 min, was accepted as the cut-off value and predicted the development of no-reflow.
A higher rate of atherosclerosis was observed in patients who developed no-reflow compared to those who did not. However, no-reflow develops more often in patients with a high SS.12 This is thought to be caused by the fact that diffuse coronary artery disease is related to impaired microcirculation resistance, which affects epicardial blood flow.23, 24 Similarly, we found a significant association between a high SS and no-reflow.
No-reflow is not a rare complication of primary PCI in STEMI patients. In the past, several scoring systems were used for the prediction of no-reflow. In their study, Yesin et al. showed that SS II could predict no-reflow.7 Wang et al. also predicted no-reflow in the model they developed.8
Distal thromboembolism is an important mechanism for the development of no-reflow. Thrombus aspiration, intracoronary Gp 2b/3a inhibitors, adenosine, calcium channel blockers and sodium nitroprusside have been used in the management of no re-flow.17 In the present study, thrombus aspiration was performed in 12 patients (6.5%), a GP2b3a inhibitor (tirofiban, absiximab) was used in 95 patients (52.1%), adenosine was used in 78 patients (42.8%), and diltiazem was used in 26 (14.2 %) patients who developed no-reflow.
Primary PCI must be performed rapidly in STEMI patients. In the above-mentioned studies, the incorporation of laboratory parameters into the scores might be helpful for implementing post-procedural measures. However, these parameters do not enable the calculation of a risk score before the procedure.7, 8 Unlike the previous scoring systems, we believe that the use of demographic and angiographic parameters that could be evaluated faster enable a more rapid evaluation independent of laboratory parameters, and thus this score would be helpful before PCI.
Several scores, such as the CHADS score, have been shown to have an association with mortality and morbidity.25 In this study, a high score created by considering the cut-off value was associated with 1-year MACE, which becomes meaningful with the mortality rate.
5 CONCLUSION
In conclusion, in our study, it was observed that the simple risk score generated from seven independent parameters predicted the no-reflow phenomenon. Considering the high mortality associated with no-reflow, we think that this newly developed scoring system may help physicians to estimate the development of no-reflow in the pre-PCI period. However, it would be appropriate to test this score in a wider range of studies with different populations.
5.1 Study limitations
This study has some limitations. First, this was a retrospective case-control type study, which can be biased compared to prospective studies. For the score, predictive efficacy needs to be further tested in a prospective and large-scale study. In our study, all patients underwent transfemoral access and received clopidogrel. Ticagrelol or prasugrel was not administered in the present study, and this may have influenced the outcomes. The relevance of the proposed risk score at centers with differing practices has not been examined. Lastly, the applicability of this predictive model in routine clinical practice is limited, as the clinical management of patients with a high probability of no-reflow is not significantly different than the management of other patients.
ACKNOWLEDGMENT
The authors thank www.metstata.com for its contributions to the statistical analysis and trial design.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest and no financial disclosures.




