A classification scheme for avian diet types
Abstract
enDescriptions of avian diets currently lack consistent terminology and standardized methods. As a consequence, most available classifications, especially for tropical birds, are inconsistent and often misleading. We identified 23 food categories most commonly eaten by birds (e.g., seeds, fruits, and insects) and proposed standard names that accurately describe the diet type associated with each food category (e.g., granivore, frugivore, and insectivore). We also propose a classification scheme for avian diet types that takes into account the number of food categories consumed and the volumetric proportion (based on stomach content analysis) of each category eaten to indicate the diet type of a species, using a binomial terminology. Given that bird diets encompass a continuum between some extremes commonly treated as distinct categories, we adopted arbitrary breaking points to delimit distinct diet types. For example, species with different proportions of insects and fruits in their diets can be classified as insectivores (IN), insectivores secondarily frugivores (INFR), frugivores-insectivores (FR-IN), frugivore secondarily insectivores (FRIN), and frugivores (FR). Because many factors can influence avian diets, the diet types of species can also be classified based on age-related, sexual, seasonal, and/or geographic variation, e.g., young are insectivores and adults are frugivores, we believe that our classification scheme provides a standardized terminology that can contribute to a more consistent and effective exchange of information about avian diets.
Un esquema de clasificación para tipos de dieta en aves
esLas descripciones de las dietas de las aves actualmente carecen de una terminología consistente y métodos estandarizados. Como consecuencia, las clasificaciones comúnmente disponibles, especialmente para aves tropicales, son inconsistentes y con frecuencia engañosas. Identificamos 23 categoría alimenticia que las aves consumen comúnmente (e.g., semillas, frutas e insectos) y proponemos nombres que describen el tipo de dieta con precisión, asociado a cada tipo de categoría alimenticia (e.g., granívoro, frugívoro e insectívoro). Adicionalmente proponemos un esquema de clasificación para los tipos de dieta en aves que tiene en cuenta el número de categorías alimenticias consumidas y la proporción volumétrica (basada en análisis de contenido estomacal) de cada una de las categorías ingeridas, con el fin de indicar la dieta de una especie usando terminología binomial. Dado que las dietas de las aves abarcan un continuo entre algunos extremos comúnmente considerados como categorías diferentes, adoptamos puntos de quiebre arbitrarios para delimitar los diferentes tipos de dieta. Por ejemplo, especies con diferentes proporciones de insectos y frutas en su dieta pueden ser clasificados como insectívoros (IN), insectívoros secundariamente frugívoros (INFR), frugívoros-insectívoros (FR-IN), frugívoros secundariamente insectívoros (FRIN) y frugívoros. Debido a que hay muchos factores que influencian la dieta de las aves, los tipos de dieta de las especies pueden ser clasificados con base en factores como la edad, el sexo, temporada y/o variación geográfica, e.g., juveniles son insectívoros y adultos son frugívoros. Consideramos que nuestro esquema de clasificación provee una terminología estandarizada que puede contribuir con un intercambio efectivo y con información más a cerca de las dietas de las aves.
Standardized terminology is important for rapid and effective exchange of information in science (Sonneveld and Loening 1993). However, in ecology and related disciplines, some terms and concepts traditionally used lack strict or concise definitions. For example, “niche” and “stability” are used differently among studies (Grimm and Wissel 1997, McInerny and Etienne 2012). Efforts to clarify and standardize terminology are often difficult because authors may favor certain terms over others and resist changes (Colautti and MacIsaac 2004). However, more rigorous standardization is needed to avoid miscommunication.
Current descriptions and classifications of avian diets represent a prime example of nonstandardized terminology and methods. For example, Kissling et al. (2007) classified all sub-Saharan species of birds based on their diet preferences using a standardized way of transferring diet descriptions from monographs and books into semiquantitative measures of diet importance. However, descriptions of avian diets in monographs and books often include non-quantitative terms such as “entirely”, “sometimes”, and “when available” (e.g., del Hoyo et al. 1992) that may be defined and used differently by different authors. Use of such non-standardized and qualitative terminology is troubling because avian diet types are commonly used as predictive variables in several analyses, such as those associated with vulnerability to habitat fragmentation (Sodhi et al. 2004), territorial systems (Stutchbury and Morton 2001), propensity to migrate (Chesser and Levey 1998), and macroecological patterns (Kissling et al. 2007).
We propose a standard classification scheme for avian diet types to improve consistency among studies, the quality of data collected to describe avian diets, and to mitigate error rates in classifying diet types across bird populations or species. Our specific objectives were to: (1) identify and delimit the foods most commonly eaten by birds, (2) propose standard names that accurately describe the diet type associated with each food category, (3) propose a classification scheme for avian diet types, and (4) identify the limitations of the classification scheme we propose and suggest approaches to circumvent them.
Diet types are not synonymous with feeding guilds
The term feeding guild is frequently used to indicate the taxonomic composition of bird diets (e.g., a species that eats insects is classified in the feeding guild insectivore). The term guild was originally defined by Root (1967) as “a group of species that exploit the same class of environmental resources in a similar way.” As explicit in this definition, the consumption of the same resource (e.g., insects) is not sufficient for delimiting a feeding guild because resources must also be exploited in a similar way. For example, giant anteaters (Tamandua tridactyla) and Rufous Gnateaters (Conopophaga lineata) eat predominantly ants and termites, but obtain food in very different ways (Oyarzun et al. 1996, Lopes et al. 2005a). Thus, the diets of birds are just one aspect of their feeding guild (Jaksic 1981); others include such things as habitat use, search strategy, time of activity, and prey size. For an attempt at classifying the foraging guilds of North American birds, see DeGraaf et al. (1985).
Diet types and food categories
We identified diet types and food categories for birds based on a review of the “food and feeding” section of all 16 volumes of the Handbook of the Birds of the World (del Hoyo et al. 1992) and references therein. The names of the diet types we propose were standardized with the suffix “-vore”, which comes from the Latin “vorare” (to devour) and is used to form nouns indicating the type of diet of an animal. Equivalent adjectives are formed by the use of the suffix “-vorous.” Because this standardization resulted in some awkward names rarely used in English scientific literature, we recommend use of the more commonly used term hematophagous rather than sanguivore and scavenger rather than necrovore. We also coined some names for which we found no largely accepted term, such as cerivore to describe the habit of eating wax. We also provide a two-letter abbreviation code for each term.
We make no effort to adjust diet categories to fit either strict botanical terminology (e.g., folivores consume food items other than leaves and granivores also eat dry fruits) or monophyletic groups (e.g., vermivores may consume several distinct and unrelated phyla of wormlike animals, piscivores may eat several groups of chordates traditionally considered to be fish because they are ectothermic aquatic vertebrates with gills and fins, even though they form an unnatural group, and algae constitute a polyphyletic group). In a similar manner, we considered fungi and lichens as vegetable matter. We tried to adjust those categories to include what we interpreted as similar food items from a birds' point of view. Here, we suggest terms to describe avian diets, briefly describe the constituent food items, and provide examples of bird species that frequently eat items in those categories.
Terminology for classifying avian diets
Vegetable matter
Algivore (AL): macroscopic, non-planktonic algae. Brant Geese (Branta bernicla) feed on marine algae, such as Ulva (Cottam et al. 1944). Lesser Sheathbills (Chionis minor) commonly eat coldwater seaweed (genus Porphyra) in intertidal habitats (Burger 1981).
Cerivore (CE): wax from bee honeycombs. Wax is commonly ingested by honeyguides (Indicatoridae) (Friedmann 1955, Downs et al. 2002) and, to a lesser extent, by some bulbuls (Pycnonotidae) (Horne and Short 1990, Fishpool and Tobias 2005). Birds that feed on other sources of wax, such as the wax-coating of bayberries and wax-myrtles (Myrica spp.) (Place and Stiles 1992) and the wax-covering of scale insects (Coccidae) (Friedmann 1955) are not considered cerivores, but as, respectively, Frugivores and Insectivores because whole fruits and insects are usually eaten. Animals that feed on wax are sometimes called wax-eaters, or as cerophagous.
Exudativore (EX): plant fluids, e.g., sap, gum and resins, honeydew (sugar-rich secretions of nymphal stages of many insects, especially aphids, coccids, and psyllids), and manna (sugar-rich fluid that oozes from damaged plants and later crystallizes). Examples of species that regularly ingest plant fluids include woodpeckers of the genus Sphyrapicus (Picidae) with adaptations for sap feeding (Tate 1973), Australian honeyeaters (Meliphagidae) that regularly ingest honeydew and manna (Paton 1980, Woinarski 1984), and Kori Bustards (Ardeotis kori) that ingest gum from acacia trees (Urban et al. 1978). Birds that consume lerp, the protective cover secreted by many Psyllidae insects (Hemiptera) (Paton 1980), should be considered as ingesting insects because the insect is usually also eaten. For example, some Australian pardalotes (Pardalotidae) are specialized lerp-feeders (Woinarski 1984). Animals that exhibit this diet type are sometimes referred as phytosuccivorous.
Iliovore (IL): consume organic mud and the associated microbiota (e.g., bacteria, protozoa, and algae). Some flamingos (Phoenicopteridae) frequently ingest mud (del Hoyo 1992). Animals that exhibit this diet type are sometimes called mud-eaters.
Folivores (FO): leaves, stems, shoots and buds, flowers, whole aquatic plants, ferns, clubmosses, and mosses. Hoatzins (Opisthocomus hoazin) (Grajal 1995) and plantcutters (Phytotoma spp.; Bucher et al. 2003) feed primarily on leaves and buds. The diet of Ostriches (Struthionidae) consists largely of leaves (Williams et al. 1993). Some tanagers (Thraupidae) also ingest leaves and buds (Munson and Robinson 1992, Guix and Ruiz 1998). Many species of screamers, swans, and geese (Anseriformes) feed extensively on aquatic vegetation and terrestrial plants (Carboneras 1992). Kakapos (Strigops habroptilus) eat clubmosses and ferns (Best 1984).
Frugivore (FR): fleshy fruits, including berries, drupes, pomes, aggregated fruits, multiple fruits, and accessory fruits. Seeds, when ingested, are usually regurgitated or defecated whole so frugivores commonly act as seed dispersers. Fruit is a staple food for birds in many families, including fruit pigeons (Columbidae) (Goodwin 1983), manakins (Pipridae) and cotingas (Cotingidae) (Snow 1992, Kirwan and Green 2012), and birds-of-paradise (Paradisaeidae) (Frith and Frith 2009). The diet of Oilbirds (Steatornis caripensis) also consists largely of fruit (Bosque et al. 1995).
Fungivore (FU): fungus and lichens. These are unusual food items for birds (Simpson 2000), but are consumed, for example, by Kakapos (Forshaw 1989) and Ruffed Grouse (Bonasa umbellus) (Tanney and Hutchinson 2011). Pygmy-parrots (Micropsitta spp.) also frequently eat lichens (Forshaw 1989). Animals that eat fungus and lichens are sometimes referred to as mycophagous.
Granivore (GR): seeds, grains, nuts, and dry fruits, such as that of pines, grasses, sedges, composites, oaks, and palms. Seeds are eaten and crushed, either during mandibulation or during gut passage, so granivores usually do not act as seed dispersers, even though they can act as such if a seed accidentally escapes destruction or attaches to the body of the bird while it feeds (Norconk et al. 1998, Herrera and Pellmyr 2002). Birds in some families of passerines frequently eat seeds, such as waxbills (Estrildidae) (Payne 2010), finches (Fringillidae) (Collar and Newton 2010), and New World sparrows (Emberizidae) (Rising 2011). Many species of macaws, parrots, and parakeets are seed predators, consuming grains and seeds of palms and flesh fruits (Forshaw 1989). Acorn Woodpeckers (Melanerpes formicivorus) feed extensively on the acorns of oaks during fall and winter (MacRoberts 1970).
Nectarivore (NE): nectar of flowers. Hummingbirds (Trochilidae) are the best known nectar-eaters, but several other families of birds have species that consume nectar, such as sunbirds (Nectariniidae), sugarbirds (Promeropidae), honeyeaters (Meliphagidae), and some Hawaiian honeycreepers (Drepanidini) (Stiles 1981, Paton and Collins 2006). Some species of Psittaculidae, such as Purple-crowned Lorikeets (Glossopsitta porphyrocephala), frequently ingest nectar (Forshaw 1989).
Palynivores (PA): pollen. Lorikeets (Psittacidae) are well-known for eating pollen. Some Darwin's finches (Geospiza spp.) also exploit this food item (Grant 1996). Animals that feed on pollen are sometimes referred to as pollinivorous.
Phytoplanktivore (PH): microscopic algae, such as diatoms, and cyanobacteria, obtained by filter-feeding. James Flamingos (Phoenicoparrus jamesi) feed extensively on diatoms (Mascitti 1998), whereas the diet of Lesser Flamingos (Phoeniconaias minor) is largely based on cyanobacteria (Kaggwa et al. 2013).
Radicivores (RA): storage organs of plants, usually in the form of carbohydrates, such as tuberous roots, taproots, corms, stem tubers, rhizomes, and bulbs. Swans (Anatidae) eat potatoes and other tubers (Carboneras 1992). Some species of francolins, pheasants, and junglefowl (Phasianinae) also ingest these food items (McGowan 1994). Animals that exhibit this diet type are sometimes referred to as rhizophagous.
Animal matter
Carnivore (CA): tetrapoda (e.g., amphibians, reptiles, birds, and mammals). Prey must be obtained alive to be considered a carnivore. Eggs of tetrapods (e.g., turtles, snakes, and birds) are also included in this food category because nest predators usually eat eggs and young. Also, eggs are a seasonal food item and no bird has a diet specialized on eggs. Birds that prey on tetrapods include raptors (e.g., eagles, falcons, and owls) (Ferguson-Lees and Christie 2001, König and Weick 2008), many seabirds (e.g., skuas; Reinhardt et al. 2000), and even some passerines (Lopes et al. 2005b). Many species of birds prey on avian eggs, including many passerines, such as crows and jays (Goodwin 1976) and tyrant-flycatchers and tanagers (França et al. 2009). Egyptian Vultures (Neophron percnopterus) that break Ostrich eggs by tossing gravel on them (Thouless et al. 1989) are a remarkable example of an egg predator.
Coprovore (CO): excreta of animals, such as ungulates, cetaceans, seabirds, and ducks. Sheathbills feed on penguin and seal excreta (Burger 1981, Favero 1996). Egyptian Vultures feed on cow dung, and obtains the carotenoid pigments that give the yellow color to their brightly ornamented heads (Negro et al. 2002). Animals that exhibit this diet type are also referred as to coprophagous.
Crustaceovore (CR): non-zooplanktonic crustacea, such as non-larval forms of Mallacostraca (e.g., crabs, lobsters, crayfishes, shrimps, prawns, mantis-shrimps, barnacles, and goose barnacles). Terrestrial crustacea, such as woodlice, are also included here. Crab Plovers (Dromas ardeola) eat crabs as a staple food (Hockey et al. 1996), as do Rufous Crab Hawks (Buteogallus aequinoctialis) (Ferguson-Lees and Christie 2001). White Ibises (Eudocimus albus) frequently prey on crayfish (Dorna et al. 2008).
Insectivore (IN): hexapoda (insects and their kin; e.g., springtails, dragonflies, cockroaches, termites, locusts, true bugs, beetles, flies, butterflies, ants, bees, and wasps), including the benthic aquatic forms (e.g., dragonflies, stoneflies, and mayflies). This category also includes birds that feed on other terrestrial arthropods, such as Chelicerata (e.g., ticks, spiders, and scorpions) and Myriapoda (e.g., centipedes and millipedes). Items included in this food category are probably among the most widespread and common food items of birds, representing the primary food of species in numerous bird families.
Molluscivore (MO): mollusks such as chitons (Polyplacophora), slugs, snails, and limpets (Gastropoda), clams, cockles, oysters, mussels (Bivalvia) and squid, cuttlefish, and octopuses (Cephalopoda). Examples of molluscivores include Snail Kites (Rostrhamus sociabilis), Limpkins (Aramus guarauna) (Reed and Janzen 1999), and Hook-billed Kites (Chondrohierax uncinatus) (Smith and Temple 1982). The primary prey of Eurasian Oystercatchers (Haematopus ostralegus) are mussels and cockles (O'Connor and Brown 1977). Squid are preyed on by many seabirds, such as albatrosses (Diomedeidae) (Croxall and Prince 1996).
Scavenger (SC): carcasses of vertebrates, scraps of food seized from predators, and other dead tissues. New World (Cathartidae) and Old World vultures are well-known for feeding on carcasses of vertebrates (Saran and Purohit 2013). Bearded Vultures (Gypaetus barbatus) are specialized for eating bones and bone marrow (Margalida and Bertran 2001). Many seabirds, such as skuas (Stercorarius spp.), also feed on carcasses of vertebrates, including discarded fish and offal from fishing vessels, galley refuse from ships, and whale blubber. Sheathbills feed on the umbilical cords and placentae of mammals, as well as feather-shafts shed by molting birds (Favero 1996, Saran and Purohit 2013). Crows (Goodwin 1976) and some raptors (Ferguson-Lees and Christie 2001) frequently eat road-killed animals.
Piscivore (PI): all kinds of fish, from the most frequently eaten Actinopterygii (ray-finned fishes) to Myxiniformes (hagfishes), Petromyzontiformes (lampreys), Chondrichthyes (sharks, rays, skates, and chimaeras), and Dipnoi (lungfishes). Fish must be captured alive. Fish eggs are also included here. In addition to several families of seabirds and wading birds, some raptors such as Ospreys (Pandion haliaetus) and Gray-headed Fish Eagles (Ichthyophaga ichthyaetus) feed primarily on fish (Ferguson-Lees and Christie 2001). Many kingfishers (Alcedinidae) are also well-known for their fishing habits (Fry et al. 1992).
Hematophagous (HM): blood of tetrapods, including consumption of damaged tissue. Few species of birds have been recorded exploiting this food item, generally in an opportunistic way. Snowy Sheathbills (Chionis albus) drink blood from the open wounds of seals, Sharp-beaked Ground-finches (Geospiza difficilis septentrionalis) and Galapagos Mockingbirds (Mimus macdonaldi) drink the blood of seabirds and iguanas, and Red-billed Oxpeckers (Buphagus erythrohynchus) drink blood and eat tissues from the wounds of mammals (Weeks 2000, Sazima and Sazima 2010).
Vermivore (VE): all kinds of “worms”, including Platyhelminthes (flatworms), Nemertea (ribbon worms), Annelida (segmented worms, such as sandworms, earthworms, and leeches), Nematoda (nematodes and roundworms), and Priapulida (priapulid worms), among others. Some shorebirds prey largely on polychaetes (Iwamatsu et al. 2007). Many species of temperate songbirds, such as Song Thrushes (Turdus philomelos), prey largely on earthworms during spring and summer (Gruar et al. 2003).
Zooplanktivore (ZO): planktonic animals, such as larvae of glassworms (Chaoborus, Diptera), amphipods (Amphipoda), krill (Euphasiaceae), brine-shrimps (Anostraca), copepods (Copepoda), forams (Foraminifera), and planktonic larval stages of many other animals. Minute benthic larvae of insects, such as those of brine flies (genus Ephydra) and chironomids (Diptera) that are obtained by filter-feeding, were also considered here. Many seabirds, such as penguins (Spheniscidae), albatrosses (Diomedeidae), and prions and petrels (Procellariidae), frequently prey on krill (Croxall et al. 1997). Chilean Flamingos (Phoenicopterus chilensis) feed on copepods, foraminifers, and amphipods (Tobar et al. 2014).
Anthropogenic food
Purgamenovore (PU): all kinds of processed food produced by humans, such as bread, cookies, snacks, popcorns, candies, milk from milk bottles, kitchen refuse, and dog food (Fisher and Hinde 1949, Lepczyk et al. 2012). This category also includes garbage obtained from landfills and similar sites because this is a generic term that encompasses a myriad of organic matter, and identifying what is being eaten can be difficult, if not impossible (Auman et al. 2008, Ottoni et al. 2009). Examples of species that frequently eat items in this category are Black Vultures (Coragyps atratus), Rock Pigeons (Columba livia), House Sparrows (Passer domesticus), and some species of gulls (Laridae). The term purgamenovore is derived from the Latin purgamen, meaning garbage or refuse.
Unusual food items that do not justify the recognition of a distinct food category
Less important food items taken rarely and/or opportunistically include tree bark and cambium, medusa, sea urchins, starfishes, tunicates, and many others that are, to the best of our knowledge, too unusual to be considered in distinct food categories, and should be included in an analysis as “other.” Good examples are sheathbills (Chionis spp.) drinking milk by interposing their bill between a pup's mouth and a female elephant seal's (Mirounga leonine) nipples (Favero 1996), or oxpeckers (Buphagus spp.) ingesting body fluids like mucous from the nose and ear wax of ungulates (Bezuidenhout and Stutterheim 1980).
Non-food material ingested
Material ingested for purposes other than nutrition should not be considered, such as hard materials like grit and gravel used to grind food (Gionfriddo and Best 1999). The same applies to soil consumed by birds, especially parrots and macaws, to detoxify dietary toxins (Diamond et al. 1999, Gilardi et al. 1999, Brightsmith et al. 2008). Consumption of mud by flamingos is a distinct case because they digest organic matter and microscopic organisms in it that can constitute as much as 20% of the dry weight of the mud (del Hoyo 1992). Plastic (Robards et al. 1995) and similar nonfood matter inadvertently ingested should also not be considered when describing bird diets, although the report of ingestion of such items has important conservation implications because it can be a significant source of mortality for birds.
Classification scheme for avian diet types
Our classification scheme considers the number of food categories ingested by a species, as well as the volumetric proportion of the items in those food categories that is ingested. Estimates, when possible, must be the volume of food at the time of ingestion, and not “as found” (Otvos and Stark 1985) to avoid bias caused by different digestion rates of food items ingested (Rosenberg and Cooper 1990).
Bird diets encompass a continuum between some extremes commonly treated as distinct categories, such as insectivores and frugivores. Thus, the dichotomy insectivores/frugivores conveys a false idea of what is usually observed in nature. For example, many species of ovenbirds (Furnariidae) and antbirds (Thamnophilidae) are insectivores (Remsen 2003, Zimmer and Isler 2003), but also feed opportunistically on fleshy fruits (Lopes et al. 2003). Conversely, strict frugivores are rare and, at least during the nestling period, the diet of most frugivores includes insects (Foster 1978, Moermond and Denslow 1985).
Given that the main objective of a classification of diet types is to provide a synthesis of the diet of one species, and not to describe all food categories ingested, we will only consider those food categories that represent 10% or more of the diet volume of a species. Food categories rarely used (i.e., low volume) will be considered of minor importance in the diet of a species (for an exception to this rule, see the classification of omnivore below). This will avoid inflating the importance of rarely consumed resources. Thus, a species with a diet composed predominantly by one food category and with the other remaining categories representing less than 10% of the diet by volume (but the sum of these less important food categories does not sum more than 35%) will be classified in the diet type designated by the main food category ingested. For example, if 88% of the diet of a species is composed by food category A, with the remaining volume represented by 7% of B, and 5% of C, this species will be classified as A (with A in capital letters). Fig. 1 demonstrates the diet types of five hypothetical species with differential consumption of fruits and insects.
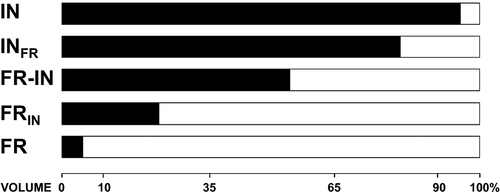
If two food categories are frequently consumed by one species (i.e., 10% of the diet volume or more), then its diet will be classified by taking into consideration the proportion of each category consumed. If the second-most representative food category consumed represents between 10 and 35% of diet volume, then this species will receive a second letter in its diet type name. This letter will be subscripted to indicate the lesser importance of this food category. For example, if the diet of one species is composed by 70% of A, 26% of B, and 4% of C, this species will be classified as AB (in capital letters), signifying that it feeds primarily on food category A and secondarily on food category B.
If the two main food categories have similar relative importance in the diet (i.e., the second-most important category represents a percent volume of more than 35%), then the diet type of this species will be categorized as A–B. The designation should always be in alphabetical order to avoid inconsistency in classification among studies, and should not reflect the marginal differences in the percentage of consumption of each food category. An example of a species classified with diet type A-B would be one with a diet consisting of 38% of A, 58% of B, and 4% of C.
The break points adopted here are arbitrary, and were based on our previous experience with studies of bird diets (Marini 1992, Marini and Cavalcanti 1998, Lopes et al. 2003, 2005a,b, Durães and Marini 2005, Ballarini et al. 2013). Break points are necessary if we want to classify variables that lie along a numerical continuum into discrete categories. Categorization of continuous data/patterns is a common way to simplify complex data, and can improve communication and our understanding of complex phenomena. For example, division of human development across lifespan in infancy, childhood, adolescence and adulthood, although based on boundaries that are arbitrarily drawn, are useful. Therefore, despite obvious drawbacks, a categorical classification of diet types can be useful, and community, guild, and functional group analyses will continue to classify birds this way.
The classification scheme we propose allows recognition of several diet types generally not recognized, such as granivore-insectivore (GR-IN) and frugivore-insectivore (FR-IN). This will avoid common inconsistencies such as the classification of birds with a mixed diet of fleshy fruits and insects (e.g., Tachyphonus) as omnivores (Willis 1979), and of species with a mixed diet of seeds and insects (e.g., Coryphospingus) as granivores (Willis 1979, Poulin et al. 1994). Although more than 1000 diet types can be recognized based on the scheme proposed here, most possible combinations, although theoretically possible, will seldom if ever, occur in nature, such as granivore-piscivore (GR-PI).
In many cases, two food categories will not suffice to accurately describe the diet of a bird species. Birds with diverse diets are often said to be “omnivores”, but we do not think that this is an accurate term to describe, for example, the diet of a bird that is largely based on vegetable matter, such as algae, tubers, and leaves, seldom ingesting animal matter (e.g., some geese), or to describe the diet of a bird that is based largely on animal matter, such as squid, fish, and krill (e.g., some seabirds). We think so because the term omnivore refers to a “heterotroph that feeds on both plants and animals, and thus operates at a range of trophic levels” (Allaby 2010). The solution we propose is to recognize three diet types that encompass diverse food categories: (1) Herbivores (HE) that feed largely on vegetable matter, (2) Faunivores (FA) that feed largely on animal matter, and (3) Omnivores (OM) that feed extensively on both plant and animal matter.
A bird will fit one of these three categories if it (1) ingests at least three food categories with a percentage of more than 10% each, or (2) the sum of the percentage of consumption of all but the main food categories is higher than 25%, but none is higher than 10%. In those cases, if the food categories used by the bird consist of at least 90% vegetable matter, the bird will be classified as an Herbivore. If those food categories consist of at least 90% animal matter, then the bird will be classified as a Faunivore. If those categories include significant portions (i.e., >10% each) of both animal and vegetable matter, the bird will be classified as an Omnivore. No bird species, to the best of our knowledge, is so generalized and opportunistic that each food category constitutes <10% of the diet.
Limitations and approaches to mitigate them
Which approach to avian diet analysis should be used?
Data collected using different methods can be used to classify the diet of a species, and a review of several methods were presented by Rosenberg and Cooper (1990) and Barrett et al. (2007). However, analysis of stomach contents (a term generically used to refer to material found in the crop and gizzard) is the main source of information about bird diets (Rosenberg and Cooper 1990). As a result, our proposed classification scheme is largely based on this method. Nevertheless, stomach contents analysis has some limitations due to differences in prey digestibility (e.g., soft-bodied animals such as earthworms become amorphous) and because liquids such as sap and nectar are difficult, if not impossible, to detect (Rosenberg and Cooper 1990, Witmer 1996). Therefore, understanding that every method has advantages and disadvantages is important and, in some cases, investigators may be forced to choose an alternative method of diet quantification better suited for their focal species (e.g., visual observation of food taken or fecal analysis). The important thing is that alternative methods should be transparent and repeatable, regardless of the type of data being analyzed.
What is being eaten?
Determining the food items being eaten is not always easy. For example, separating frugivory from granivory can sometimes be difficult because many birds that eat fleshy fruits also digest the seeds, such as House Finches (Carpodacus mexicanus; Valiente-Banuet and Rojas-Martinez 2002). Parrots often ingest the seeds of fleshy fruits, acting as seed predators (Forshaw 1989). Maui Parrotbills (Pseudonestor xanthophrys) excavate insect larvae from fruits (Mountainspring 1987). A bird visiting a flower, for example, may not be obtaining nectar, but pollen or insects too small to be seen.
Why and how to estimate volumetric proportion?
The advantage of using a volumetric method is it allows all food categories to be considered, including those that cannot be enumerated individually, such as fruit pulp and fragments of grass leaves (Hartley 1948, Rosenberg and Cooper 1990). A detailed review of the methods available is beyond the scope of this paper, but a wide range of methods have been used for estimating volumetric proportions of food categories ingested (Hartley 1948, Hellawell and Abel 1971, Hyslop 1980). No single technique is likely best-suited for all bird species, and investigators will need to choose the most appropriate technique for their particular focal species.
Diet variability
The diet of a bird species can vary in many ways (e.g., age, sex, season, and geography), and biased samples will result in biased classifications. Ontogenetic variation in diet is common, especially among nectarivores, frugivores, and granivores where young are largely fed insects and other protein-rich foods (Foster 1978, Poulin et al. 1992). Cedar Waxwings (Bombycilla cedrorum) feed largely on fruits during winter, but, during spring when fruits are scarce, flowers are a large part of their diet (Witmer 1996). Insects are often the main food of shrikes (Laniidae), but species that breed at higher latitudes may rely on small birds and mammals during winter (Yosef 2008). Geographic variation in diet also occurs, as demonstrated by Rockhopper Penguins (Eudyptes chrysocome) (Tremblay and Cherel 2003).
Investigators interested in classifying the type of diet of a bird species as a whole will need samples from all seasons in equal proportions and across the entire range of the species (preferentially proportional to the abundance of the species across its geographical range) to avoid bias. In addition, diets can also be classified based on age-related, sexual, seasonal, and/or geographic variation, e.g., young are insectivores and adults are frugivores, insectivores on breeding grounds and frugivores on wintering grounds, and so on.
Anthropogenic interference
Humans are responsible for large-scale modification of bird habitats than can have important effects on the availability of food resources. For example, distinct types of land use in farmlands result in distinct diets of Skylarks (Alauda arvensis) (Donald et al. 2001). Fishery waste supports millions of seabirds annually (Garthe et al. 1996). Garbage is an important subsidy for gulls in some areas (Weiser and Powell 2010). Bird feeders also support populations of urban and suburban bird populations (Brittingham and Temple 1992, Fuller et al. 2008), especially during temperate winters. Therefore, samples of birds from human-impacted habitats should be avoided if investigators want to describe the natural diet of a species.
How many samples are necessary?
The number of samples needed to reliably classify the diet of a species is often difficult to determine, but depends largely on the variability of the diet. Rosenberg and Cooper (1990) and Durães and Marini (2005) suggested that 10 or fewer stomach contents are normally adequate to describe the diet of a species at a specific site during a specific sampling period. Nevertheless, the diet of a species is, as discussed above, the result of a complex set of factors (Wheelwright 1986). Therefore, a considerably larger sample may be needed to describe the diet of a species throughout the year and throughout its range.
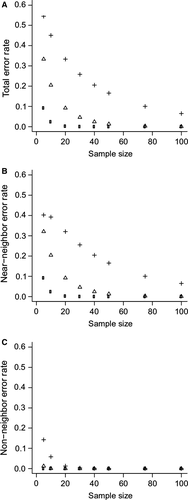
We simulated mean proportions of stomach content between two food categories to understand the effect of standard deviation among avian stomach contents and sample size on the error rate in categorization among different diet types. These simulations could be extended to all sorts of variations, but is beyond the main scope of this manuscript. We transformed proportion data of stomach contents with a logistic transformation of the odds ratio (ln[p]/ln[1-p]) to better conform to a normal distribution. We simulated mean values at the center of the ranges of stomach-content proportions for each dietary category. For example, for a species with a specialist diet that on average consumes between 90% and 100% of a single food category, we simulated a mean value of ln(0.95)/ln(0.05). We generated 100,000 means for all five diet types that exist with two different food categories. We performed individual simulations of means derived across eight sample size levels (N = 5, 10, 20, 30, 40, 50, 75, and 100) and three levels of standard deviation (SD = 1, 2, and 4). To describe the influence of sample size and standard deviation of the sampled population on error rate, we calculated an average proportion of instances across the five dietary categories where a sampled mean resulted in a misdiagnosis of the diet of that simulated distribution. We divided this error rate into two components. The first measured the proportion of means that occurred within the bounds of a neighboring diet type along the continuum (i.e., placing an IN in INFR). The second measured the proportion of means that occurred within the bounds of a non-neighboring diet type along the continuum (i.e., placing IN in IN-FR). These non-neighbor errors are considered to be more severe than near-neighbor errors.
Our simulations revealed three important patterns (Fig. 2): (1) error increases with the standard deviation of the proportion of stomach contents of a sampled population, (2) error decreases with increasing sample size, and (3) non-neighbor error rate is generally low (<0.05), even for birds with highly variable diets (SD = 4) and a relatively small sample size (~20 samples). These simulations suggest that developing a rule-of-thumb for the number of stomach samples needed to accurately classify the diet of a species is difficult because it depends on the standard deviation and overall level of specialization of the species. However, in best case scenarios (SD ~ 1), at least 10 stomachs may be needed to yield an accuracy of 0.95 or greater. More realistic cases (SD ~ 2) may require samples of >30. If a species has an extremely variable diet (SD ~ 4), very large sample sizes will be required to properly classify its diet.
Conclusions
We believe that the classification scheme proposed here can be useful for all extant species of birds, and provides the organizational rules and standardized terminology for rapid and effective exchange of information. Nevertheless, we regard this as only a first step and anticipate that tests to the scheme will probably generate modifications to improve it.
Application of the method we propose is simple, but its immediate application is hampered by a lack of good quality data for many species of birds, especially in tropical regions. What is needed, therefore, are more detailed natural history studies like those conducted at higher latitudes in the Northern Hemisphere many decades ago. For most species of birds in North America, additional samples will not be needed to apply our classification scheme. For example, Wheelwright (1986) studied the diet of American Robins (Turdus migratorius) by examining over 1900 stomach contents records. Those stomach contents were analyzed by researchers of the U.S. Biological Survey and the U.S. Fish and Wildlife Service and are currently filed on index cards at the Patuxent Wildlife Research Center in Laurel, Maryland. This is possibly the most detailed database on avian food habits in the world, with over 250,000 records (Wheelwright 1986), allowing a standard classification for most bird species in North America.
In the tropics, where avian diversity is much higher and data scarcer, many new samples are needed, and can be obtained using non-lethal methods (Rosenberg and Cooper 1990, Valera et al. 1997, Durães and Marini 2003, Diamond et al. 2007, Ceresa et al. 2014). In addition, however, for many species, many samples are already available in natural history museums and just need to be analyzed. These samples are from specimens collected and prepared as study skins and where the carcasses or stomach/crop contents were preserved in alcohol, representing important ancillary material that has only received due attention in the last few decades (Remsen 1995). We just need quantitative analyses of these samples (with a volumetric estimate of each food category).
A final recommendation is that authors using the classification system proposed here should not only classify species by diet type, but should also provide the means and standard deviations of each food category ingested. This will allow other researchers to use statistical techniques that can summarize multivariate data when this kind of analysis is more appropriate.
Acknowledgments
LEL received research fellowships from FUNARBE (FUNARPEX 2010 and 2011, FUNARPEQ 2012) and CNPq (305401/20149). MÂM received researcher fellowships from CNPq. Previous versions of this paper benefited from comments of J. V. Remsen, Jr., and D. Kissling.




