Effects of a dual-filter-based cerebral embolic protection device in transcatheter aortic valve replacement on cerebral oxygen saturation: A prospective pilot study
This is a prospective, pilot sub-analysis of the ongoing randomized PROTECT TAVI trial (Prospective Randomized Outcome Study in TAVI Patients Undergoing Periprocedural Embolic Cerebral Protection With the Claret Sentinel™ Device) (clinicaltrials.gov NCT02895737328; release date 09/06/2020). This pilot investigation complied with the Declaration of Helsinki and was approved by the local ethics committee of the Technical University of Munich (approval reference number: 290/16s).
Abstract
Purpose
The Sentinel Cerebral Protection System (Sentinel-CPS) is increasingly used in transcatheter aortic valve replacement (TAVR). However, the impact of inserting the Sentinel-CPS inside the brain-supplying arteries on cerebral perfusion and oxygenation is unknown.
Methods
Twenty patients undergoing transfemoral TAVR with (n = 10) and without (n = 10) cerebral embolic protection using the Sentinel-CPS were prospectively observed. All patients received conscious sedation and cerebral oxygen saturation (rSO2) was continuously measured with near-infrared spectroscopy (NIRS). The cumulative perioperative cerebral desaturation was calculated for each patient by multiplying rSO2 below an individualized desaturation threshold by time. In addition, rSO2 values at the time of Sentinel-CPS insertion, filter positioning, and device retraction were analyzed.
Results
There was no significant difference in cumulative cerebral desaturation in patients with Sentinel-CPS (median [IQR]) (0 [0/81] s%) and without (median [IQR]) (0 [0/23] s%), p = .762. A total of 6 patients (33.3%) experienced a perioperative decrease in rSO2 below the individualized desaturation threshold (n = 3 with Sentinel-CPS, n = 3 without Sentinel-CPS; p = 1.000). Cerebral desaturation was detected during valve deployment (n = 5) and after postdilatation (n = 1). No desaturation events occurred during Sentinel-CPS insertion, filter positioning, or retraction.
Conclusion
Our pilot study revealed no difference in cumulative perioperative cerebral desaturation between TAVR with and without Sentinel-CPS. Catheter- and filter-based manipulations in the brain-supplying arteries for Sentinel-CPS application were not associated with a decrease of cerebral perfusion and oxygenation.
1 INTRODUCTION
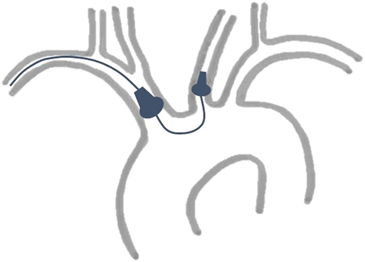
Transcatheter aortic valve replacement (TAVR) has emerged as an important treatment option in patients with aortic stenosis.1, 2 However, cerebrovascular events remain a major issue with recent data demonstrating stroke rates of up to 9.1% at 30 days.3 Dedicated mechanical devices are increasingly used to reduce cerebral embolization during TAVR such as the FDA-approved dual-filter-based Sentinel Cerebral Protection System (Sentinel-CPS) (Boston Scientific, Marlborough, MA, USA). The Sentinel-CPS is percutaneously advanced through the right radial artery, with one filter being delivered to the brachiocephalic artery and the second one to the left common carotid artery. Although recent data demonstrated an effective debris capture in 86% to 99% of patients receiving embolic protection with the Sentinel-CPS,3-6 the number of new ischemic cerebral lesions seems statistically unaffected as assessed by magnetic resonance imaging.3, 4, 7 Intravascular device manipulation might be considered as a possible mechanism of cerebral injury even though there were no safety concerns in the randomized trials. A way of intraoperative neuromonitoring, which is frequently used in cardiac and ascending or aortic arch surgery, is cerebral oximetry measured by near-infrared spectroscopy (NIRS).8, 9 NIRS oximetry noninvasively measures cerebral oxygenation (rSO2) to detect cerebral hypoperfusion or ischemia in real-time.9 Previous neurointerventional procedures, such as filter-based carotid artery stenting, demonstrated mechanically induced fluctuations in cerebral oxygenation.10-12 To date, the effects of placing the Sentinel-CPS inside the brain-supplying arteries on cerebral blood flow and oxygenation are unknown. We, therefore, sought to determine the influence of Sentinel-CPS application on cerebral oxygen saturation by using NIRS in patients undergoing transfemoral TAVR.
2 METHODS
We conducted a prospective pilot investigation between May 2018 and August 2018 at the German Heart Centre Munich as part of our ongoing single-center, randomized PROTECT TAVI trial (clinicaltrials.gov NCT02895737328). Patients were assigned to undergo transfemoral TAVR with and without Sentinel-CPS usage and received continuous NIRS oximetry intraoperatively. The objective of this pilot investigation was to determine the potential magnitude and duration of rSO2 changes during Sentinel-CPS associated intravascular manipulations, comparing cerebral oxygen saturation in TAVR with and without Sentinel-CPS application.
Inclusion criteria involved the presence of aortic stenosis eligible for TAVR, appropriate vessel diameters within the filter-landing zones (brachiocephalic artery: 9-15 mm; left common carotid artery: 6.5–10 mm), and freedom of significant stenosis, calcifications, dissections, or aneurysmatic alterations of the brachiocephalic and/or left common carotid artery as well as the absence of a true bovine arch for Sentinel-CPS application. The decision for TAVR was confirmed by the local multidisciplinary heart team comprising interventional cardiologists and cardiac surgeons. Preprocedural multislice computed tomography was used to confirm the eligibility for the Sentinel-CPS according to the official instructions for use. The pilot study complied with the Declaration of Helsinki and was approved by the local ethics committee of the Technical University of Munich (approval reference number: 290/16s). Written informed consent was obtained from the patients before enrollment.
The Sentinel-CPS consists of a six French-compatible steerable catheter (100 cm) carrying two cone-shaped, biocompatible polyurethane filters equipped with 140-µm pores to capture and remove potential debris during TAVR (Figure 1).13 The system is inserted through the right radial or brachial artery with one filter being targeted to the brachiocephalic (proximal target vessel) and the other to the left common carotid artery (distal target vessel; Figure 2). The device received its European CE mark in 2014 and is currently available in one standard size.
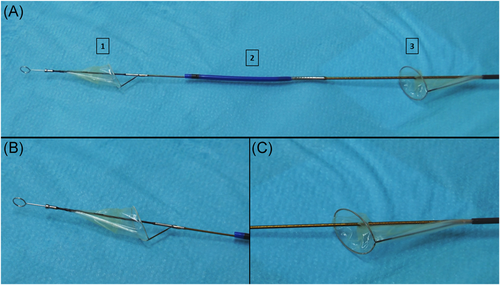
Cardiac anesthesiologists, heart surgeons, and interventional cardiologists experienced in transfemoral TAVR procedure with Sentinel-CPS usage were assigned to these implantations. Transfemoral TAVR with and without Sentinel-CPS application was performed in all patients under conscious sedation. Moderate sedation, as defined by the American Society of Anesthesiologists,14 was chosen as the targeted depth of sedation as described in detail before.15, 16 Depth of sedation was clinically assessed by the responsible anesthesiologist. Patients should feel comfortable and be arousable at any time. To achieve maximum oxygen supply, patients received 4 L/min of oxygen. In addition to standard perioperative monitoring,17 arterial and central venous pressure was monitored invasively. A temporary 5-Fr pacemaker wire was inserted through the internal jugular vein. Periprocedural hypotension (MAP < 65 mmHg) was treated with a single, 5 µg IV norepinephrine dose. A continuous norepinephrine infusion was started in case more than five bolus administrations were needed. Epinephrine was given in a similar fashion for inotropic and chronotropic support. In the case of Sentinel-CPS application, a 6-Fr sheath was placed in the right radial artery and anticoagulation with heparin was initiated (activated clotting time of 250 s). The system was delivered over a standard 0.014-coronary guidewire and advanced to the brachiocephalic trunk. Guided by the radiopaque markers, the proximal filter was deployed. Using the integrated articulation sheath, the distal part of the system was flexed toward the ostium of the left common carotid artery, where the distal filter was deployed.18 Then, standardized transfemoral TAVR was performed by our heart team. After finishing TAVR, both filters were retrieved and the Sentinel-CPS removed.
- 1.
rSO2 at baseline (rSO2-baseline) recorded upon arrival in the operating ward and before sedation and oxygen supplementation.
- 2.
rSO2 after induction of sedation (rSO2-postinduction), defined as the rSO2 value under oxygenation, 20 min after the start of sedation.
- 3.
rSO2 during Sentinel-CPS Implantation (rSO2-CPS-implantation), defined as the lowest rSO2 value during device insertion, positioning and correct filter deployment within the brachiocephalic and left common carotid arteries.
- 4.
rSO2 during valve deployment (rSO2-valve deployment), defined as the minimal rSO2 value during aortic valve implantation.
- 5.
rSO2 during postdilatation (rSO2-postdilatation), defined as the minimal rSO2 value during balloon aortic valvuloplasty following valve implantation, if necessary.
- 6.
rSO2 during Sentinel-CPS retraction (rSO2-CPS-retraction), defined as the lowest rSO2 value while retrieving both filters and removing the Sentinel-CPS device.
- 7.
rSO2 after skin closure (rSO2-skin closure), defined as the minimal rSO2 at the end of the entire procedure.
NIRS parameters were downloaded from the Masimo Root device using the Masimo Instrument Configuration Tool (MICT software) (Irvine, CA, USA).
Sentinel-CPS-related manipulation may affect cerebral perfusion and oxygenation and initiate a decrease in rSO2 as demonstrated in other neuro-interventional procedures.10-12 In accordance with previous data,15, 19 an individual desaturation threshold (rSO2-DESAT) was determined for each patient, defined as a decrease of 20% below the rSO2-baseline or an absolute value below 50%. The highest value served as threshold. In the case of rSO2 differences between both hemispheres (rSO2-Δinterhem), the lower value was used. To assess the extent of regional desaturation, an area-under-the-curve analysis was performed: rSO2-drop-Score (%sec) = [rSO2-DESAT (%)−rSO2-current (%)] × time (s). rSO2-drop-scores potentially related to Sentinel-CPS insertion, device positioning, filter deployment, and device retraction were calculated. The cumulative rSO2drop-Score (Σ_rSO2drop-score) defined as the summation of all rSO2-drop-scores during the entire surgical intervention, was generated to compare rSO2 data between TAVR patients with and without Sentinel-CPS application.
Statistical analysis was performed by using IBM SPSS Statistics 25.0 software (IBM Corp). Frequencies are given as absolute numbers and percentages. Comparison of categorical variables was calculated using Fisher's exact test. Continuous data are presented as the median and interquartile range (IQR=25th–75th percentile). Group comparisons were performed using the Mann–Whitney U test. For the statistical analysis, a two-sided 5% significance level was chosen.
3 RESULTS
In this pilot study, a total of 20 consecutive patients underwent transfemoral TAVR with (n = 10) and without Sentinel-CPS (n = 10). Two patients initially assigned to undergo TAVR with neuroprotection were excluded from the analysis. In one patient, Sentinel-CPS insertion was impossible due to a significant tortuosity of the right radial artery. In the other patient, an initially correct deployed filter dislocated and additional attempts to reposition the filter failed. Therefore, the study cohort included 18 patients with a median age of 81.5 (78.0/86.0) years. The median logistic EuroSCORE and Society of Thoracic Surgeons Predicted Risk of Mortality Score were 13.7 (7.0/20.6) and 2.3 (1.8/4.2), respectively. Further patient demographics at baseline are provided in Table 1. They were statistically similar. In total, 10 balloon-expandable Edwards Sapien 3 and 8 self-expandable Medtronic CoreValve Evolut R (Medtronic, Dublin, Ireland) valves were implanted. Balloon aortic valvuloplasty before valve deployment and after valve implantation was performed in 1 and 5 patients, respectively (Table 2). All TAVR procedures were uneventful with no complications leading to sudden hemodynamic instability and there was no unplanned, emergency conversion to general anesthesia.
| Patient characteristics | TAVR with Sentinel-CPS (n = 8) | TAVR without Sentinel-CPS (n = 10) | p value |
|---|---|---|---|
| Age (year) | 81.0 (80.3/82.8) | 84.5 (75.0/87.8) | .696 |
| Female, n (%) | 3 (37.5) | 5 (50.0) | .664 |
| Body mass index (kg/m2) | 24.6 (23.6/26.4) | 23.1 (21.0/29.1) | .408 |
| Left ventricular ejection fraction (%) | 59.0 (46.3/64.5) | 53.5 (38.5/65.5) | .696 |
| Left ventricular ejection fraction ≤50%, n (%) | 3 (37.5) | 5 (50.0) | .664 |
| Glomerular filtration rate (ml/min) | 66.5 (50.3/80.3) | 70.0 (59.8/76.8) | .762 |
| Chronic obstructive pulmonary disease, n (%) | 0 (0.0) | 1 (10.0) | 1.000 |
| Peripheral artery disease, n (%) | 4 (50.0) | 1 (10.0) | .118 |
| Systolic pulmonary arterial pressure >60 mmHg, n (%) | 1 (12.5) | 4 (40.0) | .294 |
| History of stroke, n (%) | 0 (0.0) | 0 (0.0) | – |
| History of cardiac surgery, n (%) | 0 (0.0) | 0 (0.0) | – |
| EuroSCORE I (log) (%) | 13.6 (7.0/17.3) | 13.8 (4.6/28.5) | .829 |
| EuroSCORE II (%) | 2.6 (1.8/6.7) | 3.4 (1.5/5.0) | .762 |
| Society of thoracic surgeons predicted risk of mortality (%) | 2.3 (1.9/4.2) | 2.7 (1.7/4.3) | 1.000 |
- Note: Patient data are presented as median and 25th–75th percentile unless otherwise indicated.
- Abbreviations: TAVR, transcatheter aortic valve replacement; Sentinel-CPS, Sentinel Cerebral Protection System.
| Patient characteristics | TAVR with Sentinel-CPS (n = 8) | TAVR without Sentinel-CPS (n = 10) | p value |
|---|---|---|---|
| Total procedure time (min) | 67.5 (50.3/73.8) | 51.5 (41.5/59.3) | .122 |
| Balloon-expandable valve, n (%) | 5 (62.5) | 5 (50.0) | .664 |
| Self-expandable valve, n (%) | 3 (37.5) | 5 (50.0) | .664 |
| Predilatation, n (%)a | 0 (0.0) | 1 (10.0) | 1.000 |
| Postdilatation, n (%)b | 2 (25.0) | 3 (30.0) | .588 |
- a Balloon aortic valvuloplasty before valve implantation was only performed in one patient receiving an Edwards Sapien 3 valve due to a heavily calcified aortic stenosis.
- b Postdilatation was necessary for five patients (n = 4 Medtronic CoreValve Evolut; n = 1 Edwards Sapien 3). Patient data are presented as median and 25–75th percentile unless otherwise indicated.
- Abbreviations: TAVR, transcatheter aortic valve replacement; Sentinel-CPS, Sentinel Cerebral Protection System.
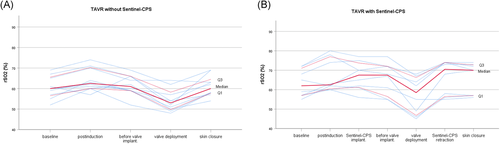
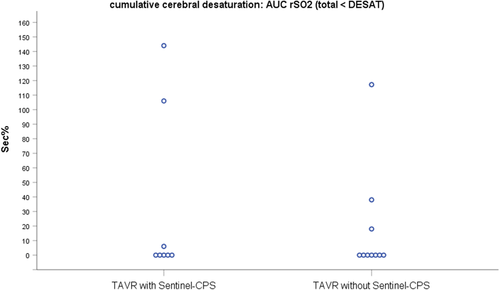
Cerebral oxygenation values at distinctive time points were comparable between both groups (Table 3). None of the eight patients receiving Sentinel-CPS experienced a decline in rSO2 during device implantation (median rSO2-CPS-implantation 67.5% [61.3/73.8]) compared to postinduction values (median rSO2-postinduction 62.5% [60.5/77.0]). Also, no decrease in rSO2 could be observed when both filters were retrieved and the Sentinel-CPS was removed (median rSO2-CPS-retraction 70.5% [56.5/74.0]). All patients (n = 18) showed a drop in rSO2 during valve deployment and balloon aortic valvuloplasty (Table 3, Figure 3). Six (33.3%) patients demonstrated a perioperative decrease in rSO2 below the individualized desaturation threshold (n = 3 with Sentinel-CPS, n = 3 without Sentinel-CPS; p = 1.000). rSO2 desaturation was more frequent in patients receiving a balloon-expandable Edwards Sapien 3 (n = 5) than a self-expandable CoreValve Evolut R valve (n = 1). Maximal calculated desaturation was 144 s% and 117 s% in TAVR with and without Sentinel-CPS, respectively (Figure 4). There was no significant difference in cumulative cerebral desaturation in patients with Sentinel-CPS (median [IQR]) (0[0/81] s%) and without Sentinel-CPS (median [IQR]) (0[0/23] s%) (p = .762).
| Patient characteristics | TAVR with Sentinel-CPS (n = 8) | TAVR without Sentinel-CPS (n = 10) | p value |
|---|---|---|---|
| rSO2-baseline (%) | 62.0 (57.0/71.0) | 60.0 (56.5/65.5) | .573 |
| rSO2-Δinterhem (%) | 3.2 (1.4/4.4) | 3.8 (2.4/8.9) | .360 |
| rSO2-postinduction (%) | 62.5 (60.5/77.0) | 62.5 (60.0/70.3) | .515 |
| rSO2-CPS-implantation (%) | 67.5 (61.3/73.8) | NA | – |
| rSO2-valve deployment (%) | 58.5 (46.8/66.3) | 53.0 (49.8/58.3) | .573 |
| rSO2-postdilatation (%) | 56.5 (52.0/61.0) | 53.0 (46.1/61.0) | .800 |
| rSO2-CPS-retraction (%) | 70.5 (56.5/74.0) | NA | – |
| rSO2-skin closure (%) | 70.0 (57.0/72.8) | 60.0 (57.8/64.5) | .237 |
- Note: Patient data are presented as median and 25–75th percentile unless otherwise indicated.
- Abbreviations: TAVR, transcatheter aortic valve replacement; Sentinel-CPS, Sentinel Cerebral Protection System; rSO2, cerebral saturation.
4 DISCUSSION
Brain protection for stroke prophylaxis during transcatheter aortic valve replacement using mechanical protection devices has become increasingly important in recent years. Randomized studies investigating the FDA approved dual-filter-based Sentinel-CPS have demonstrated a high safety profile with effective debris capture in nearly all patients (86%–99%).3-6, 20 But, even with the use of the protection device, the rate of new ischemic cerebral lesions could not be reduced as assessed by magnetic resonance imaging.3, 4, 7 This could be partly attributed to intravascular device manipulation itself. Catheter tracking and device positioning may dislodge atheromatous debris within the aortic arch and the proximal brain-supplying filter-target vessels (brachiocephalic trunk, left common carotid artery) or cause arterial injury (e.g., dissection, vasospasm)21-24 potentially affecting cerebral tissue perfusion at the microvascular level due to embolization or hypoperfusion.25 Cerebral oximetry, based on NIRS measurements, has emerged as a valuable noninvasive neuromonitoring tool to assess cerebral tissue oxygen saturation in real-time and is routinely used in cardiovascular interventions.8, 9, 26 The potential magnitude and duration of rSO2 changes during device associated intravascular manipulations in patients undergoing TAVR are unclear. To our knowledge, this is the first report investigating the use of NIRS oximetry during filter-protected TAVR in an observational pilot study.
Based on continuous NIRS measurements, our pilot series demonstrated that utilization of the Sentinel-CPS caused no cerebral desaturation during TAVR. In line with previous studies,15, 27, 28 balloon aortic valvuloplasty and valve deployment were associated with drops in rSO2, with six patients showing a transient decrease below the individualized desaturation threshold. We initially hypothesized that Sentinel-CPS-induced manipulation might cause fluctuations in cerebral oxygenation as previously described in other neuro-interventional procedures such as filter-based carotid artery stenting.10-12 However, no impairment in rSO2 could be detected by NIRS measurement during protection device insertion, filter deployment, and device retraction. The incidence of mechanically induced complications like, for example, vasospasm has been reported frequently in neuroendovascular treatment and was found to be as high as 40%.11, 25, 29, 30 Although often self-limiting, episodes of catheter-, guidewire-, or filter-induced vasospasm can cause a restriction of cerebral blood flow and lead to a reduction of ipsilateral NIRS signal, demonstrating real-time detection of cerebral ischemia.10, 11, 25 This complication is more likely to occur in patients having severe arteriosclerosis or extremely tortuous vessels, aggravating the endoluminal surface irritation and potential damage by the wires or filters.10, 11 That might be one reason for the successful application of Sentinel-CPS and the absence of detectable desaturation events related to device manipulations in our cohort, as we only enrolled highly selected patients with the absence of significant calcifications and extensive vascular tortuosity. Another important issue associated with the usage of filter-based protection devices is the occurrence of slow flow situations (7.2%)29 which might be heralded by changes in NIRS oximetry. Neuromonitoring studies suggested that middle cerebral artery flow is reduced by 10%–30% when a filter is deployed due to filter-induced flow resistance.31 Furthermore, slow or no flow can develop due to partial or complete occlusion of the filter pores caused by captured debris or thrombotic material like blood clots.32, 33 Therefore, the manufacturer's instructions clearly stipulate that the indwell time of the Sentinel-CPS is not to exceed 90 min. No slow flow events were detected by NIRS measurement in our patient cohort while filter application. The median total operation time in our patients, who received a filter-based TAVR procedure was 67.5 min, thus perhaps preventing partial or complete filter occlusion which would result in rSO2 fluctuations.
On the other hand, one might debate that device-associated fluctuations in cerebral oxygenation may go unnoticed due to the fact that NIRS only monitors parts of the frontal lobe (anterior circulation), concealing large territories of the brain from forehead sensors. But especially in neurointerventional procedures, for example, carotid artery interventions or ascending aorta surgery including the supraaortic vessels, the anterior circulation is of particular importance and thus NIRS is officially recommended as a reliable intraoperative neuromonitoring tool for these treatments.34 Also, the Sentinel-CPS application in TAVR corresponds to such a setting, as the filters are placed inside the brachiocephalic and left common carotid artery. However, mechanically- or slow flow–induced perfusion disturbances concerning the right vertebral artery might have been undetected in our patients.
Another issue in the past was that several threshold values of rSO2 have been used to define so-called significant events using NIRS.19, 35, 36 In our study an individual desaturation threshold was determined for each patient, defined as a decrease of 20% below the rSO2- baseline or an absolute value below 50%, whichever was higher. Baseline rSO2 values in our patients were within the range of an accepted normal of 55%–78% in this special TAVR population and comparable with other trials.15, 37 Our chosen threshold, therefore, complies with the current consensus and has been investigated in major randomized controlled trials of rSO2-guided procedures.15, 19, 35 However, despite such restrictions, NIRS measurement is at the moment the best available noninvasive neuromonitoring technique to control both, cerebral perfusion and oxygenation.15 Standardized NIRS oximetry, as performed in our patients, might therefore be a valuable tool to monitor rSO2 in neurointerventional settings like filter-based TAVR to detect otherwise clinically silent brain damage.
Due to the observational character of this pilot investigation, only a small sample size was empirically preestablished, which might limit the results of this study. In addition, patients were randomly assigned to undergo transfemoral TAVR with either a balloon-expandable valve or a self-expandable valve due to the predetermined nature of the ongoing PROTECT TAVI trial. Therefore, a difference in cerebral perfusion indicated by NIRS due to different implantation procedures was expected. However, this was not the focus of the study and had no effect on Sentinel-CPS application and its influence on NIRS oximetry. Furthermore, our results are based on a single specific cerebral embolic protection device and are not generalizable to other available systems. Based on these limitations, our results should be considered as hypotheses generating which need to be confirmed in larger interventional trials.
In conclusion, the TAVR procedure, in general, was associated with episodes of intraprocedural cerebral desaturation, without showing significant differences between patients with and without Sentinel-CPS. The application of the filter-based system was not accompanied by a reduction of rSO2, suggesting that mechanically induced manipulations in the brain-supplying arteries did not impair cerebral oxygenation and microcirculation status, which in turn underlines its safety profile. Due to the increasing importance of cerebral protection in transcatheter valve procedures, further large studies are warranted to confirm these initial findings. In addition, more investigations and randomized trials are required to determine the relation between NIRS oximetry and clinical as well as neuroradiological and neurocognitive evaluations.
CONFLICTS OF INTEREST
S.B. receives speaker fees from Medtronic and Boston Scientific, M.E. receives speaker fees from Medtronic. The other authors have no relevant financial or nonfinancial interests to disclose.
AUTHOR CONTRIBUTIONS
S.V. Conceptualized, methodized, validated, formally analyzed, and wrote the original draft. A.E. methodized, validated, formally analyzed, wrote, reviewed, and edited the original manuscript. M.E. reviewed, and edited the manuscript. H.R. reviewed and edited the manuscript. K.S. reviewed and edited the manuscript. S.B. reviewed and edited the manuscript. B.V. reviewed and edited the manuscript. P.T.-P. reviewed and edited the manuscript. N.P.M. methodized, validated, formally analyzed, reviewed, and edited the manuscript.
Open Research
DATA AVAILABILITY STATEMENT
All relevant data generated or analyzed during this study are included in this published article.




