Contemporary management and outcomes of acute type A aortic dissection: An analysis of the STS adult cardiac surgery database
Abstract
Purpose
Management of acute type A aortic dissection (AAAD) is challenging and operative strategies are varied. We used the STS Adult Cardiac Surgery Database (STS ACSD) to describe contemporary surgical strategies and outcomes for AAAD.
Methods
Between July 2011 and September 2012, 2982 patients with AAAD underwent operations at 640 centers in North America.
Results
In this cohort, median age was 60 years old, 66% were male, and 80% had hypertension. The most common arterial cannulation strategies included femoral (36%), axillary (27%), and direct aortic (19%). The median perfusion and cross-clamp times were 181 and 102 min, respectively. The lowest temperature on bypass showed significant variation. Hypothermic circulatory arrest (HCA) was used in 78% of cases. Among those undergoing HCA, brain protection strategies included antegrade cerebral perfusion (31%), retrograde cerebral perfusion (25%), both (4%), and none (40%). Median HCA plus cerebral perfusion time was 40 min. Major complications included prolonged ventilation (53%), reoperation (19%), renal failure (18%), permanent stroke (11%), and paralysis (3%). Operative mortality was 17%. The median intensive care unit and hospital length of stays were 4.7 and 9.0 days, respectively. Among 640 centers, the median number of cases performed during the study period was three. Resuscitation, unresponsive state, cardiogenic shock, inotrope use, age >70, diabetes, and female sex were found to be independent predictors of mortality.
Conclusions
These data describe contemporary patient characteristics, operative strategies, and outcomes for AAAD in North America. Mortality and morbidity for AAAD remain high.
1 INTRODUCTION
Acute type A aortic dissection (AAAD) remains a highly lethal disease. Without surgical therapy, mortality exceeds 50% at 30 days.1, 2 Despite significant improvements in intra-operative management and post-surgical critical care, mortality, and morbidity rate still remain high, with reported mortality rates of 25% or more in several large series.3, 4
There are currently no prospective randomized data to guide surgical strategies for AAAD. Key areas of uncertainty include choice of arterial cannulation site, temperature management on cardiopulmonary bypass, and strategies for brain protection during deep hypothermic circulatory arrest (HCA). We utilized the Society of Thoracic Surgeons Adult Cardiac Surgery Database (STS ACSD) to examine current patient characteristics, predictors and outcomes for AAAD, and describe contemporary surgical management strategies in North America.
2 MATERIALS AND METHODS
2.1 Datasource
The Society for Thoracic Surgeons National Adult Cardiac Surgery Database (STS ACSD) is the largest registry for heart surgery in the world and includes data from more than 1000 participants, representing more than 90% of all cardiac surgery centers in the United States (http://www.sts.org/national-database). Peri-operative, operative, and outcomes data are collected on patients at participating centers. The data form version 2.73 was used to collect the information included in this analysis. For details of specific definitions, please refer to https://www.sts.org/sites/default/files/documents/STSAdultCVDataSpecificationsV2_73withcorrection.pdf.
2.2 Study population
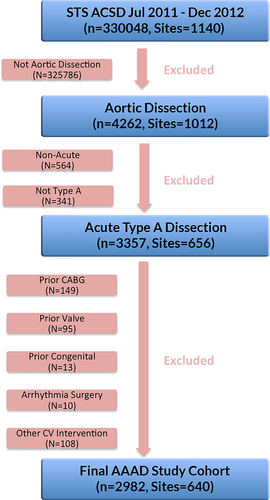
A total of 4262 patients underwent surgery for aortic dissection between July 2011 and September 2012. After excluding for prior percutaneous intervention and/or cardiac surgery, 2982 AAAD patients from 640 sites nationwide were examined in this study (Figure 1).
2.3 Statistical analysis
Categorical variables are summarized with frequency counts and percentages whereas continuous variables are summarized with r-median, mean, standard deviation, and interquartile range (IQR). Regression models were used to evaluate any association between the well-known risk factors and operative mortality. Logistic regression, accounting for within-hospital clustering of patients (GEE), was used to compute both the unadjusted and adjusted odds ratio for operative mortality to account for the hospital clustering of records. The P-values were obtained from the Global Wald Chi-square and Wilcoxon tests from comparison with the reference category for the unadjusted odds ratios. The inclusion of variables was pre-specified before carrying out the analysis and their inclusion was influenced by perceived clinical relevance and prior published literature. All analyses were performed with SAS software, version 8.2 (SAS Institute, Cary, NC).
3 RESULTS
3.1 Patient characteristics
Demographics and baseline clinical characteristics are shown in Table 1.
| Number (N = 2982) | % | Median | Mean ± Std. deviation | Interquartile range | |
|---|---|---|---|---|---|
| Demographics | |||||
| Age (years) | 2982 | 100 | 60.0 | 59.68 ± 14.01 | 50.00-70.00 |
| Gender: female | 1025 | 34.4 | |||
| Race | |||||
| Other | 80 | 2.7 | |||
| Asian | 102 | 3.4 | |||
| Hispanic | 139 | 4.7 | |||
| Black | 563 | 18.9 | |||
| Caucasian | 2067 | 69.3 | |||
| Missing | 31 | 1.0 | |||
| Risk factors | |||||
| Diabetes | |||||
| Insulin | 59 | 2.0 | |||
| No Insulin | 266 | 8.9 | |||
| Hypertension | 2375 | 79.6 | |||
| Last pre-op creatinine (mg/dL) | 2909 | 97.6 | 1.1 | 1.30 ± 1.05 | 0.90-1.40 |
| Last pre-op creatinine >2.0 mg/dL | 176 | 5.90 | |||
| Renal failure (dialysis/creatinine >2.0 mg/dL) | |||||
| Renal failure—dialysis | 58 | 2.0 | |||
| Renal failure—no dialysis | 125 | 4.2 | |||
| No renal failure | 2716 | 91.1 | |||
| Missing | 83 | 2.8 | |||
| CVD w/o CVA | |||||
| CVD-History CVA | 232 | 7.8 | |||
| CVD-No History CVA | 105 | 3.5 | |||
| No CVD | 2634 | 88.3 | |||
| Missing | 11 | 0.4 | |||
| CVD w/o CVA-time | |||||
| CVA ≤2 weeks | 90 | 3.0 | |||
| CVA >2 weeks | 142 | 4.8 | |||
| Chronic lung disease | |||||
| Severe | 45 | 1.5 | |||
| Moderate | 86 | 2.9 | |||
| Mild | 200 | 6.7 | |||
| No | 2632 | 88.3 | |||
| Missing | 19 | 0.6 | |||
| Peripheral vascular disease | 598 | 20.1 | |||
| Body surface area | 2982 | 100 | 2.0 | 2.0 ± 0.3 | 1.8-2.2 |
| Body mass index | 2958 | 99.2 | 28.3 | 29.5 ± 6.9 | 25.0-32.8 |
| Pre-operative cardiac status | |||||
| Congestive heart failure within 2 weeks | 309 | 10.4 | |||
| NYHA classification | |||||
| IV | 133 | 4.5 | |||
| III | 76 | 2.6 | |||
| II | 69 | 2.3 | |||
| I | 20 | 0.7 | |||
| Missing | 2684 | 90.0 | |||
| Myocardial infarction and time | |||||
| MI >21 days | 118 | 4.0 | |||
| MI 8-21 days | 10 | 0.3 | |||
| MI 1-7 days | 68 | 2.3 | |||
| MI >6 h but <24 h | 60 | 2.0 | |||
| MI ≤6 h | 83 | 2. 8 | |||
| No MI | 2632 | 88.3 | |||
| Missing | 11 | 0.4 | |||
| Unstable angina—no recent MI | 566 | 19.0 | |||
| Cardiogenic shock | 472 | 15.8 | |||
| Resuscitation | 156 | 5.2 | |||
| Atrial fibrillation | 243 | 8.2 | |||
| Ejection fraction | 1420 | 47.6 | 58.0 | 55.8 ± 11.0 | 50.0-62.0 |
| IABP—inotropes | 279 | 9.4 | |||
| Liver disease | 92 | 3.1 | |||
| Unresponsive neurologic state within 24 h of surgery | 99 | 3.3 |
- CVA, cerebrovascular accident; CVD, cerebrovascular disease; IABP, Intra-aortic balloon pump; NYHA, New York Heart Association; MI, myocardial infarction.
The median time from hospital arrival to operating room entry was 2.6 (IQR 1.1-6.1) h, and 79% of patients underwent operation within 24 h of arrival (Figure 2). Cardiogenic shock was present in 16% (472/2982). In addition, 5% (156/2982) underwent active resuscitation prior to surgery.
3.2 Operative characteristics
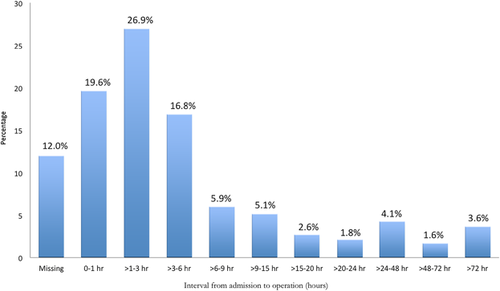
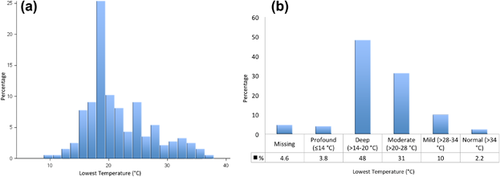
Operative characteristics are shown in Table 2. Overall procedure times (median 5.8 h), and perfusion times (median 181 min) were long. The median cross-clamp time was 102 min. There was significant variation in the lowest temperature on bypass, with a median lowest temperature of 20°C (IQR 18-25) (Figures 3A and 3B).5
| Number (N = 2982) | % | Median | Mean ± std. deviation | Interquartile range | |
|---|---|---|---|---|---|
| Status of procedure | |||||
| Emergent salvage | 82 | 2.6 | |||
| Emergent | 2466 | 82.7 | |||
| Urgent | 329 | 11.0 | |||
| Elective | 103 | 3.45 | |||
| Missing | 2 | 0.1 | |||
| Operative times | |||||
| Total operative time (hours) | 2967 | 99.5 | 5. 8 | 6.1 ± 2.3 | 4.7–7.3 |
| Time to surgery (arrival to OR) | 2516 | 84.4 | 2.6 | 6.6 ± 11.0 | 1.1 − 6.1 |
| CPB details | |||||
| Perfusion time (min) | 2929 | 98.2 | 181.0 | 195.4 ± 80.2 | 143.0 − 233.0 |
| Lowest temperature ( °C) | 2817 | 94.5 | 20.0 | 21.4 ± 5.4 | 18.0 − 25.0 |
| Lowest hematocrit | 2780 | 93.2 | 23.0 | 23.0 ± 4.8 | 20.0 − 26.0 |
| Arterial cannulation | |||||
| Aortic | 858 | 29.1 | |||
| Femoral | 1354 | 45.9 | |||
| Axillary | 914 | 31.0 | |||
| Other | 194 | 6.6 | |||
| Cross clamp details | |||||
| Aortic occlusion | |||||
| Balloon occlusion | 4 | 0.1 | |||
| Aortic cross clamp | 2909 | 97.6 | |||
| None/partial cross clamp | 63 | 2.1 | |||
| Missing | 6 | 0.2 | |||
| Cross clamp time (min) | 2889 | 96.9 | 102.0 | 112.6 ± 58.3 | 71.0 − 141.0 |
| Circulatory arrest details | |||||
| Circulatory arrest | |||||
| No circulatory arrest | 636 | 21.3 | |||
| Circ. arrest/no cerebral perfusion | 935 | 31.3 | |||
| Cerebral perfusion: | |||||
| Antegrade | 709 | 23.8 | |||
| Retrograde | 586 | 19.7 | |||
| Both | 88 | 3.0 | |||
| Unknown | 18 | 0.6 | |||
| Missing | 10 | 0.3 | |||
| Circulatory arrest time (min): | |||||
| With no cerebral perfusion | 917 | 30.8 | 25.0 | 27.2 ± 13.9 | 19.0 − 33.0 |
| With cerebral perfusion | 1302 | 43.7 | 5.0 | 16.3 ± 22.3 | 0.0 − 28.0 |
| Cerebral perfusion time (min) | 1390 | 46.6 | 30.0 | 34.3 ± 21.2 | 22.0 − 41.0 |
| Circulatory arrest + cerebral perfusion time (min) | 1296 | 43.5 | 40.0 | 50.4 ± 34.1 | 29.0 − 62.0 |
| Operative indicators | |||||
| Intra-Op TEE performed | 1957 | 65.6 | |||
| Aortic insufficiency (TEE based) | |||||
| Trivial | 917 | 46.9 | |||
| Mild | 512 | 26.2 | |||
| Moderate | 292 | 14.9 | |||
| Severe | 79 | 4.0 | |||
| N/A | 50 | 2.6 | |||
| Missing | 107 | 5.5 | |||
| Concomitant procedures | |||||
| Concomitant CABG | 427 | 14.3 | |||
| Concomitant aortic valve | 1575 | 52.8 | |||
| Valve sparing root remodeling (Yacoub) | 5 | 0.3 | |||
| Valve sparing reimplantation (David) | 36 | 2.3 | |||
| Homograft | 1 | 0.1 | |||
| Resuspension AV with replacement of ascending aorta | 712 | 45.2 | |||
| Resuspension AV without replacement of ascending aorta | 60 | 3.8 | |||
| Replacement and insertion of aortic non-valved conduit | 84 | 5.3 | |||
| Root reconstruction with valved conduit | 387 | 24.6 | |||
| Repair/reconstruction | 84 | 5.3 | |||
| Replacement | 206 | 13.1 | |||
| Concomitant mitral valve | 43 | 1.4 | |||
| Repair | 25 | 58.1 | |||
| Replacement | 16 | 37.2 | |||
| Missing | 2 | 4.7 | |||
| Concomitant endovascular procedure: | |||||
| None | 2922 | 98.0 | |||
| No debranching | 38 | 1.3 | |||
| Debranching | 10 | 0.3 | |||
| Unspecified | 1 | 0.1 | |||
| Missing | 11 | 0.3 | |||
| Concomitant other procedure | 352 | 11.8 |
- AV, aortic valve; CABG, coronary artery bypass graft; OR, operating room; TEE, transesophageal echocardiogram.
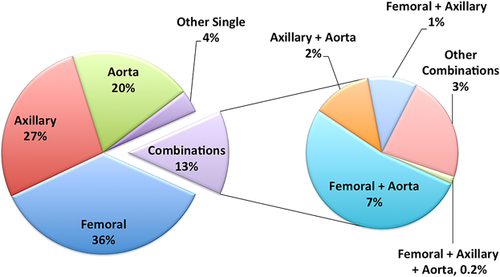
The arterial cannulation strategy used for AAAD repair is presented in Figure 4. While the STS ACSD is limited in the granularity of reporting of operative strategies, the results reported here suggest that among patients undergoing AAAD repair, a strategy of initial femoral cannulation was most commonly utilized, with axillary cannulation being the second most common approach.
3.2.1 Circulatory arrest
Circulatory arrest was not used in 21% of cases. Among the remaining 79% of patients, circulatory arrest was performed without brain protection in 40.3%, with antegrade cerebral perfusion in 30.6%, retrograde cerebral perfusion in 25.3%, and both antegrade and retrograde in 3.4% of cases.
For patients undergoing circulatory arrest without brain protection, median circulatory arrest time was 25 min (IQR 19-33). Among patients undergoing circulatory arrest with brain perfusion, median brain perfusion time was 30 min (IQR 22-41), and the median total body circulatory arrest time was 40 min (IQR 29-62).
3.2.2 Procedures performed
Concomitant CABG was performed in 14% of cases, and an aortic valve procedure in 53% of cases. Among those patients having aortic valve procedures, the following were performed: (1) root reconstruction with valved conduit (root replacement): 25.6%; (2) aortic valve resuspension: 54%; (3) isolated aortic valve replacement: 13%; (4) valve-sparing root replacement: 2.6%.
Endovascular procedures were performed in 2% of cases.
3.2.3 Blood product utilization
As expected, blood product utilization was substantial in this population. Total blood product requirement (intra- and post-operatively) included a median of 5 (IQR 2-9) units of packed red blood cells, 4 (IQR 2-8) units of fresh frozen plasma, 1 (IQR 0-3) unit of cryoprecipitate, and 3 (IQR 2-4) units of platelets.
3.3 Post-operative outcomes
Table 3 shows peri-operative outcomes including mortality and morbidity. Operative mortality for the entire cohort of 2982 patients was 17.4%. Non-fatal adverse outcomes included permanent stroke in 11%, paralysis in 2.9%, renal failure in 18%, dialysis-requiring renal failure in 8.4%, prolonged ventilation in 53%, and reoperation for any reason in 20%. The median intensive care unit and hospital length of stays were 4.7 and 9.0 days, respectively. Combined mortality or major morbidity (reoperation, deep sternal wound infection, stroke, renal failure, prolonged ventilation) occurred in 65 % of patients. Cerebral perfusion was not correlated with neurologic injury (P = 0.20). Higher temperatures seem to correlate with lower morbidity/mortality (P < 0.0001). Patients with concomitant CABG had higher combined morbidity/mortality (P = 0.0008; 72.1% mortality/morbidity with concomitant CABG versus 63.8% mortality/morbidity without concomitant CABG).
| Number (N = 2982) | % | Median | Mean ± std. deviation | Inter-quartile range | |
|---|---|---|---|---|---|
| Mortality | |||||
| Mortality—operative | 519 | 17.4 | |||
| Hospital length of stay | |||||
| LOS after surgery | 2976 | 99.8 | 9.0 | 12.8 ± 12.1 | 6.0–15.5 |
| ICU | |||||
| Total ICU hours | 2764 | 92.7 | 113.0 | 195.0 ± 250.9 | 58.0 − 232.0 |
| Total blood products | |||||
| Any blood products used | 2795 | 93.7 | |||
| Red blood cells units | 2795 | 93.7 | 5.0 | 7.0 ± 7.6 | 2.0 − 9.0 |
| Fresh frozen plasma units | 2794 | 93.7 | 4.0 | 5.9 ± 5.9 | 2.0 − 8.0 |
| Cryoprecipitate units | 2793 | 93.7 | 1.0 | 3.3 ± 6.7 | 0.0 − 4.0 |
| Platelet units | 2793 | 93.7 | 3.0 | 4.4 ± 7.3 | 2.0 − 5.0 |
| Complications | |||||
| In hospital complications | 2204 | 73.9 | |||
| Complications—operative | |||||
| Any re-operation | 595 | 19.9 | |||
| Re-op:bleeding/temponade | 260 | 8.7 | |||
| Re-op: valve dysfunction | 3 | 0.1 | |||
| Complications—infection | |||||
| Sternum—deep | 6 | 0.2 | |||
| Sternum—superficial | 15 | 0.5 | |||
| Mediastinitis | 6 | 0.2 | |||
| Sepsis | 118 | 4.0 | |||
| Complications—neurological | |||||
| Permanent stroke | 325 | 10.9 | |||
| TIA | 17 | 0.6 | |||
| Paralysis | 85 | 2.9 | |||
| Complications—pulmonary | |||||
| Prolonged ventilation | 82 | 2.8 | |||
| Pneumonia | 6 | 0.2 | |||
| Pulmonary TE | 31 | 1.0 | |||
| Deep venous TE | 110 | 3.7 | |||
| Complications—renal | |||||
| Renal failure if no prior RF | 486 | 17.9 | |||
| Renal failure if no prior dialysis | 238 | 8.4 | |||
| Complications—vascular | |||||
| Iliac/femoral dissection | 7 | 0.2 | |||
| Acute limb ischemia | 81 | 2.7 | |||
| Aortic dissection | 18 | 0.6 | |||
| Complications—summary | |||||
| Major complications (mortality, re-operation, deep sternal infection, stroke, renal failure, prolonged ventilation) | 1938 | 65.0 | |||
- ICU, intensive care unit; LOS, length of stay; RF, renal failure; TE, thromboembolic; TIA, transient ischemic attack.
The patients with other types of concomitant procedure were also more likely to have higher mortality/morbidity (P < 0.0001; 76.1% with concomitant procedures versus 63.5% without concomitant procedures).
3.3.1 Risk factors associated with mortality
Multivariable logistic regression analysis yielded the following significant pre-operative risk factors associated with mortality: resuscitation prior to surgery, unresponsive neurologic state within 24 h of surgery, age >70, cardiogenic shock, diabetes, inotrope use, female sex (Table 4).
| 95% confidence interval | ||||
|---|---|---|---|---|
| Predictors | Odds ratio | Lower | Upper | P-value |
| Creatinine of 0.5-5 per 1 mg/dL increase (not on dialysis) | 1.94 | 1.46 | 2.59 | <.0001 |
| Age ≥70 years | 1.70 | 1.31 | 2.21 | <.0001 |
| BMI per 5 Kg/m2 increase | 1.06 | 0.99 | 1.15 | 0.1030 |
| Female | 1.39 | 1.12 | 1.72 | 0.0031 |
| Diabetes: any | 1.48 | 1.08 | 2.03 | 0.0153 |
| Hypertension | 0.81 | 0.62 | 1.05 | 0.1152 |
| CVA <2 weeks | 0.72 | 0.33 | 1.55 | 0.4018 |
| Peripheral vascular disease | 1.29 | 1.00 | 1.66 | 0.0489 |
| Cardiogenic shock | 1.49 | 1.10 | 2.00 | 0.0094 |
| Resuscitation | 4.24 | 2.72 | 6.60 | < 0.0001 |
| Inotropes | 1.46 | 1.05 | 2.04 | 0.0243 |
| Liver disease | 1.12 | 0.57 | 2.17 | 0.7462 |
| Unresponsive neurologic state within 24 h of surgery | 1.81 | 1.00 | 3.26 | 0.0496 |
| Immunosuppressive treatment | 0.99 | 0.56 | 1.74 | 0.9688 |
| Cardiovascular disease | 0.89 | 0.62 | 1.27 | 0.5188 |
| Race: | ||||
| Black vs White | 0.77 | 0.58 | 1.04 | 0.0868 |
| Hispanic vs White | 1.03 | 0.65 | 1.63 | 0.9104 |
| Asian vs White | 0.65 | 0.36 | 1.18 | 0.1588 |
| Other vs White | 2.16 | 1.23 | 3.79 | 0.0072 |
| Renal failure: | ||||
| Renal failure and no dialysis vs none | 0.53 | 0.26 | 1.08 | 0.0808 |
| Renal failure and dialysis vs none | 2.14 | 0.90 | 5.05 | 0.0839 |
| Chronic lung disease: | ||||
| Mild vs none | 1.25 | 0.86 | 1.81 | 0.2517 |
| Moderate vs none | 1.35 | 0.78 | 2.33 | 0.2780 |
| Severe vs none | 0.86 | 0.33 | 2.27 | 0.7643 |
| Aortic insufficiency: | ||||
| Trivial vs none | 0.61 | 0.38 | 0.99 | 0.0438 |
| Mild vs none | 0.85 | 0.61 | 1.20 | 0.3647 |
| Moderate vs none | 0.84 | 0.60 | 1.17 | 0.2988 |
| Severe vs none | 0.85 | 0.62 | 1.15 | 0.2915 |
| MI: | ||||
| <24 h vs >21 days or no MI | 2.13 | 1.41 | 3.22 | 0.0003 |
| 1-21 days vs >21 days or no MI | 1.33 | 0.75 | 2.34 | 0.3265 |
| CHF and NYHA IV: | ||||
| CHF not NYHA IV vs no CHF | 1.08 | 0.68 | 1.74 | 0.7367 |
| CHF and NYHA IV: CHF and NYHA IV vs no CHF | 1.36 | 0.84 | 2.22 | 0.2152 |
| Aortic dissection volume>3 (or 2.57 per 1 year) | 0.70 | 0.55 | 0.89 | 0.0041 |
- BMI, body mass index; CHF, congestive heart failure; CVA, cerebrovascular accident; MI, myocardial infarction; NYHA, New York Heart Association.
- Bold items refer to statistical significance ( > 0.05).
3.3.2 Disposition
Most patients (60%) were discharged to home, followed by discharge to an extended care facility (30%). A small number of patients were discharged to nursing homes (3.7%), other hospitals (4%), or hospice (1%).
3.4 Center experience with AAAD
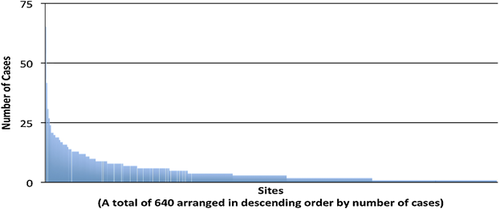
During the study period, one or more primary AAAD repairs were performed at only 640/1140 (56%) of centers reporting data to the STS ACSD, with a median of three cases performed at each center during the study period. Only 11% of centers (n = 72) performed 10 or more cases (Figure 5). The higher the hospital volume, the lower the unadjusted odds ratio (OR) for mortality (hospital volume ≥3, OR = 0.67, P = 0.004; hospital volume ≥10, OR = 0.68, P = 0.0001), and OR = 0.98 with each additional case increase (P < 0.0001).
4 DISCUSSION
This study represents a comprehensive and contemporary review of patient characteristics, operative management, and outcomes of patients with AAAD in North America. Key findings include a mortality rate of 17%, a stroke rate of 11%, and large variations in operative approaches with significant differences in cannulation strategy, cerebral protection strategy, and temperature management. In addition, only a few centers have significant experience with AAAD.
The overall mortality in this report of 17% is lower than the IRAD experience of 25%3 and the National Inpatient Sample analysis of 26%.4 However, the lower mortality rate could also be due to the exclusion of re-operative patients from this analysis. Mortality in the German Registry for Acute Aortic Dissection Type A (GERAADA) was similar at 20%.6 In contrast, single-center series have reported mortality rates of 2.8% to 9%.7-9 This discrepancy in outcomes between large registries and single-center series suggests opportunities for improvement in outcomes if best practices are identified and implemented.
This study highlights a remarkably large variation in practice and techniques used to treat AAAD in North America. The site used for arterial inflow was most commonly the femoral artery, with a substantial number of cases reporting axillary and direct aortic cannulation. Femoral cannulation enables rapid institution of cardiopulmonary bypass and has a long history of use in AAAD. However, this is in contrast to the Europeans where only 25% cannulate the femoral artery,10 and the Canadians where only 17% cannulate the femoral artery.11 Femoral cannulation can cause extension of dissection into the iliofemoral vasculature, which can lead to pressurization of the false lumen and malperfusion at the onset of cardiopulmonary bypass.12 There is some evidence that femoral cannulation is associated with increased embolic events,13, 14 although other studies have shown that there is no difference in stroke risk with femoral cannulation.15, 16 Axillary artery cannulation has more recently gained popularity as a cannulation site for aortic and arch surgery.17-20 This is especially so in Europe and Canada.10, 11 It simplifies antegrade cerebral perfusion during systemic circulatory arrest. There is some evidence that axillary artery cannulation improves outcomes for AAAD.20, 21 Other groups have proposed that direct aortic cannulation can be safe.22-24 Other potential sites of cannulation include the left ventricular apex, innominate artery,25 and carotid artery. However, the utilization of these approaches was unable to be determined in this study.
Perfusion management in this study was strikingly heterogeneous. Utilization of circulatory arrest in operations for AAAD is common as it allows for examination of the aortic arch for tears and a more complete resection of diseased ascending aorta. However, 22% of cases in this study were not performed under circulatory arrest. Among patients undergoing operation with hypothermic circulatory arrest, cerebral perfusion strategies varied greatly with upwards of 40% without any adjunctive cerebral perfusion during the period of circulatory arrest compared with only 29% in the GERAADA registry and 18% in the Canadian survey.10, 11 A large retrospective study from GERAADA found that for circulatory arrest times less than 30 min, hypothermic circulatory arrest alone compared with antegrade cerebral perfusion yielded similar results.26
Among those with adjunctive cerebral perfusion in this study, slightly more had antegrade cerebral perfusion (44%) as compared to retrograde cerebral perfusion. This is in contrast to the Europeans where 69% had antegrade cerebral perfusion and the Canadians where 72% had antegrade cerebral perfusion.10, 11 There is growing evidence to suggest that antegrade cerebral perfusion is associated with better long-term survival.15, 27 Others have reported use of combined antegrade and retrograde cerebral perfusion with good results as well.28 However, the necessity for and optimal route of cerebral perfusion is still controversial.
The minimum temperature achieved during circulatory arrest also varied greatly. Lansman et al and Griepp et al popularized profound hypothermia for organ protection during aortic arch surgery.29, 30 There is emerging evidence to suggest that mild to moderate hypothermia is comparable to deep hypothermia in terms of operative risk,31-33 and the optimal temperature during circulatory arrest remains unclear. One has to weigh the risks of potentially increased coagulopathy, increased length of operation, and increased systemic inflammatory response with deeper degrees of hypothermia versus the potential benefits of better organ protection.
The type of procedures performed to treat AAAD is largely dictated by the extent of the dissection. In this experience, more than half of patients required management of the aortic valve either with replacement or repair via resuspension. More than a quarter of patients required root replacement. Valve-sparing root replacement was performed very infrequently. Treatment of the aortic arch and descending thoracic aorta cannot be determined in this study as the information is not available in the database.
Stroke remains a devastating complication after AAAD repair and is a major cause of mortality. The rate of 11% in this report is similar to other large studies of AAAD despite significant differences in cerebral perfusion strategies. This is considerably higher than most other cardiac surgical procedures. Cerebral protection via antegrade or retrograde cerebral perfusion has been shown to decrease neurologic complications in surgery involving the aortic arch.34 However, in this study, cerebral perfusion was not correlated with lower neurologic injury.
Out of more than 1000 centers participating in STS ACSD, only 640 centers reported more than one case during the study period. Among those centers with experience in the treatment of AAAD, the majority performs fewer than three cases a year. This case distribution implies that few surgeons (and few centers) are able to accumulate substantial experience with the treatment of these complicated cases. There is a correlation between hospital experience and lower mortality. However, it is still unclear whether individual surgeon experience, rather than hospital experience, affects mortality.
4.1 Limitations
The STS ACSD collects limited data regarding aortic surgery and aortic dissection and the exact type and extent of procedure performed cannot be determined based on the current data elements. Several important pre-operative clinical factors that affect outcomes such as malperfusion are not collected in this database. There is also no information regarding a patient's family or genetic history. Another major limitation is the lack of information on patients undergoing non-operative management as the STS ACSD is a surgical database. Finally, multivariable logistic regression analysis for operative mortality was only performed using available pre-operative risk factors.
5 CONCLUSIONS
Acute type A aortic dissection remains a highly lethal and morbid condition. Surgery to treat the disease is a relatively uncommon procedure with only about 3000 cases per year in North America and individual center and surgeon experience is limited. There are large variations in surgical practice, particularly with regards to temperature management on cardiopulmonary bypass, approach to brain protection, and arterial cannulation strategy. Future studies should examine intra-operative characteristics that might affect outcome including type of cannulation, lowest temperature on cardiopulmonary bypass, type of cerebral perfusion, and management of the aortic arch. Large multicenter trials will be required to definitively answer these important questions. Given the high mortality and morbidity of surgical treatment of AAAD and current heterogeneous treatment strategies, these trials are imperative.