Novel single-entry point injection technique for masseter hypertrophy treatment using botulinum neurotoxin based on patient-reported comfort
Abstract
Introduction
Botulinum neurotoxin (BoNT) injections are widely used for the treatment of masseter muscle hypertrophy in Southeast Asia. However, there remains a lack of consensus regarding the optimal injection technique. This study aimed to compare the efficacy and patient discomfort associated with single-entry point injections versus multiple three-point injections for masseter muscle hypertrophy treatment with BoNT.
Materials and Methods
Sixteen participants, comprising both male and female Korean adults aged 22–63, were enrolled in the study. On the left side of the face, single-entry point injections were administered, followed by multidirectional injections, while on the right side, three-point injections were given. Pain intensity during the procedure was assessed using visual analogue scale scores.
Result
Our results revealed that participants experienced lower levels of pain with single-entry point injections compared to three-point injections (average visual analogue scores of 3.31 and 5.19, respectively).
Conclusion
These findings highlight the potential benefits of single-entry point injections in reducing patient discomfort during masseter muscle hypertrophy treatment with BoNT. We advocate for further research to validate these findings and encourage practitioners to consider single-entry point injections as a viable option for enhancing treatment outcomes in their clinical practice.
1 INTRODUCTION
The widespread demand for facial contouring and rejuvenation has driven the extensive adoption of botulinum neurotoxin (BoNT) injections in aesthetic medicine.1, 2 Among the target muscles for facial contouring, the masseter muscles are significant in their contribution to the shape and symmetry of the lower third of the face. Hypertrophy of the masseter muscles may result in a bulky, angular jawline, contributing to a more masculine appearance. Such hypertrophy is often caused by factors such as biting force, chewing patterns, dietary choices, or para-functional habits such as bruxism and other temporo-mandibular joint disorders. This is often presents an aesthetic concern for individuals, particularly in Southeast Asia, where the prevailing beauty standards lean towards a softer and narrower facial contour.3
BoNT acts by inhibiting the release of acetylcholine at the neuromuscular junction, thus reducing muscle contraction and altering facial contours.4 Its application to address masseter muscle hypertrophy is widely practiced.5 While consensus guidelines and various studies propose different injection techniques, such as six-point or three-point injections for each masseter, our clinical observations across Southeast Asia, where we predominantly treat patients from this region, have shown that single-entry point injections are associated with less discomfort compared to multiple injections.2, 3, 5, 6
The aim of this study is to compare the discomfort experienced during single-entry point injection versus multiple-point injections for masseter muscle hypertrophy in Southeast Asian patients.
2 MATERIALS AND METHODS
We recruited participants who expressed interest in improving their jawline contour and were suitable candidates for addressing bilateral masseter muscle hypertrophy with BoNT. The study enrolled 16 participants, including seven males and nine females of Korean descent, aged between 22 and 63 years. Each participant received an injection with a single-entry point (Figure 1 and Video S1) on the left side of their face and three-point injections on the right side (Figure 2 and Video S2). A 1 and a half inch 30G needle was used for the injections. The dose for each side of the masseter was 25 units per side. Exclusion criteria included individuals under 18 years old, pregnant or lactating women, those who had undergone facial aesthetic treatments such as BoNT, filler, biostimulator, or energy-based devices to the lower third of the face within the past 3 months, and individuals with a history of hypersensitivity or allergic reactions to BoNT.
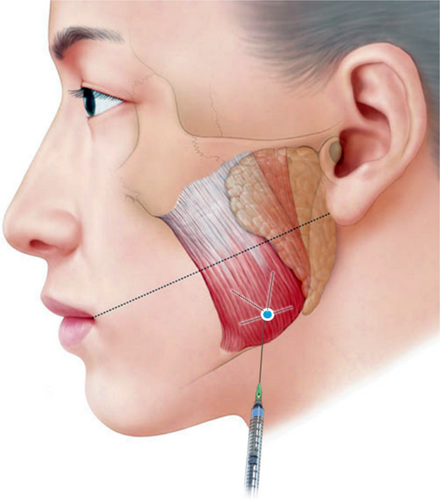
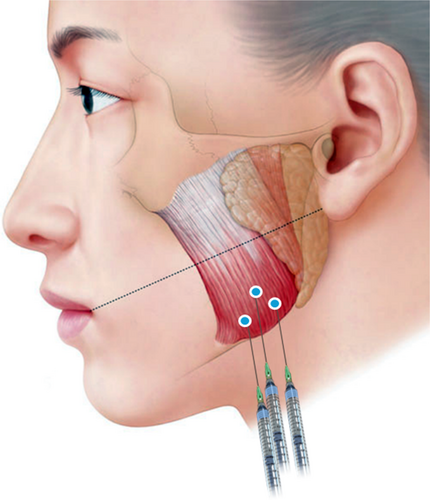
The visual analogue scale (VAS), a validated subjective measure for acute pain, was utilized to assess the intensity of pain experienced by participants throughout the procedure.7 This scale ranges from 0 to 10, where 0 signifies the absence of pain and 10 indicates the highest level of pain imaginable.
Informed consent was obtained from all participants involved in the study. The study adhered to the principles of the Declaration of Helsinki, and Institutional Review Board review was deemed unnecessary and waived for this study due to the nature of the investigation.
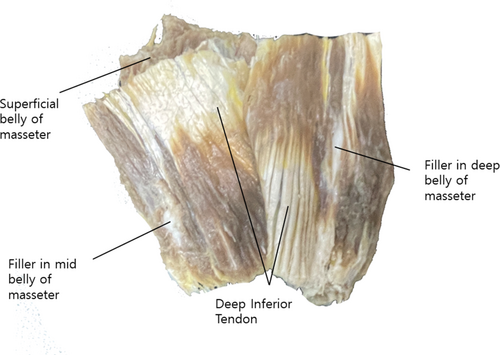
A masseter has been harvested from the cadaver (74 year old Korean male) and injected with the stained filler in multiple direction and observed the spread of the fillers in the layers.
3 RESULTS
Table 1 provides a summary of the results obtained. The results revealed that participants who received a single-entry point injection on the left side had VAS scores ranging from 2 to 6, with an average score of 3.31. Conversely, those who received a three-point injection on the right side showed VAS scores ranging from 4 to 6, with an average score of 5. These findings suggest that participants generally experienced lower levels of pain during the single-entry point injection compared to the three-point injection (Table 2). The significance difference with p < 0.05.
| Age (years) | Gender | Left (one-point injection) | Right (three-point injection) |
|---|---|---|---|
| 28 | Male | 3 | 6 |
| 32 | Female | 2 | 5 |
| 42 | Male | 3 | 5 |
| 33 | Female | 4 | 6 |
| 42 | Female | 3 | 6 |
| 22 | Female | 3 | 5 |
| 36 | Female | 2 | 4 |
| 34 | Male | 4 | 5 |
| 53 | Male | 6 | 6 |
| 43 | Female | 4 | 5 |
| 26 | Female | 3 | 4 |
| 63 | Female | 2 | 5 |
| 57 | Female | 3 | 4 |
| 53 | Male | 4 | 5 |
| 22 | Male | 5 | 6 |
| 36 | Male | 3 | 4 |
| Statistic | Left injection (one-point) | Right injection (three-point) |
|---|---|---|
| Mean | 3.31 | 5.00 |
| Standard deviation | 1.15 | 0.73 |
| Median | 3.00 | 5.00 |
| 1st quartile (Q1) | 3.00 | 4.00 |
| 3rd quartile (Q3) | 4.00 | 5.75 |
| t-statistic | −5.48 | |
| p-value | 0.00001 |
The masseter muscle, harvested from a 74-year-old Korean male cadaver, was injected with stained filler in multiple directions. The observations revealed that the filler spread uniformly across all layers of the masseter muscle. This comprehensive distribution indicates that the injection technique effectively permeates the entire muscle, ensuring thorough coverage and potentially enhancing the efficacy of the treatment.
4 DISCUSSION
Surgical intervention of masseter muscle hypertrophy involves ostectomy of the mandibular angle, dissection of the masseter muscle, and removing subcutaneous fat through liposuction.8-10 While the literature indicates that surgical intervention can be effective and long-lasting in severe cases of masseter hypertrophy, potential postoperative complications include sequelae from general anesthesia, pain, trismus, postoperative bleeding, haematoma, oedema, infection, scarring, and facial nerve paralysis.9, 10 In contrast, BoNT type A offers a non-invasive alternative with fewer associated risks and complications, making it an attractive option for patients.1-3, 5, 6, 11 Nevertheless, certain adverse events have been documented, such as headaches, ecchymosis, hematoma, muscle weakness, dry mouth, and unnatural facial expressions.12, 13 While typically mild and short-lived, resolving in a few weeks to several months in certain patients, it is important to recognize that these adverse events can have a negative impact on aesthetics.4 Employing precise techniques for dilution, storage, and injection, along with comprehensive patient screening to exclude those with contraindications, can prevent these undesirable side effects.12
Various injection techniques exist for administering BoNT in the treatment of masseter muscle hypertrophy.6 Traditionally, BoNT is injected into the most prominent bulge of the masseter muscle.14 Some researchers propose for a multi-point injection technique to improve toxin distribution within the muscle.5, 15 Alternatively, others recommend using the fewest injection sites possible or even a single injection site. Sermswan et al.'s16 cadaveric study aimed to determine optimal injection techniques by investigating the distribution of injections within the masseter muscle. Thirty masseter muscles from Asian cadavers were dissected to observe dye spreading patterns post-injections at different points within the muscle. Findings suggested the three-point technique as most suitable for safe zone injections in masseter hypertrophy. However, the study's main limitation lies in the supine positioning during dissection, potentially impacting dye distribution towards the posterior muscle fibers due to gravitational effects. This gravitational influence may have led to reduced primary spreading dimensions. Additionally, factors such as muscle contraction, blood flow, and pressure in living individuals were not considered. Another cadaveric study by Bae et al.17 aimed to delineate safe injection zones by examining the relationship between the risorius muscle and masseter muscle. The authors recommended a three-point injection technique to minimize the risk of damage to surrounding structures.
We chose to compare the discomfort levels associated with three injection points with a single-entry point injection, aligning with the common practice among practitioners in Southeast Asia, based on our clinical expertise. Following the single-entry point injection, a multidirectional injection technique was employed, as illustrated in Figure 1. The BoNT was administered in a retrograde fanning manner. All injections were directed towards the lower half of the masseter muscle, following the recommendation by Yi et al.2 This approach aims to prevent damage to the parotid duct and superficial aponeurosis, which may occur if injections target the upper region. Moreover, the lower part of the masseter muscle is more densely innervated, making it more effective for treatment. Care was taken to avoid superficial injections into the anterior section of the masseter muscle to prevent BoNT diffusion to the risorius muscle, which could result in asymmetrical smiling.17, 18
Our study findings offer evidence supporting the superiority of single-entry point injections over multiple three-point injections in terms of patient comfort for treating masseter muscle hypertrophy with BoNT. While single-entry point injections for this indication are not common, we urge practitioners to contemplate this approach in their clinical practice, given its potential for reducing patient discomfort and enhancing treatment outcomes.
Regarding the efficacy of previous study, the masseter muscle thickness measured by ultrasonography was 9.8 ± 1.3 mm (relaxed state) and 12.4 ± 1.4 mm (contracted state) in females, and 11.3 ± 1.2 mm (relaxed state) and 14.7 ± 1.4 mm (contracted state) in males.19 Additionally, the skin and subcutaneous tissue thickness around the masseter region is less than 1 cm in most cases.20 A 1.5-inch needle, which is 3.81 centimeters in length, would be sufficient to ensure adequate dispersion of the toxin into the muscle belly. Furthermore, injecting into the masseter muscle from various angles in a cadaver confirmed that all layers were effectively reached (Figure 3), suggesting that the efficacy of the one-point technique is comparable to that of existing techniques.
Further research involving larger sample sizes and controlled studies is needed to validate these findings and determine the optimal injection technique for minimizing discomfort while maintaining treatment efficacy.
5 CONCLUSION
In conclusion, our findings support our initial observation that single-entry point injections offer a less uncomfortable alternative for addressing masseter muscle hypertrophy. Participants reported experiencing less pain levels with single-entry point injections compared to three-point injections. This suggests that single-entry point injections may present a viable option for treating masseter muscle hypertrophy in Southeast Asian patients, offering a more comfortable treatment experience without compromising efficacy. Additional research and clinical trials are required to validate these findings and enhance injection techniques for improved patient outcomes.
AUTHOR CONTRIBUTIONS
Conceptualization: Jovian Wan; Jong-Seo Kim; Hee-Jin Kim; Kyu-Ho Yi, Youngjin Park; Soo Yeon Park. Writing—Original Draft Preparation: Jovian Wan; Jong-Seo Kim; Hee-Jin Kim; Kyu-Ho Yi, Youngjin Park; Soo Yeon Park. Writing—Review & Editing: Jovian Wan; Jong-Seo Kim; Hee-Jin Kim; Kyu-Ho Yi. Visualization: Jovian Wan; Jong-Seo Kim; Hee-Jin Kim; Kyu-Ho Yi. Supervision: Jovian Wan; Jong-Seo Kim; Hee-Jin Kim; Kyu-Ho Yi.
ACKNOWLEDGMENTS
All authors have reviewed and approved the article for submission.
FUNDING INFORMATION
The authors received no financial support for the research, authorship, and publication of this article. The products utilized in this study were donated by the injectors for the purposes of this study.
CONFLICT OF INTEREST STATEMENT
The authors declared no potential conflicts of interest with respect to the research, authorship, and publication of this article. This study was conducted in compliance with the principles set forth in the Declaration of Helsinki.
ETHICS STATEMENT
All of Korean cadavers are from institute which is all donated and areapproved for research purposes.
Open Research
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study are available in the supplementary material of this article.




