Evaluation of pathogen distribution and antimicrobial resistance in breast plastic surgery infection
Xueshang Su and Jintian Hu have contributed equally to this work.
Abstract
Background
The demand for mammaplasty has increased in recent years, and infection remains one of the common and serious post-operative complications. In this study, we analyzed the pathogen distribution and antibiotic susceptibility of breast plastic surgery infections, and compared the differences in pathogenic species between surgical procedures.
Methods
The number of each species was counted in the microbial samples of breast plastic surgery infections in Plastic Surgery Hospital of Chinese Academy of Medical Sciences from January 2011 to December 2021. The in vitro antibiotic sensitivity testing data were analyzed using WHONET 5.6 software. The surgical techniques, the period of infection, and other details were gathered in accordance with the clinical data.
Results
There were a total of 42 cases included, and 43 different types of pathogenic bacteria, mostly gram-positive bacteria, were found. CoNS (13/43) and Staphylococcus aureus (22/43) made up the majority. The most prevalent of the five Gram-negative bacteria was Pseudomonas aeruginosa. Results of drug sensitivity tests indicate that S. aureus is highly sensitive to vancomycin, cotrimoxazole, and linezolid, whereas CoNS is highly sensitive to vancomycin, linezolid, and chloramphenicol. Both of these bacteria show high resistance to erythromycin and penicillin. Breast augmentation, breast reconstruction, and breast reduction surgery were the most frequently associated breast surgery procedures in this study with infections, with the highest number of infections occurring following breast augmentation with fat grafting, breast reduction surgery, and breast reconstruction with autologous tissue. Various breast plastic surgery procedures have different common pathogens of infection, but the most prevalent are CoNS and S. aureus. Additionally, the majority of the infections in this study were in the early stages.
Conclusions
Gram-positive bacteria were the predominant cause of breast plastic surgery infections, and the types of infection strains, the period of infection onset, and the antibiotic susceptibility of prevalent strains varied between breast plastic procedures.
1 INTRODUCTION
With the progress of the medical technology and the change of perception, the demand for breast surgery is increasing. Breast augmentation, breast reconstruction following breast cancer surgery and breast reduction are the most common breast plastic surgery operations. These surgeries are generally meant to improve the patient's breast appearance and meet their aesthetic needs. Although breast plastic surgery is frequently thought of as clean surgery, Kataria et al. proposed that clinical investigations have revealed that the infection rate for breast surgery is higher than that of other clean surgeries.1 Infection is still a frequent postoperative complication and the primary reason for surgical failure, which has a significant negative impact on patients' quality of life and places financial and mental strain on them. Antibiotic overuse and the rise of drug-resistant bacteria have made infection treatment more challenging in recent years. Although it is widely suggested that perioperative prophylactic antibiotic use should not exceed 24 h, 52% of surgeons prefer to use antibiotics for more than 24 h, 72% of plastic surgeons will prolong the use of antibiotics after surgery with expander, according to a poll with 650 respondents.2 Therefore, antibiotic selection is crucial to delay the emergence of drug-resistant strains and strengthen the effect of infection prevention and control. In order to provide recommendations for the prevention and control of infection in breast plastic surgery, this study examined the different species and antimicrobial susceptibility of common infectious pathogens after breast plastic surgery, and compared the common strains causing infections in by various surgical techniques. It is reported as follows.
2 METHODS
2.1 Clinical specimens
From January 2011 to December 2021, data on patients with infections following breast surgery at plastic surgery hospital were collected retrospectively. In this study, the presence of one of the following symptoms was considered as infection, including erythema, local warmth, swelling, purulent discharge, delayed wound healing beyond expectations, new or increasing pain, increasing malodour.3 All specimens were subjected to routine smears, gram staining, inoculation and culture, isolation, and identification in accordance with National Clinical Laboratory Procedures (4th edition). After eliminating duplicate strains, 43 bacterium strains were identified. Duplicate strains refer to multiple bacterial cultures from the same patient that detect the same pathogen. Isolated strains were mostly derived from wound secretions and abscess extracts. This research has been approved by the ethics committee.
2.2 Microbe species identification and antimicrobial resistance test
Microbial samples were cultured and inoculated using blood and maikangkai dishes (Antu, Zhengzhou). Different types of samples were inoculated in the respective Petri plates and incubated in the corresponding incubators for 18–24 h as needed before being removed for colony morphology observation. The pathogens including Gram-negative bacteria and Gram-positive bacteria were identified by API identification strip (bioMérieux) based on instruction. It should be noted that the culture conditions of this study are only applicable to common strains, and nontuberculous mycobacteria and anaerobic bacteria are not routinely cultured in our hospital. If clinicians suspect nontuberculous mycobacterial infection or anaerobic infection based on clinical manifestations, the samples will be sent to professional institutions for testing. However, nontuberculous mycobacterial infection or anaerobic bacteria infection has not been found in our hospital so far. The antibiotic susceptibility was tested by Kirby-Bauer Paper flakes diffusion method. The susceptibility paper was produced by the oxoid. The test judgment standard was based on the 2021 Clinical and Laboratory Standards Institute document M100.4 The 11 antibiotics used in this study included methicillin, cotrimoxazole, penicillin, clindamycin, tetracycline, levofloxacin, erythromycin, vancomycin, chloramphenicol, gentamicin, and linezolid. The quality control strains included Staphylococcus aureus (ATCC25923), Escherichia coli (ATCC25922), Pseudomonas aeruginosa (ATCC27853), and Enterococcus faecalis (ATCC29212). The identified strains were stored at −80°C. The bacterial culture of hands of health care workers and environment were carried out monthly in our hospital, and the results were all qualified. All infections in this study were not caused by health care workers or hospital environment.
2.3 Statistical analysis
Microsoft Excel 2016 was used to enter data, process it, and calculate percentages. The categorical data was represented using frequencies and percentages. WHONET 5.6 software and SPSS 22.0 were used for data analysis. The detection rate of pathogenic bacteria, distribution of pathogenic bacteria in various breast plastic surgery and distribution of pathogens in relation to onset of infection were analyzed by trend Chi-square tests. p-Values < 0.05 were considered statistically significant.
3 RESULTS
3.1 The overall distribution of pathogens
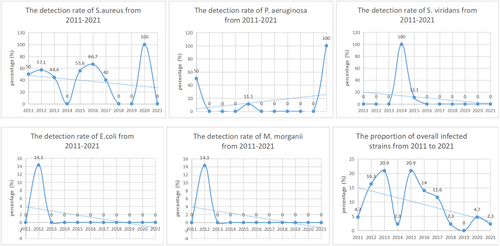
A total of 42 cases of infection following breast surgery were included, 43 strains of pathogenic bacteria were detected, among which 38 strains were Gram-positive bacteria. Staphylococcus aureus and CoNS were the most common strains, accounting for 81.4% of all infected strains. There were 5 strains of Gram-negative bacteria, including 3 isolates of P. aeruginosa, 1 isolate of E. coli, and 1 isolate of Morganella morganii. As shown in Table 1. The detection rates of S. aureus, Streptococcus viridans, E. coli, M. morganii, and the overall fraction of infected strains all showed a downward trend from 2011 to 2021 (p < 0.05), whereas P. aeruginosa detection rate indicated an increased trend (p < 0.05). No significant trend change was observed for CoNS or Granulicatella adiacens (p > 0.05). As shown in Figure 1.
| Species | Staphylococcus aureus | Coagulase negative staphylococcus | Pseudomonas aeruginosa | Streptococcus viridans | Escherichia coli | Morganella morganii | Granulicatella adiacens | Total |
|---|---|---|---|---|---|---|---|---|
| 2011 | 1 (50.0) | 0 (0.0) | 1 (50.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (4.7) |
| 2012 | 4 (57.1) | 1 (14.3) | 0 (0.0) | 0 (0.0) | 1 (14.3) | 1 (14.3) | 0 (0.0) | 7 (16.3) |
| 2013 | 4 (44.4) | 5 (55.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 9 (20.9) |
| 2014 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (2.3) |
| 2015 | 5 (55.6) | 2 (22.2) | 1 (11.1) | 1 (11.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 9 (20.9) |
| 2016 | 4 (66.7) | 1 (16.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (16.7) | 6 (14.0) |
| 2017 | 2 (40.0) | 3 (60.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 5 (11.6) |
| 2018 | 0 (0.0) | 1 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (2.3) |
| 2019 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 2020 | 2 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (4.7) |
| 2021 | 0 (0.0) | 0 (0.0) | 1 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (2.3) |
| Total | 22 (51.2) | 13 (30.2) | 3 (7.0) | 2 (4.7) | 1 (2.3) | 1 (2.3) | 1 (2.3) | 43 (100.0) |
| Χ 2 | 19.765 | 0.096 | 41.524 | 44.570 | 22.668 | 22.668 | 0.000 | 18.860 |
| p-Value | 0.000 | 0.756 | 0.000 | 0.000 | 0.000 | 0.000 | 1.000 | 0.000 |
3.2 Antimicrobial susceptibility testing of S. aureus and CoNS to common antibiotics
According to the drug sensitivity results of S. aureus and CoNS (Table 2), in this study, S. aureus was more sensitive to vancomycin, cotrimoxazole and linezolid, while CoNS was more sensitive to vancomycin, linezolid and chloramphenicol. There were no vancomycin-resistant or linezolid-resistant strains found. Both of them had high resistance rates to penicillin and erythromycin, and the resistance rate of CoNS to penicillin was 100%. In this study, there were 2 strains (9.1%) of MRSA and 5 strains (38.5%) of MRCNS. It is worth noting that the number of P. aeruginosa, S. viridans, E. coli, M. morganii and G. adiacens was small, the results of antimicrobial susceptibility testing of them were not described.
| Antibiotics | Staphylococcus aureus | Coagulase negative staphylococcus | ||
|---|---|---|---|---|
| S | R | S | R | |
| Methicillin | 81.8 | 9.1 | 46.2 | 38.5 |
| Cotrimoxazole | 96.4 | 9.1 | 61.5 | 15.4 |
| Penicillin | 9.1 | 90.9 | 0 | 100 |
| Clindamycin | 40.9 | 50.0 | 53.8 | 46.2 |
| Tetracycline | 77.3 | 22.7 | 61.5 | 23.1 |
| Levofloxacin | 72.7 | 13.6 | 69.2 | 23.1 |
| Erythromycin | 27.3 | 63.6 | 30.8 | 61.5 |
| Vancomycin | 100 | 0 | 100 | 0 |
| Chloramphenicol | 77.3 | 9.1 | 76.9 | 7.7 |
| Gentamicin | 68.2 | 22.7 | 69.2 | 23.1 |
| Linezolid | 90.9 | 0 | 100 | 0 |
- Abbreviations: R, resistance rate; S, sensitive rate.
3.3 Distribution of pathogenic bacteria for infections following different breast plastic surgery
The 42 cases of infection following breast plastic surgery included 16 cases of breast augmentations (breast augmentation with fat grafting, breast swelling operation with silicon capsule implant, breast augmentation by artificial material injection), 10 cases of breast reconstructions (breast reconstruction with autologous tissue, breast reconstruction with prosthetic materials), and 7 cases of breast reduction procedures. Breast augmentation with fat grafting, breast reduction procedure and breast reconstruction with autologous tissue were the most prevalent. Distribution of Gram-positive bacteria and Gram-negative bacteria in various breast plastic surgery procedures is shown in Table 3, and the difference was not statistically significant (p > 0.05). And distribution of specific pathogenic bacteria in various breast plastic surgery procedures is shown in Table 4.
| Surgical procedure | G+ (n) | G− (n) | X 2 | p-Value |
|---|---|---|---|---|
| Breast augmentation with fat grafting | 7 | 1 | 3.639 | 0.966 |
| Breast reduction procedure | 6 | 1 | ||
| Breast reconstruction with autologous tissue | 6 | 1 | ||
| Breast swelling operation with silicon capsule implant | 3 | 1 | ||
| Breast augmentation by artificial material injection | 4 | 0 | ||
| Breast injectable material removal surgery | 3 | 1 | ||
| Breast reconstruction with prosthetic materials | 3 | 0 | ||
| Else | 5 | 0 |
- Abbreviations: G−, Gram-negative bacteria; G+, Gram-positive bacteria.
| Surgical procedure | Staphylococcus aureus | Coagulase negative staphylococcus (CoNS) | PPseudomonas aeruginosa | Streptococcus viridans | Escherichia coli | Granulicatella adiacens | Morganella morganii | Total |
|---|---|---|---|---|---|---|---|---|
| Breast augmentation with fat grafting | 3 | 3 | 0 | 0 | 1 | 1 | 0 | 8 (19.0%) |
| Breast reduction procedure | 4 | 2 | 1 | 0 | 0 | 0 | 0 | 7 (16.7%) |
| Breast reconstruction with autologous tissue | 6 | 0 | 0 | 0 | 0 | 0 | 1 | 7 (16.7%) |
| Breast swelling operation with silicon capsule implant | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 4 (9.5%) |
| Breast augmentation by artificial material injection | 1* | 3* | 0 | 1 | 0 | 0 | 0 | 4 (9.5%) |
| Breast injectable material removal surgery | 1 | 2 | 1 | 0 | 0 | 0 | 0 | 4 (9.5%) |
| Breast reconstruction with prosthetic materials | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 3 (7.1%) |
| Else | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 5 (11.9%) |
| Total | 22 | 13 | 3 | 2 | 1 | 1 | 1 | 42 (100%) |
- * A case of polybacterial infection with S. aureus and CoNS.
3.4 Onset of infection
In this study, of the 42 cases of infection, 37 (88.1%) cases happened within 1 month after surgery (early infection), 5 (11.9%) cases occurred more than 1 month after surgery (late infection). The five late infections included one case of Liposuction after autologous fat injection and four cases of Breast augmentation by artificial material injection. The distribution of pathogenic bacteria in early and late infection is shown in Table 5, and the difference was not statistically significant (p > 0.05).
| Species | Early infection (n) | Late infection (n) | X 2 | p-Value |
|---|---|---|---|---|
| Staphylococcus aureus | 20 | 2 | 5.668 | 0.540 |
| Coagulase negative staphylococcus | 10 | 3 | ||
| Streptococcus viridans | 1 | 1 | ||
| Pseudomonas aeruginosa | 3 | 0 | ||
| Escherichia coli | 1 | 0 | ||
| Granulicatella adiacens | 1 | 0 | ||
| Morganella morganii | 1 | 0 |
4 DISCUSSION
A perfect breast shape is essential for achieving physical harmony, which has a significant impact on social interaction, quality of life, and psychological condition. People's expectations of their external appearance have steadily improved in recent years, and the demand for breast plastic surgery has gradually increased. Furthermore, the incidence of breast cancer is increasing year after year, with a younger tendency. Breast reconstruction following breast cancer surgery has also grown in importance in breast plastic surgery. Infection, a common complication of breast plastic surgery, has a negative impact on the surgical outcome and patient prognosis. A thorough understanding of the causes of infection, pathogenic bacteria, and antibiotic susceptibility is beneficial in preventing and controlling infection and reducing infection-related loss.
Some studies have indicated that the bacteria of breast infection is similar to the common bacteria of breast skin; therefore, it is hypothesized that breast infection may be related to exposure to skin bacteria during surgery.5, 6 Several investigations have determined that S. aureus and CoNS are the most prevalent strains on breast skin.7, 8 In this study, the most prevalent breast infection strains were S. aureus and CoNS, which was consistent with the theory that skin was the source of infection strains. Many studies have suggested that there are endogenous bacteria in the breast, and bacteria in the mucosa of the intestinal tract, oropharynx, and urogenital tract can be translocated to the breast via lactation and lymphatic system, which may be potential risk factors for infection. In many breast surgeries, the surgical incision is made in the areola to minimize the visual impact of the scar left by the incision,9 Washer et al. hypothesized that the higher risk of infection associated with such procedures was due to the endogenous flora of the nipple-areolar complex.10, 11 In addition, some breast plastic surgery involves the implantation of foreign bodies. Animal experiments have shown that the presence of subcutaneous foreign bodies reduces the number of pathogenic bacteria necessary for infection, and the absence of a microenvironment in the implantation material reduces neutrophil function, increasing the likelihood of infection.9
In a 58-sample investigation of infection following breast augmentation, 84.5% of the infected bacteria were Gram-positive bacteria, with S. aureus being the most frequent.12 The results of this experiment is similar with our study. There were 5 Gram-negative bacteria cases in this investigation, with P. aeruginosa accounting for the highest proportion. In recent years, an increasing number of Gram-negative bacteria have been identified in breast implant infections, particularly in the early infection stages.13 Pseudomonas aeruginosa has been found as the most prevalent Gram-negative bacteria causing breast prosthesis infection.14 And according to certain research, it was the second most prevalent infection strain in breast plastic surgery after S. aureus.9 Granulicatella adiacens and M. morganii were rare in other studies of breast infection, but in this study, G. adiacens and M. morganii caused 1 case of infection respectively, which may be related to opportunistic infection when the patient's immunity is reduced.
Walker et al. found CoNS as the most prevalent Gram-positive bacteria associated with breast prosthesis infection.14 In this study, infection of breast prosthesis is recorded in breast reconstruction with prosthetic materials and breast swelling operation with silicon capsule implant. In the former, the most prevalent infection strain was CoNS, whereas in the later, the proportion of S. aureus and CoNS was same, which may be related to the small sample size. During breast reduction surgery, Lohmeyer et al. discovered CoNS in 53% of breast tissue samples.7 However, S. aureus was the most common pathogen of breast reduction surgery infection in our study, I think this difference can be explained by the fact that CoNS can exist as normal bacteria without causing infection. Several studies have demonstrated that the risk of infection is 10 times greater for women receiving reconstruction surgery after mastectomy than for those getting breast augmentation for cosmetic purposes.11 Infection is the most common complication of breast reconstruction following breast cancer surgery.15 This may be related to poor blood supply in the breast skin following breast cancer surgery, and radiotherapy reduces the body's anti-infection capacity.7 Moreover, with the rise in popularity of nipple-sparing mastectomy, the lactation duct transected at the base of the nipple has become an open channel for pathogen entry and a potential infection focal site, which increased the risk of infection.16 In this study, a total of 10 individuals were infected with S. aureus following breast reconstruction. CoNS was the most prevalent infection strain in breast reconstruction with prosthetic materials, while S. aureus was the most prevalent infection strain in breast reconstruction with autologous tissue. However, Yoon et al. found that CoNS was the most common strain, followed by S. aureus, in a study on the microorganisms in the drainage fluid after breast reconstruction.5 This difference may be attributable to the fact that the study routinely analyzed all drainage fluids and CoNS is the most prevalent cause of occult infection.7 However, only samples displaying clinical signs of infection were included in our investigation.
Russell et al. proposed that breast infections are typically polymicrobial and that broad-spectrum antibiotics should be administered until culture findings are available.17 However, only one case of polybacterial infection was found in this study. Whether breast infections are often polybacterial needs more research. And the long-term use of broad-spectrum antibiotics to accelerate the development of bacterial resistance is still an unsolved serious problem, and antibiotic therapy was associated with high capsular contracture rate,12 thus it should be administered with caution. When first-line antibiotic treatment does not respond well, it should be considered.7, 18
In this study, early infection accounted for 88.1%, S. aureus was the most prevalent infection strain. There were 4 cases of infection after breast augmentation by artificial material injection, all of which were late infections, which is consistent with Corcione et al. ‘s view that late infection is frequently caused by inert bacteria (such as CoNS).11
Some studies suggest breast reconstruction infections following breast cancer surgery are more prevalent in the late stages,13, 19 but in this study, all breast reconstruction infections were in the early stages. Lalani believed that breast augmentation infections were more prevalent in the early stages.9 In this study, both autologous granulated fat injection and silicone gel prosthesis implantation were considered to be in the early stages of breast augmentation. However, breast augmentation by artificial material injection was in the late stage, suggesting that the timing of infection may be related to the surgical procedure.
5 CONCLUSIONS
This study summarized the bacterial species, drug sensitivity test, the differences in bacterial species and infection time between different types of plastic surgery of infection after breast plastic surgery in our hospital. However, due to the small sample size, further studies are still needed.
AUTHOR CONTRIBUTIONS
Sien Zhan, Jintian Hu, Xueshang Su, Fengli Jiang, and Yuanyuan Wu performed the research. Sien Zhan, Jintian Hu, Xueshang Su, and Fengli Jiang designed the research study. Fengli Jiang, Jintian Hu, and Ximeng Jia contributed essential reagents or tools. Fengli Jiang, Yuanyuan Wu, Ximeng Jia, and Jintian Hu analyzed the data. Jintian Hu and Xueshang Su wrote the paper. All authors have read and approved the final manuscript.
ACKNOWLEDGMENTS
We are grateful that this work was supported by the National Natural Science Foundation of China (81671933) and the Key Medical Discipline Research Project of Beijing Shijingshan District.
FUNDING INFORMATION
The authors have no financial interest to declare in relation to the content of this article.
ETHICS STATEMENT
The authors confirm that the ethical policies of the journal, as noted on the journal's author guidelines page, have been adhered to and the medical ethics review board of Plastic Surgery Hospital, Chinese Academy of Medical Sciences approved the proposal (20235).
Open Research
DATA AVAILABILITY STATEMENT
Research data are not shared.




