Regulation of epidermal proliferation and hair follicle cycling by synthetic photostable retinoid EC23
Abstract
Background
Retinoid signaling is an important regulator of the epidermis and skin appendages. Therefore, synthetic retinoids have been developed for therapeutic use for skin disorders such as psoriasis and acne.
Aims
In previous studies, we showed how the photostable retinoid EC23 induces neuronal differentiation in stem cell-like cell populations, and here, we aim to investigate its ability to influence epidermal and hair follicle growth.
Methods
EC23 influence on skin biology was investigated initially in cultures of monolayer keratinocytes and three-dimentional in vitro models of skin, and finally in in vivo studies of mice back skin.
Results
EC23 induces keratinocyte hyperproliferation in vitro and in vivo, and when applied to mouse skin increases the number of involucrin-positive suprabasal cell layers. These phenotypic changes are similar in skin treated with the natural retinoid all-trans retinoic acid (ATRA); however, EC23 is more potent; a tenfold lower dose of EC23 is sufficient to induce epidermal thickening, and resulting hyperproliferation is sustained for a longer time period after first dose. EC23 treatment resulted in a disorganized stratum corneum, reduced cell surface lipids and compromised barrier, similar to ATRA treatment. However, EC23 induces a rapid telogen to anagen transition and hair re-growth in 6-week-old mice with synchronously resting back skin follicles. The impact of EC23 on the hair cycle was surprising as similar results have not been seen with ATRA.
Conclusions
These data suggest that synthetic retinoid EC23 is a useful tool in exploring the turnover and differentiation of cells and has a potent effect on skin physiology.
Abbreviations
-
- 13CRA
-
- 13-cis retinoic acid
-
- ATRA
-
- All-trans retinoic acid
-
- DMSO
-
- Dimethylsulfoxide
-
- DMEM
-
- Dulbecco's modified Eagles medium
-
- TEER
-
- Trans epithelial electrical resistance
-
- TEM
-
- Transmission electron microscopy
1 INTRODUCTION
Retinoids are derivatives of vitamin A that elicit their biological activity through nuclear receptors and drive gene transcription of a large range of target genes. They play an important role in many aspects of cellular homeostasis across multiple organ systems.1 All-trans retinoic acid (ATRA) is the most bioactive product of vitamin A and regulates many aspects of biology including embryonic development,2, 3 patterning and differentiation of the nervous system,4 eye development, and epithelial maintenance.5, 6
Retinoids are also known to be important regulators of epidermal cells.7 For example, retinoids promote proliferation and inhibit differentiation in cultured keratinocytes along with inducing changes in expression levels of keratins.8 Furthermore, transcriptomic analysis revealed several differentiation-related genes are suppressed by retinoic acid confirming that retinoic acid inhibits both early and late stages of keratinocyte differentiation. Conversely, proliferation-associated genes regulating cell division are induced and cell cycle inhibitors down-regulated.9 This suggests that retinoids promote a proliferative phenotype while reducing cellular differentiation, which could have profound effects on epidermal structure and function. However, retinoid treatment in vivo is often more complex compared to in vitro studies; application of retinoic acid to human and mouse skin resulted in increased proliferation of basal keratinocytes, leading to an increased number of cell layers and thickening of the suprabasal strata with potent and chronic treatments associated with reduced terminal differentiation and impaired barrier function.10, 11
As retinoids have such a wide-spread effect on many aspects of skin homeostasis including keratinocyte proliferation, differentiation, skin surface lipids, sebum production, and extracellular matrix deposition, it makes them attractive molecules for use in skin therapeutics and cosmetics. Retinoids have long-since been used to treat conditions such as psoriasis, acne, and neoplasms and are often included in anti-aging preparations.7
While this wide range of activity suggests an important pharmacological use for natural retinoids, these benefits are dampened by two factors, the innate toxicity of these compounds,12 and by ATRA-mediated resistance, whereby ATRA is quickly broken down by cytochrome P450 enzymes, before beneficial effects are observed.13 This has prompted the synthesis of more stable analogues, capable of mediating the same biological processes, with an increased therapeutic value, particularly if they exhibit decreased toxicity and/or increased potency when compared with their natural counterparts.14
EC23 is a synthetic retinoid that exhibits enhanced photostability, resisting photoisomerisation in conditions that have dramatic effects on ATRA.15-17 EC23 is a potent agonist of both RARβ and RARγ but also activates RARα at nanomolar concentrations.18 Evaluation of its biological activity using pluripotent stem cells revealed that EC23 and ATRA both induced neuronal differentiation, as expected.15 The enhanced stability and potency of EC23 alongside its hypothesized role in skin prompted the evaluation of this synthetic retinoid during epidermal proliferation and differentiation studies.
2 RESULTS
2.1 Retinoid compounds induce keratinocyte proliferation and suppress differentiation in vitro
Initial investigations compared the biological effect of EC23 on cultured keratinocytes in vitro to those observed with ATRA. Both ATRA and EC23 resulted in an increased expression of the proliferation marker Ki67 in 2D cultured HaCaT keratinocytes (Figure 1Ai–iii,B). However, EC23 treatment resulted in a significant rise in the percentage of Ki67-positive cells compared with both the vehicle control and ATRA.
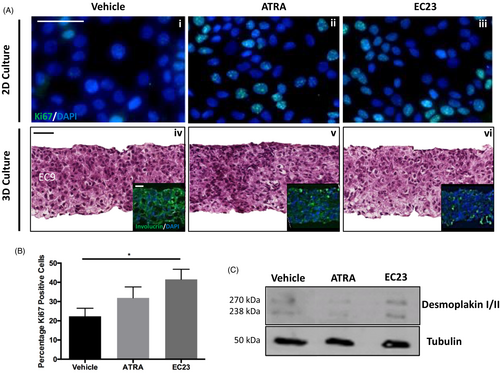
It is well documented that retinoids are able to supress junctional protein expression19-21 and have detrimental effects on skin's barrier integrity.7, 22, 23 Here, we have investigated the effect of retinoid treatment on desmoplakin expression in differentiated HaCaT cells. Western blot (Figure 1C) data suggest that both ATRA and EC23 induce downregulation of desmoplakin, a crucial component of desmosome junctions, following 4 days treatment.
However, investigating the nature of cell-to-cell interactions is limited in 2D culture, particularly as the skin is a three-dimensional tissue upon which many microenvironmental factors act. For this reason, we cultured HaCaT cells within a 3D porous, polystyrene scaffold: Alvetex® Scaffold. The use of Alvetex® Scaffold to bioengineer a multitude of tissue types (including skin equivalents) is well-documented.24-28 HaCaT cells were cultured within Alvetex® Scaffold for 14 days and then treated with retinoids for 4 days. H&E staining (Figure 1Aiv–vi) suggests that cultures treated with retinoid compounds are more densely populated by cells than their control counterpart and involucrin staining suggests reduced differentiation with retinoid treatment, particularly EC23. These 3D data, supporting the 2D findings, indicate that retinoids enhance keratinocyte proliferation, with EC23 potentially to a greater degree than ATRA.
Although 3D culture restores a more physiological geometry to cultures, the response of immortalized cell lines can be far-removed from the native tissue. We therefore employed a fully human epidermal equivalent to bridge the gap between in vitro and in vivo. Epidermal equivalents were treated with 0.1 μM EC23 or ATRA for 3 days. Histological analysis (Figure 2Ai–iii) reveals all models consist of a multi-layered, stratified tissue with expected morphology; however, retinoid treated epidermis appeared thicker than their control counterpart. Quantification revealed (Figure 2B), that 0.01 μM of either compound had little effect on epidermal thickness but 0.1 μM ATRA and to a greater degree EC23, significantly increased epidermal thickness compared with the vehicle control. This suggests both retinoid compounds exert a dose-dependent effect on epidermal thickness.
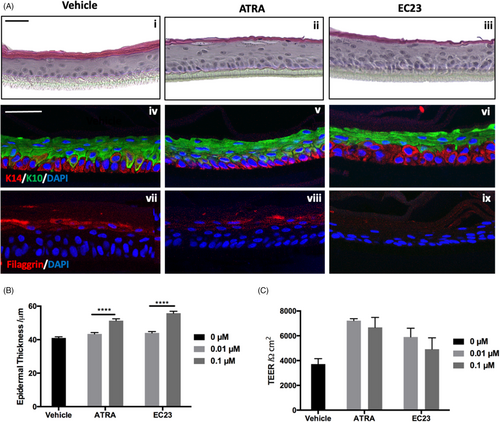
Epidermal equivalents were also stained for epidermal differentiation biomarkers: basally located keratin-14 (Figure 2Aiv–vi), suprabasal keratin-10 (Figure 2Aiv–vi) and filaggrin, a marker of terminal differentiation (Figure 2Avii–ix). EC23 treated epidermal constructs contain multiple keratin-14 positive cell layers, and fewer keratin-10 positive layers compared with ATRA and vehicle treatments. This strengthens the hypothesis that EC23 maintains a proliferative phenotype and suppresses differentiation.
Filaggrin staining is abundant in vehicle treated control tissues (Figure 2Avii); however, staining is diffuse with ATRA treatment (Figure 2Aviii) and barely visible with EC23 treatment (Figure 2Aix). This reduced expression of barrier-related proteins has consequences on barrier function as determined by trans-epithelial electrical resistance (TEER) (Figure 2C) which is initially increased with retinoid treatment but decreases dose-dependently and is reduced following EC23 treatment compared with ATRA. This suggests that reduced terminal differentiation has down-stream consequences on the integrity of the epidermal barrier.
2.2 Retinoid treatment induces keratinocyte proliferation and has implications on epidermal differentiation in vivo
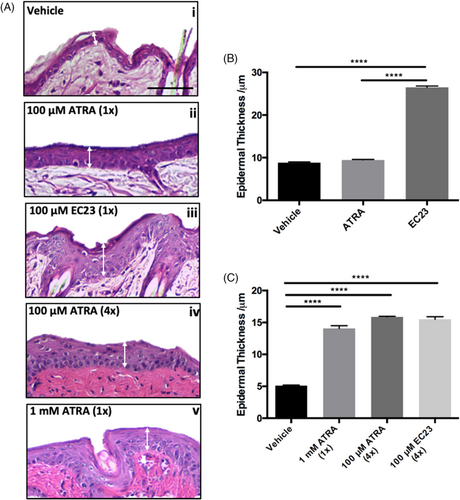
A more complex animal model was then studied to test the effects of both naturally occurring ATRA and synthetic EC23. This involved the topical application of 100 μM of each compound to the back of shaved mice. Initial data supplement in vitro findings with EC23 resulting in significant thickening of the epidermis (Figure 3A). A single dose of 100 μM ATRA resulted in little (not significant) epidermal thickening, whereas 100 μM EC23 resulted in significant epidermal thickening (Figure 3B). ATRA was able to generate the same level of epidermal thickening as EC23, but only after repeated application (four times) or at a higher (10-fold) concentration (Figure 3C). This highlights the enhanced potency of EC23, as treatment at the same concentration results in a response almost threefold greater than ATRA.
The hyperproliferative effect of EC23 is also reflected by enhanced keratin-14 staining in the suprabasal layers of the epidermis (Figure 4C) with more keratin-14 positive layers present than with ATRA (Figure 4B) or vehicle treatment (Figure 4A). This complements the previously described in vitro data, as the same phenomenon was observed within the bioengineered epidermal model (Figure 2).
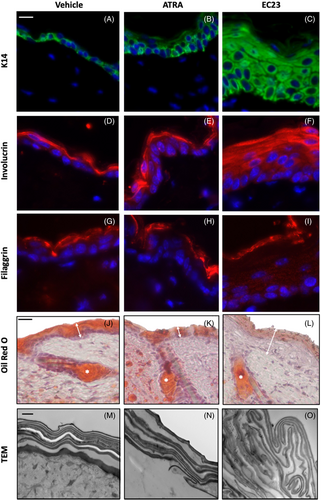
Involucrin, a keratinocyte differentiation marker involved in cornified envelope formation, is expressed in all treatment conditions (Figure 4D–F). This suggests that the early stages of keratinocyte differentiation are unaffected by either retinoid treatment. Broad suprabasal expression of involucrin following EC23 treatment (Figure 4F) suggesting that although the epidermis is hyperproliferative, keratinocytes have still committed to differentiation.
Filaggrin (Figure 4G–I) is abundantly expressed in vehicle treated samples (Figure 4G). However, to a certain degree with ATRA (Figure 4H) treatment but more so with EC23 (Figure 4I), staining becomes diffuse and less intense. This, in combination with in vitro data, suggests that treatment with both ATRA but to a greater extent, EC23 impairs terminal differentiation.
Retinoids are well-known to reduce sebum production and are widely used as a treatment for severe acne.29-32 We found that Oil-red O staining was reduced with both ATRA (Figure 4K) and EC23 (Figure 4L) treatment and in-keeping with the other parameters measured, EC23 reduced skin surface lipids significantly more than ATRA treatment.
Transmission electron microscopy (TEM) analysis (Figure 4M–O) revealed that the organization of cornified envelopes was disrupted with EC23 treatment. Abnormal and unevenly spaced cornified envelopes were identified within a thicker hyperkeratotic stratum corneum indicating that the accelerated proliferation and excess involucrin-positive suprabasal cell layers are accompanied by disorderly assembly of the stratum corneum.
To evaluate the effect of retinoid compounds on sustaining epidermal proliferation, mouse back skin was treated with a single dose of 1 mM ATRA, 100 μM EC23 or DMSO and samples were harvested up to 14 days following treatment. Immunofluorescence analysis of Ki67 (Figure 5A) and quantification (Figure 5B) confirms that ATRA and EC23 enhance epidermal proliferation in vivo in a similar manner to previous in vitro findings (Figure 1). Interestingly, EC23 maintained enhanced proliferation for a longer period of up to 7 days, whereas ATRA samples return to control levels sooner. This suggests not only enhanced potency of EC23 within this tissue, but also reflects the enhanced stability of EC23 and potentially reduced catabolism.
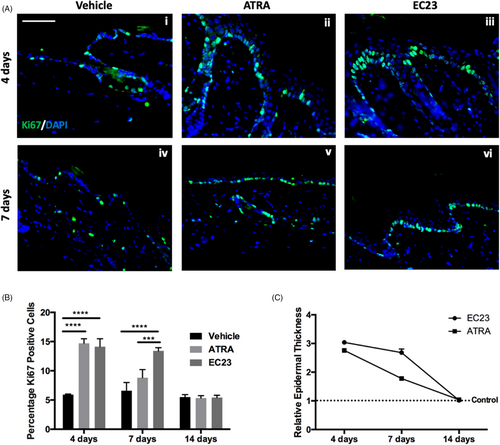
The long-lasting effects of EC23 are also demonstrated by its effects on epidermal thickness (Figure 5C). A single application of both 100 μM EC23 and 1 mM ATRA induce epidermal thickening to a similar degree and by 7 days post-treatment the thickness of ATRA treated samples has reduced by over 50%, whereas EC23 treated samples are still almost 3-times thicker than control levels. By 14-day post-treatment, both EC23 and ATRA treated skin has returned to control epidermal thickness levels, suggesting that although the effects of EC23 are sustained for longer than ATRA, they are reversible.
2.3 EC23 treatment disrupts the epidermal barrier and induces significant hair re-growth in vivo
Finally, the effects of chronic retinoid treatment on mouse back skin were investigated, which had effects on both barrier function and hair follicle cycling. Barrier dysfunction is a common side effect of medical and cosmetic retinoid treatment33 and due to the effects observed here in vitro, it was hypothesized that EC23 treatment would significantly reduce the barrier function of chronically treated mouse back skin.
A toludine blue penetration assay34 was used to test the permeability of the skin (Figure 6A). Mouse back skin was treated chronically with 100 μM ATRA, EC23 or vehicle control once every 7 days. A single treatment of EC23 rendered the epidermal barrier leaky and allowed penetration of the dye. However, ATRA had to be repeatedly applied at least 4 times to have a similar effect. This further supports our findings that EC23 is a more potent and bioactive molecule than ATRA.
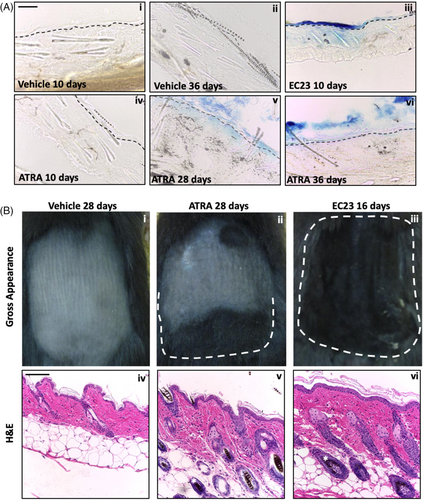
Hair loss is a common side effect of systemic retinoid treatments.35-37 Localisation of signaling components suggests endogenous RA synthesis participates in hair cycle regulation.38, 39 Mice that had their back skin synchronously in telogen were treated with the retinoids and changes in the hair cycle followed during five treatments up to 50 days. Surprisingly, all mice in the EC23 cohort exhibited hair re-growth and anagen hair follicles after two first treatments (Figure 6Biii,vi). ATRA required three repeated applications before the mice began to show at least partial hair growth in the treated area (Figure 6Bii,v).
3 DISCUSSION
In this study, we have investigated the effect of a photostable, synthetic retinoid, EC23 on skin structure and function. Although EC23 is a well-characterized RAR agonist and has been demonstrated to readily induce neuronal differentiation,14, 16, 25, 40, 41 it has yet to be studied in the context of skin biology. Here, we describe its potent ability to induce keratinocyte proliferation leading to epidermal thickening and hair re-growth. We assess EC23's function in multiple model systems ranging from in vitro cell cultures, to bioengineered human tissues in vitro to finally confirming our findings in vivo using a murine model.
Retinoids are a class of compounds readily used in skincare products due to their ability to direct gene expression and modulate a multitude of aspects of skin physiology.42-47 These include anti-aging effects such as increased collagen deposition,48-51 increased epidermal thickness,52-54 and a reduction in sebum production.55-57 Although retinoids are potent actives, a balance between biological activity and irritation exists with side effects including erythema, pruritis, and burning. For this reason, there has been a substantial effort to develop synthetic compounds, designed to overcome unwanted side effects.58
EC23 is a synthetic retinoid designed to overcome the stability issues associated with ATRA, which breaks down readily when exposed to light and heat. EC23 contains a non-isomerisable linker unit that provides complete stability and unlike ATRA, does not break down readily in laboratory conditions.17, 59 EC23 has been well-characterized in terms of stem cell differentiation and neuronal induction.14, 16, 25, 41, 60-62 In this study, we have assessed the effects of this compound on epidermal physiology in multiple model systems to establish the biological effects of EC23 in skin.
We have demonstrated enhanced keratinocyte proliferation both in vitro and in vivo following single dose EC23 treatment when compared with ATRA. Along with enhanced proliferation maintained for a longer period (7-day post-treatment) post-EC23 treatment before levels returned to control levels at 14-day post-treatment. This suggests both that EC23 is more potent than ATRA but also has longer lasting biological effects, potentially due to reduced metabolism. It is also interesting to observe proliferation did return to control levels in the long-term, which suggests that although the effects of EC23 are long-lived, they are not permanent. This finding is also supported by an increased number of keratin-14 positive cell layers both in vitro and in vivo.
The enhancement of keratinocyte proliferation reported, translates to epidermal thickening. We tested the effect of EC23 on an epidermal equivalent which has previously shown responsiveness to commonly used retinoids.63 3D engineered tissues provide a more physiological platform for investigations and bridge the gap between 2D cell culture and native tissues.64, 65 We demonstrate significant epidermal thickening of this tissue construct following EC23 treatment to a greater degree than ATRA. Similarly, in vivo application to mouse back skin also induced epidermal thickening of almost threefold compared to controls, whereas to achieve the same level of thickening ATRA treatment had to be either repeated 4 times or concentration increased 10-fold. Again, this further evidences increased potency of EC23 and like Ki67 expression; increased epidermal thickness was maintained for longer post-EC23-treatment but ultimately returned to control levels following 14 days.
It is well-documented that a balance exists between retinoid potency and unwanted side effects of topically applied retinoids.66 At a cellular level, these can include effects on terminal keratinocyte differentiation, lipid composition, and junctional proteins which can disrupt the epidermal barrier.10, 20, 56, 67, 68 Here, we demonstrate that EC23 treatment downregulates desmoplakin expression in vitro, a known consequence of retinoid treatment. We also demonstrate reduced terminal keratinocyte differentiation following EC23 both in vitro and in vivo through reduced filaggrin staining. However, increased involucrin staining following EC23 treatment suggests that although keratinocytes differentiation is affected, they are undergoing early differentiation changes. Furthermore, we also demonstrate reduced epidermal lipids and structural changes to the stratum corneum, all of which contribute to the integrity of skin as a barrier.
These cell and tissue level changes translated into functional barrier deficits, detected in tissues both in vitro and in vivo. In vitro this was a dose-dependent decrease in TEER, a measurement which reflects barrier function of the tissue.69 Similarly, in vivo this was demonstrated by a dye permeability assay. These data reveal that EC23 again has more potent biological activity than ATRA, particularly as a single dose of EC23 rendered mouse skin leaky, whereas multiple re-applications of ATRA were required to induce the same response.
Finally, we found that EC23 has a striking effect on promoting telogen to anagen transition. Repeated application of ATRA also resulted in earlier hair re-growth than control treatment but the effect was modest. Retinoids and their receptors are known to modulate hair cycling, but the effects are complex. Conditional epidermal deletion of RARα leads to delayed anagen entry and eventually to alopecia,70 which demonstrates how retinoid signaling is required for normal progression of the hair cycle. However, elevated levels of endogenous retinoic acid via depletion of a RA degrading enzyme Cyp26b1 lead to arrest of hair growth.71 Likewise, it was found that cultured anagen stage human scalp hair follicles enter catagen when treated with ATRA.72 The complex regulation is also reflected by differential expression of RA-binding proteins CRABP1 and CRABP2 during the hair cycle.73 It may be hypothesized that epidermal hyperproliferation and compromised barrier may elicit a wound healing type of response that is known to promote formation of new follicles.74
In this study we describe for the first time the biological effects of EC23, a synthetic, photostable retinoid compound on several models of skin biology. Our findings have been consistent across each approach in that EC23 has a more potent effect than naturally occurring ATRA and induces keratinocyte proliferation, epidermal thickening with effects on epidermal differentiation, barrier function, and hair re-growth. These effects may be attributed to EC23's increased stability, potency and even potentially reduced metabolism within target cells, making this synthetic molecule an attractive target for further studies in potential clinical and biotechnological applications.
4 MATERIALS AND METHODS
4.1 Cell culture
HaCaT cells were cultured in Dulbecco's Modified Eagle Medium (DMEM) (ThermoFisher Scientific) supplemented with 10% FBS (Merk Sigma) and 1% penicillin/streptomycin (Merk Sigma), and passaged at 70%–80% confluence. Cultures were incubated at 37°C, 5% CO2 in a humidified environment. EC23 (Reprocell) and ATRA (Merk Sigma) were prepared in dimethylsuloxide (DMSO) (Merk Sigma) and stored at −80°C.
Alvetex® scaffolds (Reprocell) were used to culture HaCaT in 3D. 1 x 106 cells were pipetted onto the center of each scaffold, and cells were allowed to attach for 30 min at 37°C, grown 48 h submerged, and raised to air liquid interface (ALI) for further 14 days in the presence of retinoids or DMSO.
In vitro epidermal constructs were generated as previously described.24 At 10 days ALI, models were treated with retinoids or DMSO and harvested after 3 days of treatment.
4.2 Trans epidermal electrical resistance
TEER was measured from epidermal models in DPBS using a Millicell® ERS-2 Voltohmmeter and standard probe.
4.3 In vivo trials
Experimental procedures were performed under a UK government Home Office license (60/3941). Individual treatment groups were housed separately throughout the study (maximum of four animals per cage), with access to food and water ad libitum. All mice used were sex-matched C57/BL6 (female) and 6 weeks of age, in order to ensure that all mice were in telogen. Prior to treatment, a 2 × 3 cm area was shaved on the dorsal region of each mouse (three mice per condition). Retinoid stock solutions were diluted accordingly in acetone.
4.4 Paraffin embedding
In vitro 3D cultures and mouse skin samples were fixed in 10% formalin (Merk Sigma), gradually dehydrated in ethanol (30%–100%) followed by Histoclear (ThermoFisher Scientific), and embedded in paraffin wax (ThermoFisher Scientific) using plastic molds (CellPath). Using a microtome (LEICA RM2125RT), 5 μm sections were transferred to charged superfrost microscope slides (ThermoFisher Scientific) for down-stream processing.
4.5 Histological analysis
Sections were H&E stained as previously described.24 Briefly, samples were deparaffinized and rehydrated before staining in Mayer's hematoxylin (Sigma-Aldrich) followed by alkaline alcohol. Samples were counterstained in eosin (Sigma-Aldrich) and mounted with DPX (Thermo Fisher Scientific). Images were captured using a Leica ICC50 high-definition camera mounted onto a Brightfield Leica microscope. Viable epidermal thickness was measured from H&E stained sections using Image J software and data analyzed using GraphPad Prism where an ANOVA was used to calculate significance as appropriate: * = p < 0.05, ** = p < 0.01, *** = p < 0.001, **** = p < 0.0001.
4.6 Oil red O staining
Formalin fixed sections were rinsed in water followed by 60% isopropanol (Merk Sigma). Samples were stained with a 0.5% Oil Red O stain in isopropanol (Merk Sigma) followed by rinses in 60% isopropanol. Samples were counterstained with Mayer's hematoxylin (Merk Sigma) and mounted using an aqueous mount (Merk Sigma).
4.7 Immunofluorescence
Immunofluorescence of 2D culture and paraffin wax sections was conducted as previously described.25 Primary antibodies targeting ki67 (Merk Sigma), keratin-14 (Covance), keratin-10, involucrin, and filaggrin (Abcam) were used along with relevant fluorescent secondary antibodies (donkey anti-rabbit Alexa Fluor 488 or 594 or donkey anti-mouse Alexa Fluor 488 or 594, Thermo Fisher Scientific).
All images were captured using Zeiss 880 with Airyscan confocal microscope with ZEN software.
4.8 Immunoblotting
Protein concentrations of lysates were determined using the Bicinchoninic Acid (BCA) Protein Assay kit (ThermoFisher Scientific). Immunoblots were probed with antibodies against desmoplakin (mAb 1:1000 dilution) and β-tubulin (mAB,1:2000 dilution, Merk Sigma) using ECL Plus Western Blotting Detection System (GE Healthcare).
4.9 Toluidine blue penetration assay
Skin samples were processed for Toluidine Blue penetration assay as previously described.34
4.10 Transmission electron microscopy
Skin samples were fixed in Karnovsky's fixative (2.5% glutaraldehyde and 4% paraformaldehyde in Sorensen's buffer (pH 7.4) (Agar Scientific), before being embedded in araldite resin (Agar Scientific). Ultra-thin (70 nm) sections were cut using a diamond knife (Agar Scientific) on a Reichert Ultracut S Ultramicrotome (Leica). Sections were stained with 1.5% uranyl acetate (VWR) and lead citrate (VWR) and analyzed with a H7600 transmission electron microscope (Hitachi).
AUTHOR CONTRIBUTION
Project design and supervision were provided by CAA, AM, and SP. Two-dimensional cell culture, animal work ,and subsequent down-stream processing and analysis were performed by RN and NR. Three-dimensional cell culture, subsequent down-stream processing, and analysis and manuscript drafting were performed by KG and VM. All co-authors reviewed and approved the final manuscript. SP is the principal investigator and corresponding author.
ACKNOWLEDGMENTS
The research was funded by a research grant obtained from the Medical Research Council in the form of a CASE studentship. The authors would like to thank Mrs Christine Richardson for expert help with electron microscopy.
CONFLICT OF INTEREST
S. Przyborski is affiliated with Reprocell Europe. All other authors state they have no conflicts of interest.
ETHICAL APPROVAL
In vivo experimental procedures were performed under a UK government Home Office license held by Professor C Ambler (60/3941). Individual treatment groups were housed separately throughout the study (maximum of four animals per cage), with access to food and water ad libitum.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




