Foreign body reaction to polyacrylamide filler (Aquafilling®) injected nine years previously: A complication of SARS-CoV-2 infection or merely a coincidence?
To the Editor,
Aquafilling® (BIOTRH, spol, s.r.o., Czech Republic) is a kind of synthetic nonbiodegradable hydrophilic gel, which is employed around the world for breast augmentation to correct mild undesirable results after silicone implants.1 It is composed of 98% physiological saline and 2% poly (acrylamide-co-N, N0-methylene-bis-acrylamide). Beside breast and buttock augmentation, it is used for face rejuvenation in some countries. However, in some countries, the use of Aquafilling® is prohibited, hence polyacrylamide gel has relatively high rate of complication risk.2 Here, we describe a female patient who had suffered from inflammatory reaction during SARS-CoV-2 infection and subsequently developed foreign body reaction to Aquafilling®, which was injected for face rejuvenation nearly a decade ago.
A 36-year-old woman presented with a 5-month history of asymptomatic nodules on her left cheek. Nine years earlier, she underwent Aquafilling® injection for midface rejuvenation. After that time, she had never undergone an aesthetic procedure. She had developed abrupt onset painful red swelling on her left cheek 6 months before her presentation to our clinic (Figure 1A). She had hospitalized with the presumptive diagnosis of soft tissue infection and treated with parenteral antibiotics. During her hospital stay, due to the presence of an influenza-like illness, she was also tested for coronavirus. Nasopharyngeal swab RT-PCR-based SARS-CoV-2 detection was positive. The erythematous swelling gradually displayed resolution in the following 6 weeks, with residual disfiguring nodules. Then, she had undergone hyaluronidase injection without improvement in other private clinics.
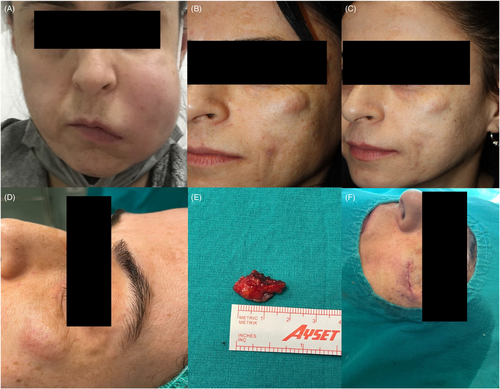
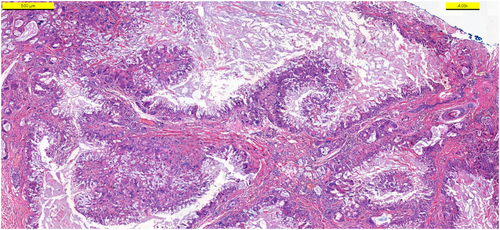
On dermatological examination, there was a nontender, noninflamed, mobile nodule on her left cheek (Figure 1B). Smaller skin-colored xhanthelasma-like papules were present around eyelids and perioral area. The patient was initially treated with systemic corticosteroid (oral methylprednisolone, 1 mg/kg, daily) with minimal resolution (Figure 1C). Surgical removal of the nodule was performed (Figure 1D–F). Histopathological examination revealed that deep dermis was diffusely infiltrated by histiocytic cells surrounding amorphous foreign body material and focally forming granuloma-like structures (Figure 2). Considering clinical and pathological characteristics of the patient, the diagnosis of foreign body reaction was made.
Aquafilling® is not approved by US Food and Drug Administration (FDA). Moreover, there are many reports on increasing concerns about long-term safety of Aquafilling®.1, 3 Based on these reports, breast deformity is the main complication, which may require surgical removal of the material and reconstruction. Among the patients with breast or buttock augmentation, the most common complication is induration and masses, with the frequency of 83%.1 On the contrary, there is scant literature on Aquafilling® associated reaction in the face area, probably due to limited experience as a facial augmentation agent and availability of various licensed HA fillers for use.
Foreign body granuloma (FBG) is a well-known late inflammatory reaction (LIR) of polyacrylamide gels.2 The exact pathogenesis and triggers of FBG still remain elusive. They may occur even several years after the injection and are often refractory to treatment. Recently, a report has described late-onset reactions at different time points in a patient injected Aquafilling® for facial rejuvenation.4 Similar to the patient described by Kong et al., there was no history of systemic disease or prior trauma in our patient. On the contrary, the complication of our patient occurred much more later than injection. Regarding possible triggers in such a late reaction, our patient was unique due to the presence of filler reaction accompanying native coronavirus disease. Considering the association of SARS-CoV-2 infection and foreign body reaction to polyacrylamide gels and/or any other permanent filler, we could not find any report in the literature. However, the literature review highlights LIRs including with hyaluronic acid (HA) dermal fillers after SARS-CoV-2 infection or vaccination.5-7 Bachour et al. postulate that LIRs toward HA might occur within a few hours up to several weeks after the SARS-CoV-2 infection.5 Based on these reports, immunologic cross-reaction mediated by CD4 + T-cell and macrophage activation in the cytokine storm of coronavirus seem to be responsible LIRs associated with HA fillers. Of note, SARS-CoV-2-induced Type IV hypersensitivity might be eligible for our patient. Based on the reports suggesting the role of biofilm formation in LIRs associated with permanent fillers, we might speculate that active coronavirus infection, as an external triggering factor, could trigger the transformation of dormant biofilm to active form. This may explain why our patient developed inflammatory swelling. On the contrary, it is not straightforward to justify clear relationship because of some questionable points. For instance, we cannot explain why the reaction was asymmetrically affected left side despite she was injected same agent equally in both sides.
In conclusion, the use of non-biodegradable products in particular unapproved ones poses more risk for late-onset complications requiring surgical intervention. Considering ongoing coronavirus infection and vaccination program, aesthetic practitioners should consider all potential risks and avoid inappropriate filler materials for face rejuvenation. Emergency providers should also be aware of apparent manifestations of reactions associated with fillers because of commonly being the first point of contact. Patients should also be correctly informed about the risk of SARS-CoV-2-related filler and implant reactions.
ACKNOWLEDGMENT
The patient in this manuscript has given written informed consent to the publication of her case details.
FUNDING INFORMATION
The author(s) reported there is no funding associated with the work featured in this article.
CONFLICT OF INTEREST
No potential conflict of interest was reported by the author(s).
ETHICAL APPROVAL
None.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.




