Role of HIF-1α in pathogenic mechanisms of keloids
Abstract
Backgrouds and Objective
Keloids are defined as overrepairing products that develop after skin lesions. Keloids are characterized by the proliferation of fibroblasts and the overaccumulation of extracellular matrix components (mainly collagen), leading to a locally hypoxic microenvironment. Hence, this article was aimed to review hypoxia in pathogenesis of keloids.
Methods
We reviewed and summarized the relevant published studies.
Results
Hypoxia results in the accumulation of hypoxia-inducible factor 1α (HIF-1α) in keloids, contributing to overactivation of the fibrotic signaling pathway, epithelial–mesenchymal transition, and changes in metabolism, eventually leading to aggravated fibrosis, infiltrative growth, and radiotherapy resistance.
Conclusion
It is, therefore, essential to understand the role of HIF-1α in the pathogenic mechanisms of keloids in order to develop new therapeutic approaches.
Abbreviation
-
- DAMPs
-
- damage-associated molecular patterns
-
- ECM
-
- extracellular matrix
-
- EMT
-
- epithelial–mesenchymal transition
-
- ERK1/2
-
- extracellular signal-regulated kinase 1/2
-
- GLUT1
-
- glucose transporters 1
-
- HBOT
-
- hyperbaric oxygen therapy
-
- HIF-1
-
- hypoxia-inducible factor-1
-
- HRE
-
- hypoxia-response element
-
- HS
-
- hypertrophic scars
-
- KFs
-
- keloid fibroblasts
-
- MAPKs
-
- mitogen-activated protein kinases
-
- mTOR
-
- mammalian target of rapamycin
-
- MyD88
-
- myeloid differentiation factor 88
-
- NFs
-
- normal fibroblasts
-
- NF-κB
-
- nuclear transcription factor-κB
-
- ODDD
-
- oxygen-dependent degradation domain
-
- PAI-1
-
- plasminogen activator inhibitor-1
-
- PDGF
-
- platelet-derived growth factor
-
- PH
-
- pleckstrin homology
-
- PI3K
-
- phosphatidylinositol-3-kinase
-
- PIP2
-
- phosphatidylinositol diphosphate
-
- PIP3
-
- phosphatidylinositol triphosphate
-
- PKD1
-
- 3-phosphoinositide-dependent protein kinase 1
-
- PTEN
-
- phosphatase and tensin homolog deleted on chromosome ten
-
- ROS
-
- reactive oxygen species
-
- RTK
-
- receptor tyrosine kinase
-
- TAC
-
- tricarboxylic acid cycle
-
- TGFR
-
- transforming growth factor beta receptor
-
- TGF-β
-
- transforming growth factor-β
-
- TLRs
-
- Toll-like receptors
-
- VEGF
-
- vascular endothelial growth factor
-
- α-SMA
-
- α-smooth muscle actin
1 INTRODUCTION
Skin is considered to be the largest organ in the human body and functions to protect the internal tissues and organs from lesions, radiation, infections and extreme temperature. However, the skin is also vulnerable to damage, and wound healing is thus an important process in the human body. A hypoxic microenvironment plays an important role in wound healing in the event of skin lesions, such as burns, infections or trauma,1 by promoting various wound-healing processes, including cell proliferation, epithelial–mesenchymal-transition (EMT) and immunochemotaxis.2-9 As the wound-healing process gradually slows down, it generally results in mature scars. However, keloids are defined as overrepairing products that develop after skin lesions, and are characterized by the overabundant accumulation of extracellular matrix (ECM) and progressive invasion into the adjacent normal skin, exceeding the original wound margin.10 Patients with keloids often suffer from severe physical and psychological symptoms, including pain, pruritus, and depression, which may affect their self-confidence and appearance. Although surgical excision combined with radiotherapy has some curative potential for keloids, obstacles to successful therapy include recurrence and multiple keloids, which are difficult to excise. There is thus a need to explore the pathogenesis of keloids in order to develop more reliable treatments.
Hypoxia-inducible factor-1 (HIF-1) is considered to be the main regulator of hypoxia and plays an important role in fibrotic diseases and malignant neoplasms.11, 12 Numerous studies have demonstrated that keloids have a hypoxic microenvironment due to fibroblast proliferation and collagen synthesis. This leads to the accumulation of HIF-1α, which can, in turn, activate the fibrotic pathway through various mechanisms, resulting in the secretion of cytokines leading to angiogenesis, EMT and changes in the levels of glycolysis in keloids, further leading to tumor-like biological behavior.13-16 It is, therefore, important to explore and understand the role of HIF-1 in the pathogenic mechanisms of keloids in order to develop more effective treatments. There have been few reviews on the specific mechanisms linking HIF-1 and keloids. The current review thus aims to explain the mechanisms of HIF-1 in regulating fibrosis, EMT, glucose metabolism and angiogenesis in keloids, and discusses some new potential ways of targeting HIF-1 in keloid therapy. The crosstalk of hypoxia signaling in keloids and the biological functions of HIF-1α in different cells are illustrated in Figure 1-3.
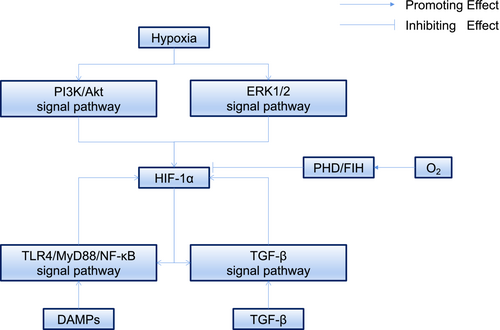
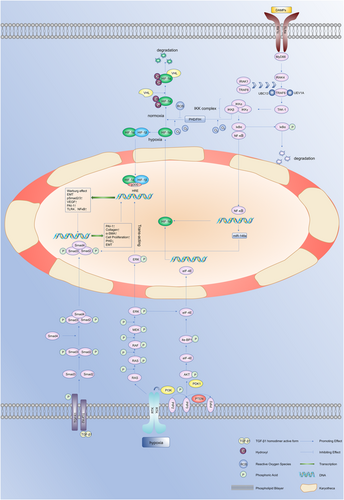
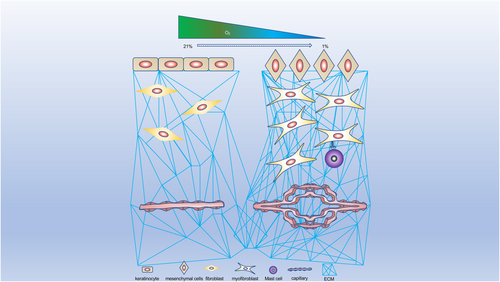
2 PHOSPHOINOSITIDE 3-KINASE (PI3K)/AKT AND EXTRACELLULAR SIGNAL-REGULATED PROTEIN KINASE (ERK)1/2 PATHWAYS MEDIATES HIF-1Α EXPRESSION IN KELOIDS
HIF-1 is an ubiquitous protein in human and mammalian cells,17 and is composed of two subunits, HIF-1α (120 kilodaltons) and HIF-1β (91–94 kilodaltons).18 HIF-1α is regulated by an oxygen-dependent degradation domain and is highly sensitive to oxygen.19 Under normoxic conditions, HIF-1α is hydroxylated by the prolyl hydroxylase domain (PHD) through two proline residues within the oxygen-dependent degradation domain and is then recognized by the von Hippel–Lindau protein, resulting in its degradation after ubiquitination.20 In addition, asparaginyl hydroxylase, also known as factor inhibiting HIF (FIH), can also hydroxylate HIF-1α residue 803. This prevents the coactivator p300/cyclic adenosine monophosphate response element-binding protein-binding protein (CBP) from binding with HIF-1α, which, in turn, prevents HIF-1α from binding with the hypoxia-response element (HRE).21-24 Because hydroxylation is an oxygen-dependent reaction, this process is inhibited under hypoxia, thus stabilizing HIF-1α, which then combines with HIF-1β to form HIF-1. HIF-1 then translocates into the nucleus, recruiting p300/CBP to recognize HRE, resulting in the activation of downstream target genes.25 High expression of HIF-1α thus often indicates that the cells or tissues have experienced hypoxia.
Lei et al. found that keloid fibroblasts (KFs) were more sensitive to hypoxia than normal fibroblasts (NFs),26 indicating that the signaling pathway regulating HIF-1α expression in keloids was abnormally activated and sensitive. There is substantial evidence for the overexpression of HIF-1α in keloids.13-15 Excessive fibrosis and proliferation of KFs increase local oxygen consumption and lead to an insufficient energy supply, resulting in local relative hypoxia. This, in turn, leads to abnormal activation of many signaling pathways, which play key roles in the transcriptional enhancement and stability of HIF-1α.
We consider the relationship between the abnormally activated pathway and HIF-1α in keloids under hypoxic conditions below. The PI3K/Akt/mammalian target of rapamycin (mTOR) signaling pathway allows cells to respond to changes in the external environment and plays an important role in many diseases.27 When receptor tyrosine kinases (RTKs) are stimulated by various signal,28 they activate PI3K resulting in the phosphorylation of phosphatidylinositol diphosphate (PIP2) to phosphatidylinositol triphosphate (PIP3), regulating signaling proteins such as Akt.29 Under normal conditions, PIP3 is dephosphorylated by phosphatase and tensin homolog deleted on chromosome ten (PTEN) to PIP2, which plays an important role in the negative regulation of the PI3K/Akt/mTOR pathway. Activation of the PI3K/Akt pathway may be beneficial for cell growth and survival in extreme environments, through stabilization of HIF-1α. PI3K can regulate the synthesis of Akt and its downstream protein mTOR, which can phosphorylate eukaryotic translation initiation factor 4 E-binding protein (4 E-BP1), resulting in HIF-1α translation.30
ERK1/2 belongs to the mitogen-activated protein kinase (MAPK) family.31 Each MAPK pathway comprises a three-tiered cascade including a MAPK, MAPK kinase (MAP2K) and MAP2K kinase (MAPK3K). In the ERK1/2 pathway, Ras protein (as a small G protein) acts as an upstream activator, Raf as MAP3K, MAPK/ERK kinase (MEK) as MAP2K and ERK as MAPK, forming the Ras/Raf/MEK/ERK pathway.32 Following stimulation by growth factors, Ras becomes activated initiating the Ras/Raf/MEK/ERK cascade and resulting in phosphorylation of 4 E-BP1 and increased HIF-1α translation.33
Zhang et al. found that the PI3K/Akt pathway was activated through stimulation of RTKs by hypoxia in KFs, leading to significantly increased HIF-1α mRNA transcription and protein translation, upregulation of plasminogen activator inhibitor-1 (PAI-1) promoter activity and expression of PAI-1 protein.34 Moreover, PTEN expression levels in keloids were shown to be significantly lower than in normal skin tissues, which may also increase the expression of HIF-1α.35, 36 In contrast to the PI3K/Akt signaling pathway, inhibition of ERK1/2 did not effectively reduce the expression of HIF-1α, but did reduce the expression of PAI-1, the downstream target gene product of HIF-1α, indicating that ERK1/2 does not directly increase the expression of HIF-1α but can affect the expression of PAI-1 through transacting factors.34 Mast cells can also induce HIF-1α overexpression in KFs by activating the PI3K/Akt and ERK1/2 pathways through direct co-culture with KFs, suggesting that a hypoxic microenvironment might affect the immune microenvironment and thus the pathogenic mechanisms of keloids.37
3 BIOLOGICAL EFFECTS OF HIF-1Α IN DIFFERENT CELLS: PROMOTING KELOID FORMATION
3.1 KFs and HIF-1α
Fibroblasts play an important role in the process of wound healing by synthesizing and secreting collagen and forming the ECM, which is an important component of granulation tissue. However, KFs become overactivated, leading to the deposition of large amounts of collagen and ECM, causing serious fibrosis of the skin and leading to keloid formation. Numerous studies have shown that HIF-1α is highly expressed in keloids and promotes collagen synthesis.14 The accumulation of HIF-1α in fibroblasts leads to the activation of various signaling pathways, thus amplifying and activating a series of functions, such as fibrosis and glycolysis. Alterations in these signaling pathways (such as transforming growth factor (TGF)-β/Smad and Toll-like receptor 4 (TLR4)/nuclear factor (NF)-κB) and metabolic function-related signaling pathways play important roles in promoting keloid progression.
3.1.1 HIF-1α amplifies TGF-β/Smad signaling pathway in KFs
TGF-β/Smad is an important signaling pathway in fibrotic diseases such as renal fibrosis, liver fibrosis and keloids.38-41 TGF-β is a member of the TGF-β superfamily.42 TGF-β binds to the TGF-β receptor 2 (TGFR2) in the homodimer active form, and the latter then recruits and activates TGFR1,43 which, in turn, phosphorylates Smad2 and Smad3. Smad2 and Smad3 then bind to Smad4 to form a complex, which is transported to the nucleus. Finally, Smad3 binds to the promoter of genes encoding proteins such as PAI-1, collagen and fibronectin to initiate their transcription and translation, leading to fibrosis.38 Besides, the TGF-β/Smad signaling pathway also specifically reduced PHD transcription and translation, thus increasing HIF-1α stability.44
In keloids, the TGF-β/Smad signaling pathway can promote the proliferation and activation of KFs, reduce apoptosis and increase the secretion of collagen and PAI-1, resulting in fibrosis.45-48 The rapid proliferation of fibroblasts and the synthesis of large quantities of collagen means that keloids have a hypoxic environment, resulting in the accumulation of HIF-1α.16 Lei et al. found that HIF-1α expression induced by hypoxia (1% O2) could significantly upregulate TGFR2 expression and pSmad2/3 protein levels, and promote the proliferation and inhibit the apoptosis of KFs.26
HIF-1α may also amplify fibrosis by affecting the TGF-β/Smad signaling pathway, which induces the transformation of fibroblasts into myofibroblasts. During wound healing, fibroblasts may transform into α-smooth muscle actin (α-SMA)-positive myofibroblasts and secrete more ECM than normal fibroblasts.49 Myofibroblasts gradually disappears along with the formation of a mature and stable scar.50, 51 However, cellular markers of myofibroblasts can often be detected in keloid tissue, and pseudotemporal ordering in single-cell sequencing also confirmed that fibroblasts in keloids tended to transform into myofibroblasts.36, 52, 53 The abnormally active state of myofibroblasts in keloids may be closely related to the hypoxic microenvironment, causing an interaction between TGF-β/Smad and HIF-1α. Zhao et al. found that hypoxia combined with exogenous TGF-β significantly improved the phosphorylation level of Smad2/3 compared with exogenous TGF-β alone, while α-SMA and collagen levels were also significantly increased. Removal of exogenous TGF-β also led to increased phospho (p) Smad2/3, collagen and α-SMA levels compared with the blank control group under hypoxic conditions, but this process was significantly inhibited by the addition of the specific Smad3 inhibitor, SIS3. These results indicated that HIF-1α interacted with the TGF-β/Smad signaling pathway via Smad3, resulting in the transformation of fibroblasts into myofibroblasts, which secreted more collagen and thus amplified the fibrotic effect.54
3.1.2 Mutual promotion of HIF-1α and TLR4/myeloid differentiation factor 88 (MyD88)/NF-κB in KFs
TLRs are transmembrane signal transduction receptor proteins that play a key role in the innate immune system.55 TLR4 is involved in immune recognition by recognizing bacterial lipopolysaccharides and damage-associated molecular patterns (DAMPs).55, 56 When the human body is injured, DAMPs are released and recognized by TLR4, which phosphorylates interleukin-1 receptor-associated kinase 4 (IRAK4) through the adaptor protein MyD88, enabling IRAK4 to recruit IRAK1, which, in turn, recruits the adaptor protein TNF receptor-associated factor 6 (TRAF6). TRAF6 then forms a complex with ubiquitin ligases (UBC13, UEVLA) to activate TGF-β-activated kinase 1, which phosphorylates the inhibitor of NF-κB (IκB) kinase (IKK) complex (composed of IKKα, IKKβ and IKKγ). IKKβ then phosphorylates IκBα leading to IκBα ubiquitination and degradation, releasing NF-κB to transfer into the nucleus to initiate target gene expression.57 Under normoxic conditions, PHD and FIH cause inactivation of IKKβ, but IKKβ is active during hypoxia, allowing NF-κB to transfer into the nucleus to mediate HIF-1α transcription.58-60 At the same time, the binding of HIF-1α to HRE initiates NF-κB and TLR transcription, thus amplifying the NF-κB signal.61 Hypoxia thus leads to mutual activation between HIF-1α and NF-κB, amplifying the effects of both signals.
The TLR4/MyD88/NF-κB signaling pathway plays an important role in many fibrotic diseases, including systemic scleroderma, hepatic fibrosis and renal fibrosis.62, 63 Chen et al. found that TLR4 expression was significantly higher in KFs compared with NFs, and TLR4 was likely to increase the expression of TGF-β, amplify the Smad4 signaling pathway and promote keloid fibrosis.64 KFs with hypoxia express higher levels of HIF-1α, TLR4, MyD88 and NF-κB than KFs, NFs with normoxia and NFs with hypoxia, indicating that hypoxia causes mutual promotion and activation between HIF-1α and NF-κB in keloids. NF-κB can promote the expression of pSmad2/3 and TGFR2, enhance the intensity and duration of TGF-β/Smad signaling by miR-148a, and help to amplify fibrosis signaling.26, 65
3.1.3 Effect of HIF-1α on metabolism of KFs
The body's metabolism depends largely on aerobic respiration and anaerobic glycolysis to maintain its energy supply. Aerobic respiration involves the cellular production of 2, 2 and 34 adenosine triphosphate (ATP) molecules from one glucose molecule through glycolysis, the tricarboxylic acid cycle (TAC) and the electron transport chain, respectively, in the presence of sufficient oxygen. Under hypoxic conditions, the reductive coenzymes required by the TAC cannot be regenerated, and cells thus obtain ATP by glycolysis. In the case of oxygen deficiency, HIF-1α activates the transcription of genes encoding glucose transporter 1 (GLUT1) and lactate dehydrogenase A (LDH-A), which allow glucose to be absorbed more quickly by the cells, to synthesize ATP and lactic acid.66, 67
The rapid growth of tumors generates a hypoxic microenvironment, meaning that tumors often need to obtain their energy through anaerobic glycolysis. Keloids are characterized by the rapid proliferation of KFs and oversynthesis of ECM, and these tumor-like behaviors suggest that they may show metabolic reprogramming similar to malignant tumors. According to Su et al., the activities of glycolytic pathway enzymes including hexokinase, glyceraldehyde-3-phosphate dehydrogenase and LDH were higher in KFs than in NFs, while KFs also had higher rates of ATP and lactic acid generation compared with NFs.68 Following the addition of glycolysis inhibitors, the inhibition rate of ATP synthesis was significantly higher in KFs than in NFs, but the opposite result was shown after the addition of oxidative phosphorylation inhibitors. This could indicate that the metabolism of KFs has undergone a metabolic reprogramming similar to tumor cells, to meet the energy demands caused by the rapid proliferation of KFs.68 Vinaik et al. found that glycolytic enzymes (such as GLUT1) were also abnormally elevated in keloids.69 Under hypoxia, HIF-1 regulation depends on glycolysis-related proteases, resulting in a significantly higher rate of ATP synthesis in hypoxic compared with normoxic KFs.70 Wang et al. showed that KFs had higher mRNA and protein levels and higher activity of glycolytic enzymes compared with NFs, but lower mitochondrial complex activity.76 In addition, HIF-1α was upregulated in KFs under hypoxia, leading to increased proliferation and collagen synthesis, attenuation of mitochondrial activity, enhanced cell antioxidant capacity and increased expression of reactive oxygen species (ROS).71 These results indicate that keloids show tumor-like metabolic reprogramming, allowing them to accelerate ATP synthesis through anaerobic glycolysis and the Warburg effect to meet the growing energetic demands for rapid proliferation and collagen synthesis. HIF-1α regulates the process of glycolysis in a dose-dependent manner, which increases the rate of ATP generation, damages mitochondrial function and enhances the antioxidant capacity of keloids. At the same time, the activation of GLUT1 caused by anaerobic metabolism means that the increase in ROS will inactivate FIH and PHD, which may stabilize HIF-1α and further promote glycolysis, providing another important reason why keloids are more sensitive to hypoxia.25, 71, 72 In addition, the HIF-1α/pyruvate kinase M2 (PKM2) signaling pathway can increase the expression of PKM2, a key enzyme of glycolysis, which can, in turn, interact directly with TGF-β inducible factor homeobox 2 and mediate the recruitment of histone deacetylase into the E-cadherin promoter sequence, inhibiting E-cadherin transcription and inducing EMT, which may be another important pathophysiological mechanisms of keloid formation.73, 74
3.1.4 HIF-1α enhances sensitivity to platelet-derived growth factor (PDGF) in KFs
PDGF is an important cytokine for the proliferation and migration of vascular smooth muscle cells and fibroblasts, and also stimulates collagen synthesis in the late stage of wound healing.75 Under chronic hypoxia, the accumulation of HIF-1α leads to decreased expression of protein tyrosine phosphatases of the PDGF receptor (PDGFR), which inhibits the negative regulation of PDGF and promotes the proliferation and migration of vascular smooth muscle cells and fibroblasts.76, 77 Keloid tissue expresses more PDGF than normal skin, and KFs have about four- to five-fold higher levels of PDGFR than NFs, resulting in increased sensitivity of KFs to PDGF.78, 79 This may be related to the fact that TGF-β1 upregulates PDGFR and hypoxia mediates the expression of HIF-1α, making sensitive KFs continuously stimulated by PDGF, thus promoting keloid formation.80 However, more studies are needed to clarify the role of PDGF in keloids.
3.2 HIF-1α induces EMT in keloid keratinocytes
After wounding, keratinocytes at the wound edge lose cell adhesion and acquires stronger migratory capability to close the defect.1 This process relies on the EMT process. EMT plays an important role in the progression and metastasis of malignant and fibrotic diseases. Cells adopt a mesenchymal phenotype after EMT, increasing their ability to synthesize collagen and cross the endothelial barrier.7, 81 HIF-α induces EMT by upregulating EMT-related transcription factors or inhibitors, activating EMT-related signaling pathways and regulating EMT-related inflammatory cytokines and epigenetic regulators.82 HIF-1α and TGF-β can both induces EMT by up regulating the Snail1, ZEB1, TWIST1 and PKM2 pathways.73, 83-85 Numerous studies have found that HIF-1α and TGF-β/Smad signaling pathways could impact each other to influence EMT.86, 87
Hahn et al. found that EMT genes were significantly upregulated and cell adhesion-related genes were significantly downregulated in keloid-derived keratinocytes, resulting in reduced intercellular adhesion and increased migration and invasion abilities of keratinocytes.88 Hahn et al. also showed that keratinocytes derived from keloids showed EMT after stimulation by TGF-β.89 A study by Ma et al.13 found that keloid-derived keratinocytes lost their typical cobblestone appearance and adopted a spindle-shaped morphology under conditions of 1% O2. At the same time, keratinocyte markers (E-cadherin, ZO-1) were partially lost and mesenchymal markers (vimentin, fibronectin) were significantly increased. HIF-1α silencing resulted in inhibition of EMT, indicating that HIF-1α activated EMT-related signaling and enhanced the invasive ability of keloids. EMT is an important mechanism in the occurrence and progression of keloids. Mutual crosstalk between HIF-1α and TGF-β pathways may jointly induce EMT, and further studies are warranted to explore the specific mechanisms so as to develop potential novel therapies.
3.3 HIF-1α promotes vascular endothelial cell proliferation and differentiation and angiogenesis in keloids
During wound healing, vascular endothelial cells respond to vascular endothelial growth factor (VEGF), which can activate endothelial cells to break down the ECM in granulation tissue, promoting endothelial cell proliferation and migration and contributing to the formation of new capillaries.1 VEGF is a highly specific mitogen of endothelial cells that promotes angiogenesis in a dose-dependent manner.90, 91 The VEGF gene promoter contains an HRE structure. Under hypoxia, HIF-1 translocates into the nucleus and binds with the HRE to enhance VEGF mRNA transcription.92, 93 In the early stage of wound healing, local hypoxia recruits macrophages, neutrophils and other cells to the wound to secrete VEGF and thus promote the proliferation and migration of vascular endothelial cells, leading to the formation of new capillaries. In malignant tumors, the increased expression of HIF-1 caused by local hypoxia stimulates the expression of VEGF and provides oxygen for tumor growth, which is an important mechanism by which tumor cells can obtain more energy.94
Keloids are a type of benign tumor, with the characteristics of rapid proliferation and invasive growth leading to an insufficient oxygen supply, which, in turn, stimulates the secretion of VEGF to form new capillaries to meet the nutrient and energy demands. Fujiwara et al. reported that VEGF mRNA levels were increased six-fold in KFs compared with NFs, and KF-conditioned medium had a seven-fold greater ability to induce vascular endothelial cell activation than NF-conditioned medium.95 VEGF expression levels in KFs were significantly higher under hypoxic compared with normoxic conditions.96 In addition, expression levels of HIF-1α and VEGF, the rate of fibroblast proliferation, capillary density and the collagen I/III ratio were all significantly higher in keloids compared with normal skin, indicating that locally high oxygen consumption caused by collagen secretion and fibroblast proliferation results in keloid hypoxia, leading to the expression of HIF-1-mediated VEGF to form capillaries, to partially compensate for the increased oxygen consumption.97-100 However, this can only partly compensate for the oxygen consumption and promote metabolism, meaning that the keloids need to secrete more VEGF to meet their metabolic needs.
3.4 The role of HIF-1α in hypertrophic scars
Hypertrophic scars (HS) and keloids show similar clinical presentations including itch, pain and elevation. However, HS ceases to grow and gradually regresses, softens and fattens over time, and scar symptoms spontaneously attenuate, which are quite different from keloids. Zhang et al. measured different stages of HS oxygen tension through transcutaneous pressure of oxygen. The author found that the oxygen tension in HS was lower compared with normal skin at scar early stage and reached the bottom at scar regressive stage.101 Then, the oxygen tension started to recover and became near normal at mature stage. Meanwhile, the microvessel density and the expression level of HIF-1α negatively correlated with oxygen tension.101 Qi et al. also found that oxygen tension gradually raised up when HS became mature, accompanied with HIF-1α and its downstream factor VEGF downregulated.102
There are few related studies on the pathogenesis of HIF-1α in HS, thus the specific mechanism is still unclear. Wang et al. found that hsa-miR31-5p could be induced and expressed under hypoxia. It could inhibit the function of FIH and stabilize HIF-1α, which led to HS fibroblasts proliferation and collagen synthesis.103 Liu et al. found that HS fibroblasts cultured with cobalt chloride(a classical hypoxic-mimetic reagent) could result in loss of 5-hydroxymethylcytosine and increase expression of phosphorylated focal adhesion kinase, promoting scar contracture.104 Li et al. found that p53 was downregulated under hypoxia. It made p53 difficult to combine with p300, promoting HIF-1α more easily to combine with p300 and exert its transcriptional effect.105 Overall, the pathophysiological mechanisms of HIF-1α in hypertrophic scars are not completely known. HS and keloids both present hypoxia, but HS can gradually recover. Perhaps it is because KFs display tumor cell-like biological behaviors such as over proliferation, contributing to continuous hypoxic state. The detailed mechanism needs further investigation.
4 INHIBITORS OF HIF-1Α SIGNALING PATHWAY AND RELATED TREATMENTS FOR KELOIDS
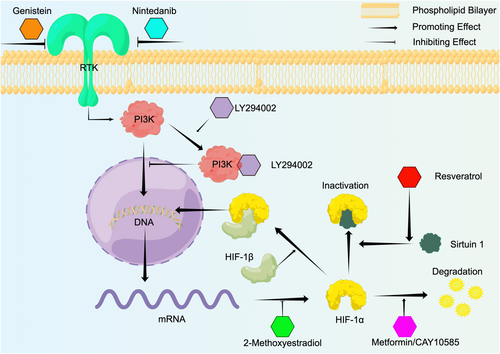
The current treatment approaches for keloids mainly include surgical excision, or surgical excision combined with radiotherapy and hormone injection. However, some multiple keloids are difficult to excise, and even surgical excision combined with radiotherapy has a recurrence rate of 10%–23%, indicating the difficulties in treating keloids.106 As noted above, HIF-1α plays an important role in keloid fibrosis, invasion and radiotherapy resistance, and targeting HIF-1α may thus represent a promising approach for the potential treatment of keloids. Here, we listed some potential treatments or targeted drugs of HIF-1α aiming to provide new ideas for future research (as shown in Figure 4 and Table 1).
| Pathway | Inducer/Ligands | Receptor | Function |
|---|---|---|---|
| PI3K/Akt signal pathway | Hypoxia | RTKs | Inducing transcription of HIF-1α |
| ERK1/2 signal pathway | Hypoxia | RTKs | Inducing transcription of HIF-1α |
| HIF-1α signal pathway | Hypoxia | NA | Amplifying fibrotic signaling like TGF-β signal |
| Initiating NF-κB and TLR transcription | |||
| Metabolic reprogramming | |||
| EMT in keloid keratinocytes | |||
| Promoting angiogenesis by stimulating VEGF | |||
| Decreasing PDGFR degradation | |||
| TGF-β signal pathway | TGF-β homodimer | TGFR1 and TGFR2 | Myofibroblasts transition, ECM deposition, EMT process and PHD reduction |
| TLR4/MyD88 signal pathway | DAMPs | TLR4 | Inducing transcription of HIF-1α and amplifying TGF-β signal |
- Abbreviations: DAMPs, damage-associated molecular patterns; ECM, extracellular matrix; EMT, epithelial–mesenchymal transition; ERK1/2, extracellular signal-regulated kinase 1/2; HIF-1α, hypoxia-inducible factor 1α; MyD88, myeloid differentiation factor 88; NF-κB, nuclear transcription factor-κB; PDGFR, platelet-derived growth factor receptor; PHD, prolyl hydroxylase domain; PI3K, phosphatidylinositol-3-kinase; RTK, receptor tyrosine kinase; TGFR, transforming growth factor beta receptor; TGF-β, transforming growth factor-β; TLR, Toll-like receptors; VEGF, vascular endothelial growth factor.
4.1 Hyperbaric oxygen therapy (HBOT)
HBOT is treatment method in which patients are placed in a compression chamber at above atmospheric absolute pressure with 100% oxygen to alleviate hypoxic symptoms.107 HBOT is widely used for treating infected wounds, improving the survival of skin flaps, carbon monoxide poisoning, and reducing inflammation and radiotherapy resistance of malignant tumors.108, 109 Song et al. found that the recurrence rate of keloids after surgery combined with HBOT was significantly reduced compared with a control group that only underwent surgical resection, and immunohistochemistry after secondary surgery in recurrent patients showed that HIF-1α and VEGF levels were significantly lower in the HBOT group compared with the control group.110 In addition, Zhang et al. found that the capillary density was significantly decreased and EMT was inhibited in keloids after HBOT.111 HBOT increases the oxygen concentration in the body and reduces HIF-1α levels in keloids, thus contributing to inhibition of EMT, glycolysis and radiotherapy resistance.
4.2 HIF-1α transcription inhibition
LY294002 is a PI3K-specific inhibitor that effectively inhibits the activity of HIF-1α. LY294002 can specifically bind with PI3K, inhibiting the transcription of HIF-1α in KFs, thus affecting HIF-1α and translation of the downstream gene PAI-1.34 Nintedanib and genistein are non-specific receptor tyrosine kinase inhibitors that inhibit PI3K/Akt/mTOR-mediated HIF-1α transcription by binding to RTKs, thus blocking the expression of VEGF and other downstream genes. Genistein inhibited HIF-1α transcription in KFs under hypoxia.34 Zhou et al. showed that nintedanib effectively inhibited the synthesis of collagen, PAI-1 and α-SMA and attenuated the proliferation and migration of KFs.112
4.3 HIF-1α translation inhibition
2-Methoxyestradiol (2ME2) is a natural derivative of oestradiol that can inhibit HIF-1α at the translational level by depolymerizing microtubules, resulting in downregulation of downstream genes such as VEGF.113 Long et al. found that 2ME2 significantly reduced HIF-1α expression of KFs in vitro, leading to inhibition of VEGF and enhancement of radiation-induced KF apoptosis, thus reducing the radiotherapy resistance of keloids.114
4.4 HIF-1α stabilization inhibition
Metformin is commonly used for the treatment of diabetes and also possesses anti-tumor activity. Lei et al. showed that metformin upregulated the E3 ubiquitin ligase parkin in keloids and promoted the degradation of HIF-1α, attenuating the proliferation of KFs through inhibition of the TGF-β/Smad signaling pathway. Metformin can also inhibit EMT through HIF-1α/PKM2-pathway-mediated glycolysis in KFs.74 CAY10585 was shown to promote HIF-1α degradation and significantly reduce the expression of collagen in KFs.14
4.5 HIF-1α/DNA binding inhibition
Resveratrol is a natural polyphenol compound extracted from plants that is widely found in berries such as grapes, and which has demonstrated various effects, including anti-cancer activity and in the treatment of pulmonary hypertension.115-117 Resveratrol effectively increased the expression of sirtuin 1 under hypoxia, allowing it to bind to HIF-1α, leading to HIF-1α deacetylation and inactivation.118, 119 Si et al. accordingly showed that resveratrol inhibited the proliferation and collagen synthesis of KFs by downregulating HIF-1α.120
5 CONCLUSIONS AND SUMMARY
Keloids are a skin disease involving abnormal dermal hyperplasia and fibrosis, which can seriously affect the patient's quality of life and present a challenge to plastic surgeons. Although surgical excision combined with radiotherapy can have a good therapeutic effect, some cases still recur and some multiple keloids cannot be surgically removed. HIF-1α plays important roles in keloid fibrosis, EMT and metabolic reprogramming, associated with keloid progression and radiotherapy resistance. HIF-1α is, therefore, expected to become an important therapeutic target for keloids, warranting further basic and clinical research to bring new hope to patients suffering from keloids.
AUTHOR CONTRIBUTIONS
ZQ participated in the data collection and drafted the article. MZ, WZ, ZL, LS, NZ, and XL participated in the data collection. XW reviewed and amended the article. All authors read and approved the final manuscript.
ACKNOWLEDGMENT
The author acknowledged the assistance from Rongjia Zhu and Robert Chunhua Zhao.
FUNDING INFORMATION
This work was supported by the National Natural Science Foundation of China [grant numbers 81971846].
CONFLICT OF INTEREST
The authors declare that they have no competing interest.
ETHICS STATEMENT
The authors confirm that the ethical policies of the journal, as noted on the journal's author guidelines page, have been adhered to.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in Pubmed at https://pubmed.ncbi.nlm.nih.gov/.