Biochemical and hormonal abnormalities in adult female acne
Abstract
Background
Prevalence of adult patients with acne is increasing and women comprise majority of the cases. There is lack of data on biochemical and hormonal abnormalities in adult female acne (AFA).
Aims
To evaluate biochemical and hormonal parameters in 60 patients of AFA.
Methods
A cross-sectional observational study conducted from November 2018 to March 2020 in Dermatology outpatient department of a tertiary care hospital in North India. Adult females (age > 25 years) with a clinical diagnosis of acne were included in the study.
Results
60 cases of AFA were included. The age ranged between 26–41 years with mean age at presentation being 29.45 years. 53.3% patients had persistent acne while 46.7% had late-onset acne. 50% patients had history of premenstrual flare-up of their acne. Raised FBG was found in 25% patients. 10% had raised serum insulin levels. HOMA-IR index was deranged in 55% patients. At least one lipid alteration was reported in 91.6% of patients. In hormonal parameters, raised TT was present in 6.7%, LH in 3.3%, FSH in 18.3%, prolactin in 3.3%, and TSH in 15%. No association was found between acne severity and biochemical and hormonal parameters.
Conclusions
Our study highlighted the importance of measuring lipid profile in AFA and calculating HOMA-IR index for measuring insulin resistance rather than simply measuring serum insulin levels. In our study, additional parameter deranged in significant number of patients was FBG. Hence, we recommend routine screening of lipid profile, FBG and calculation of HOMA-IR index in AFA.
1 INTRODUCTION
Acne is a chronic inflammatory disease of pilosebaceous unit characterized by formationof comedones, papules, pustules, and nodules of varying degree of inflammation and depth.1 It is principally a disorder of adolescence. However, the prevalence of adult patients with acne is increasing and women comprise majority of cases. Adult acne is defined as acne that develops (late-onset acne) or continues (persistent acne) after 25 years of age.2 Increased sebum production, follicular hyperkeratinization, Propionibacterium acnes colonization and inflammation play an interactive pathogenic role in acne. There are 2 clinical types: (1) Persistent acne (2) Late-onset acne. Both types of adult acne are more common in women.3 Hormonal alteration, stress, increased use of cosmetics and exposure to hot and humid conditions might play a role in increased prevalence of adult acne in females.4 In past few years, acne is considered as a chronic systemic disease, rather than a transient pubertal dermatological disease.5 Lipid alterations like high serum total cholesterol (TC), triglycerides (TG) and low high density lipoprotein (HDL) have also been linked to acne.6
1.1 Objective
The primary objective was to determine biochemical and hormonal abnormalities in adult female acne (AFA). The secondary objective was to describe the clinical characteristics, aggravating factors and other comorbidities in AFA.
2 MATERIALS AND METHODS
This cross-sectional observational study was conducted from November 2018 to March 2020 in Dermatology outpatient department of a tertiary care hospital in North India. Adult females (age > 25 years) with clinical diagnosis of acne were included in study. Postmenopausal women, pregnant and lactating women, patient taking oral contraceptive pills or acnegenic drugs, hormonal therapy were excluded from the study. A detailed history including age of onset, duration, seasonal aggravation, and premenstrual flare were taken. Clinical assessment of each patient included site and morphology of lesion, severity and grading of acne and associated features like seborrhea, patterned alopecia, hirsutism etc. Acne was graded according to the Investigator Global Assessment score tool for Acne vulgaris severity grading. Body mass index (BMI) was graded according to Asian classification of adult nutritional status. Hirsutism was diagnosed by a Modified Ferriman-Gallwey score and alopecia was graded by Ludwig scale. Subjects underwent a detailed hormonal (Total testosterone, luteinizing hormone, follicle stimulating hormone, thyroid stimulating hormone and prolactin) and biochemical (fasting blood glucose, lipid profile, and insulin) assessment on day 2–5 of their menstrual cycle. Fasting blood glucose (FBG) and serum lipids were measured by spectrophotometric analyzer (Beckman-Coulter fully automated AU series analyzer) by the respective kits. Raised FBG was considered ≥100 mg/dl. Dyslipidemia was considered as: Serum triglycerides (TG) ≥150 mg/dl, High Density Lipoprotein (HDL) <50 mg/dl, Low Density Lipoprotein (LDL) >100 mg/dl and Total Cholesterol (TC) ≥200 mg/dl. Serum Insulin, Testosterone (TT), Prolactin (PRL), Follicle Stimulating Hormone (FSH), Luteinizing Hormone (LH), and Thyroid Stimulating Hormone (TSH) were measured by electromagnetic chemiluminescence (E-CLIA) by Roche e-411 fully automated immunoassay analyzer. Hyperinsulinemia was considered as levels of serum Insulin >25μIU/ml. Raised TT and PRL were considered as levels >48 ng/dl and >26.72 ng/ml respectively. Raised FSH and LH was defined as levels >10 and >15 IU/L respectively. LH/FSH ratio is approximately 1:1 during follicular phase. LH/FSH ratio >1 indicates an abnormality in LH or FSH secretion. Raised TSH was considered as levels >5.6μIU/ml.
Homeostasis Model of Assessment of Insulin Resistance: HOMA-IR index >2.41 was considered as insulin resistance.
Transabdominal ultrasound pelvis was done with the help of Toshiba Xario ultrasound machine by using curvilinear/linear probe of 3.5 and 7.5 MHz frequency respectively. On ultrasound pelvis, PCOS was diagnosed when there was antral follicle count (AFC) of ±12 and/or ovarian volume of 10 ml or more.
The data collected was entered in a Microsoft excel spreadsheet and for analysis statistical package for social sciences (SPSS) software version 23 was used.
2.1 Statistical analysis
Continuous variables were expressed as mean ± standard deviation, median and range. Categorical variables were expressed as frequencies and percentages. To check normality of data Shapiro–Wilk test was used. Fisher's exact test was used to find out the association of acne severity with biochemical parameters, hormonal parameters and obesity. A p value was considered significant if <0.05.
3 RESULTS
Our study included 60 clinically diagnosed cases of AFA.
3.1 Demographic characteristics
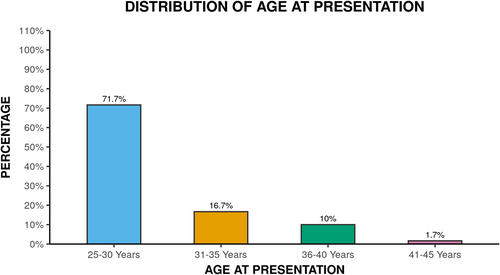
The age ranged between 26–41 years with mean age at presentation being 29.45 ± 4.18 years. Majority of patients 43 (71.7%) were in age group of 25–30 years as shown in Figure 1. The mean age of onset was 24.97 ± 5.66 years. Overall mean disease duration was 4.39 ± 4.60 years.
3.2 Clinical characteristics of acne
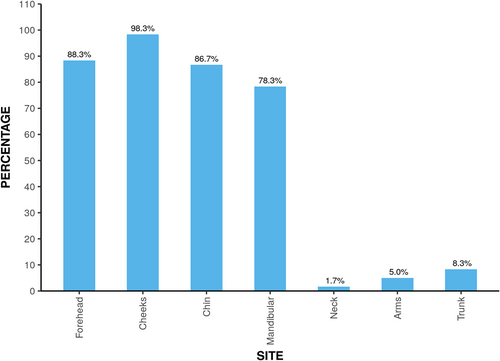
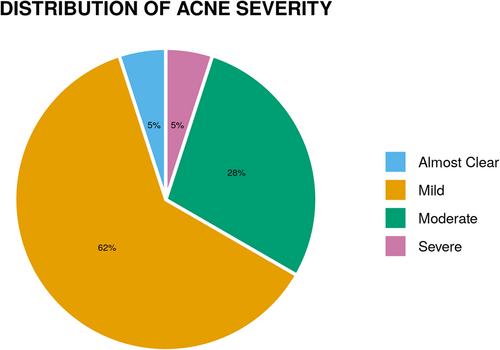
Majority of the patients 21 (35%) had their onset between 26–30 years. 32 (53.3%) patients had persistent acne while 28 (46.7%) had late-onset acne. Papules were most common morphological pattern present in 60 (100%) patients, followed by comedones (81.7%), pustules (63.3%), papulopustular (10%), nodules (5%), and scarring (3.3%). Different sites affected are shown in Figure 2. Distribution of acne severity is depicted by Figure 3.
3.3 Associated features including comorbidity
The commonest associated feature was seborrhea in 30 (50%) cases, followed by acrochordons (8.3%), acanthosis nigricans (6.7%) and patterned alopecia (1.7%). Hirsutism was not seen in any of the patient. 24 (40%) had positive family history of acne. A total of 9 (15%) reported irregular cycles. The BMI ranged between 18.83–40.9 kg/m2 with mean (SD) BMI was 25.65 (3.63) kg/m2. Majority, 32 (53.3%) were pre-obese and had BMI between 25–29.9 kg/m2.
3.4 Aggravating factors
Overall, 27 patients reported seasonal aggravation. 30 (50%) patients reported aggravation on exposure to sunlight. 30 (50%) patients had history of premenstrual flare-up of their acne. Overall, 40 (66.6%) patients reported acne aggravation by stressful life events.
3.5 Biochemical/hormonal and radiological parameters
Biochemical and hormonal abnormalities deranged in AFA patients in our study are shown in Table 1. Raised FBG was found in 25% patients. 6 (10%) had raised serum insulin levels. HOMA-IR index was found to be deranged in 33 (55%) patients. At least one lipid alteration was reported in 91.6% of the patients. Serum TG was raised in 20%, decreased HDL (66.7%), raised TC (38.3%), and raised LDL (50%).
| Biochemical parameters | Total number of patients (%) |
|---|---|
| Raised FBG | 15 (25%) |
| Raised serum insulin | 6 (10%) |
| HOMA-IR | 33 (55%) |
| Fasting lipid profile | |
| Raised TG | 12 (20%) |
| Raised TC | 23 (38.3%) |
| Raised LDL | 30 (50%) |
| Decreased HDL | 40 (66.7%) |
| Hormonal Parameters | |
| Raised TT | 4 (6.7%) |
| Raised LH | 2 (3.3%) |
| Raised FSH | 11 (18.3%) |
| Raised LH/FSH | 22 (36.7%) |
| Raised TSH | 9 (15%) |
| Raised Prolactin | 2 (3.3%) |
- Abbreviations: FBG–Fasting blood glucose, FSH–Follicle stimulating hormone, HDL–High density lipoprotein, HOMA-IR–Homeostasis model assessment of insulin resistance, LDL–Low density lipoprotein, LH–Luteinizing hormone, TG–Triglyceride, TC–Total cholesterol, TT–Total testosterone, TSH–Thyroid stimulating hormone.
In hormonal parameters, raised TT was present in only 4 (6.7%) patients. Raised LH and FSH was seen in 2 (3.3%) and 11 (18.3%) patients respectively. 22 (36.7%) cases had LH/FSH ratio >1. Serum prolactin and TSH was raised in 2 (3.3%) and 9 (15%) patients respectively.
USG was suggestive of PCOS in 2 cases only.
3.6 Association
No association was found between acne severity and biochemical and hormonal parameters. (Tables 2 and 3). Similarly, no association was found between acne severity and BMI.
| Parameters | Acne severity | Fisher's exact test | |||||
|---|---|---|---|---|---|---|---|
| Biochemical parameters | Almost clear | Mild | Moderate | Severe | Total | X^2 | p value |
| FBG <100 mg/dl | 3 (100.0%) | 27 (73.0%) | 13 (76.5%) | 2 (66.7%) | 45 (75.0%) | 1.212 | 0.950 |
| ≥100 mg/dl | 0 (0.0%) | 10 (27.0%) | 4 (23.5%) | 1 (33.3%) | 15 (25.0%) | ||
| TG <150 mg/dl | 3 (100.0%) | 29 (78.4%) | 13 (76.5%) | 3 (100.0%) | 48 (80.0%) | 1.693 | 1.000 |
| ≥150 mg/dl | 0 (0.0%) | 8 (21.6%) | 4 (23.5%) | 0 (0.0%) | 12 (20.0%) | ||
| HDL ≤50 mg/dl | 1 (33.3%) | 25 (67.6%) | 11 (64.7%) | 3 (100.0%) | 40 (66.7%) | 3.043 | 0.484 |
| >50 mg/dl | 2 (66.7%) | 12 (32.4%) | 6 (35.3%) | 0 (0.0%) | 20 (33.3%) | ||
| TC <200 mg/dl | 2 (66.7%) | 22 (59.5%) | 10 (58.8%) | 3 (100.0%) | 37 (61.7%) | 2.031 | 0.734 |
| ≥200 mg/dl | 1 (33.3%) | 15 (40.5%) | 7 (41.2%) | 0 (0.0%) | 23 (38.3%) | ||
| LDL <100 mg/dl | 1 (33.3%) | 17 (45.9%) | 9 (52.9%) | 3 (100.0%) | 30 (50.0%) | 3.635 | 0.438 |
| ≥100 mg/dl | 2 (66.7%) | 20 (54.1%) | 8 (47.1%) | 0 (0.0%) | 30 (50.0%) | ||
| Insulin ≤25 mIU/L | 3 (100.0%) | 34 (91.9%) | 15 (88.2%) | 2 (66.7%) | 54 (90.0%) | 2.354 | 0.432 |
| >25 mIU/L | 0 (0.0%) | 3 (8.1%) | 2 (11.8%) | 1 (33.3%) | 6 (10.0%) | ||
| HOMA-IR ≤2.41 | 1 (33.3%) | 19 (51.4%) | 7 (41.2%) | 0 (0.0%) | 27 (45.0%) | 3.323 | 0.443 |
| >2.41 | 2 (66.7%) | 18 (48.6%) | 10 (58.8%) | 3 (100.0%) | 33 (55.0%) | ||
- Abbreviations: FBG–Fasting blood glucose, HDL–High density lipoprotein, HOMA-IR–Homeostasis model assessment of insulin resistance, LDL–Low density lipoprotein, TG–Triglyceride, TC–Total cholesterol.
| Parameters | Acne severity | Fisher's exact test | |||||
|---|---|---|---|---|---|---|---|
| Hormonal parameters | Almost clear | Mild | Moderate | Severe | Total | X^2 | p value |
| TT ≤48 ng/dl | 3 (100.0%) | 35 (94.6%) | 15 (88.2%) | 3 (100.0%) | 56 (93.3%) | 1.233 | 0.729 |
| >48 ng/dl | 0 (0.0%) | 2 (5.4%) | 2 (11.8%) | 0 (0.0%) | 4 (6.7%) | ||
| LH ≤15 mIU/ml | 2 (66.7%) | 36 (97.3%) | 17 (100.0%) | 3 (100.0%) | 58 (96.7%) | 9.115 | 0.192 |
| >15 mIU/ml | 1 (33.3%) | 1 (2.7%) | 0 (0.0%) | 0 (0.0%) | 2 (3.3%) | ||
| FSH ≤10 mIU/ml | 3 (100.0%) | 31 (83.8%) | 13 (76.5%) | 2 (66.7%) | 49 (81.7%) | 1.542 | 0.680 |
| >10 mIU/ml | 0 (0.0%) | 6 (16.2%) | 4 (23.5%) | 1 (33.3%) | 11 (18.3%) | ||
| Prolactin ≤26.72 ng/ml | 3 (100.0%) | 35 (94.6%) | 17 (100.0%) | 3 (100.0%) | 58 (96.7%) | 1.286 | 1.000 |
| >26.72 ng/ml | 0 (0.0%) | 2 (5.4%) | 0 (0.0%) | 0 (0.0%) | 2 (3.3%) | ||
| TSH ≤5.6 mIU/L | 3 (100.0%) | 30 (81.1%) | 16 (94.1%) | 2 (66.7%) | 51 (85.0%) | 2.874 | 0.399 |
| >5.6 mIU/L | 0 (0.0%) | 7 (18.9%) | 1 (5.9%) | 1 (33.3%) | 9 (15.0%) | ||
- Abbreviations: FSH–Follicle stimulating hormone, LH–Luteinizing hormone, TT–Total testosterone, TSH–Thyroid stimulating hormone.
4 DISCUSSION
Although acne has traditionally been viewed as a disorder of adolescent, the prevalence of adult patients with acne is rising.4 In our study, mean age at presentation was 29.45 ± 4.18 years which was similar to studies by George et al.7 and Khunger et al.4 Majority of patients, 71.7% were in the age group of 25–30 years in our study. This age is of great social and psychological importance for adult female and becomes even more important for working women. This is similar to studies by Khunger et al.4 and Dreno et al.8 where 79.4% and 53.2% of patients were in age group of 26–30 years. However, Di Landro et al.,9 found majority (56.9%) of patients were in age group of 30–39 years.
Majority (35%) of the patients, had their onset between 26–30 years. This is in contrast to studies by Dreno et al.8 and Chlebus et al.10 where majority had their onset in the adolescent. We found persistent acne (53.3%) more common than late-onset acne (46.7%) which is in consonance to other studies.4, 10, 11 However, Sardana et. al.,12 found late-onset acne (56.6%) to be more common than persistent acne (43.3%). Family history of acne was present in 40% cases in our study. This has been reported in 10%–56.8% of the cases from various studies.8, 12 Seasonal aggravation was seen in 45% patients. Out of these, 25 had summer aggravation while only 2 had winter aggravation. Similarly, seasonal aggravation was noted in 44.5% of patients in a study from South India.7 Summer aggravation may be due to the ultraviolet radiation induced inflammation and generation of squalene peroxides which are comedogenic in nature.4 50% patients in our study reported aggravation on exposure to sunlight. Khunger et al.4 and George et al.7 noticed aggravation on sun exposure in 33.2% and 26.4% of patients respectively. This was slightly lesser than our study.
15% patients reported irregular menstrual cycles in our study. In a study from India, menstrual irregularity was noted in 25.8% of patients which was higher than our study.12 50% patients reported premenstrual flare-up of acne in our study. Premenstrual flare has been reported in 30%–78% of the cases.12, 13 Our results were also in this range. Premenstrual flare may be due to hydration induced cyclical narrowing of pilosebaceous orifice between days 16–20 of menstrual cycle.7
Stress has also been implicated in pathogenesis of AFA. It increases levels of pro-inflammatory cytokines and corticotrophin releasing hormone and thus increasing the level of cortisol.14 We noted that 66.6% patients had history of stressful life events. Stress as a worsening factor has been reported in 50%–71% of patients.13
Acne is clearly exacerbated by obesity associated disorders such as hyperandrogenism and hirsutism. Androgens, insulin and growth hormone are frequently elevated in obese patients.15 In our study, 53.3% cases were pre-obese with BMI between 25–29.9 kg/m2. Tanghetti et al.16 reported BMI ≥25 kg/m2 in 51.9% of their patients similar to our study.
Papules were the most common morphological pattern in our study. This is similar to other studies, where papules were also the most common morphological pattern.12, 13, 17 In our study, scarring was found in only 2 patients. Khunger et al.4 and Tanghetti et al.16 reported scarring in 76.4% and 63% of patients respectively.
Cheeks were most common site affected in our study. Similarly, cheeks were the most common site affected in other studies.4, 9, 16 Our study reported acne on trunk along with face in only 8.3% patients. Since, pure truncal involvement is not cosmetically distressing, it may be possible that fewer patients are seeking treatment for only truncal acne.
Majority (61.7%) of patients had mild acne, followed by moderate, severe and almost clear acne. In a multicenter case-control study, most of the cases had moderate acne (50.2%) followed by mild (42%) and severe (7.8%).9 However, Capitanio et al.18 found comedonal acne in 85% of patients. This variation in severity of acne in different studies may be due to the difference in acne grading systems used and partially due to difference in the study population and geographical area.
Signs of clinical hyperandrogenism like hirsutism, androgenetic alopecia, irregular menses and hyperseborrhea could exist even in absence of raised serum androgens due to end organ hypersensitivity. Cutaneous 5α reductase converts TT to DHT which is further metabolized to 3α androstenediol glucuronide which is a surrogate marker of peripheral androgen excess.19 The commonest associated feature was hyperseborrhoea present in 50% of our cases. Both Sardana et al.12 and Bansal et al.17 reported hyperseborrhoea in 50% of patients which was in consonance to our study.
None of our patient had hirsutism. Khunger et al.4 and Kaminsky et al.11 reported hirsutism in 5.7% and 2% patients respectively. Only 1 patient had patterned alopecia in our study. Khunger et al.4 observed androgenetic alopecia in 1.8% of patients.
Acanthosis nigricans and acrochordons are considered as signs of insulin resistance.20 In our study, 6.7% patients had acanthosis nigricans (AN) and 8.3% had acrochordons. There is only 1 study which had evaluated AN in adult acne and was found in 5% of patients.11
In biochemical parameters, we evaluated FBG, Insulin, HOMA-IR index, and lipid profile. Our study showed that 25% patients had raised FBG. In a case-control study by Balta et al.,21 no statistically significant difference in mean FBG was found between cases and controls. Insulin and IGF-1 increases sebum production and also increases synthesis and bioavailability of androgen.22 Raised insulin levels suppresses sex hormone binding globulin (SHBG) concentration, increases androgen levels and contributes to acne. IGF-1 stimulates lipid synthesis in sebaceous glands through induction of sterol response element binding protein-1 (SREBP1). This overstimulated SREBP1 increases sebum production and enhances concentration of monounsaturated fatty acids in sebum and thus, increases colonization of P. acnes and acne formation.22 10% patients had raised serum insulin in our study. Khunger et al.4 reported raised insulin in only 2 (0.86%) patients which was lesser than our study. Shrestha23 in her study evaluated C-peptide which was found to be altered in 10.3% of patients. C-peptide is a measure of endogenous insulin secretion and is more stable than insulin.24
In our study, insulin resistance was evaluated using HOMA-IR index and was raised in 55% cases. Balta et al.21 did not find any statistically significant difference in mean HOMA-IR index of cases and controls.
We noticed at least one lipid alteration in 91.6% patients. 20% patients had raised TG, 50% had raised LDL and 38.3% had raised TC while 66.7% had decreased HDL. Our study reported lipid alterations in higher percentage of patients as compared to a study from Nepal in which lipid alterations were reported in 15.4% of patients.23 Likewise, da Cunha et al.,6 reported increase in TC in 17.35%, TG in 8.22%, LDL in 39.72%, and low HDL in 11.42% of patients. In our study, lipid profile was deranged in higher percentage of patients as compared to other studies. Dietary factors, genetics, race, smoking, and socioeconomic status could account for it.
Since adult acne is more common in females, so underlying hormonal abnormalities have been put as an important underlying factor. Androgens such as TT, dehydroepiandrosterone sulfate (DHEA-S) and DHT regulate genes which are responsible for sebaceous gland growth and sebum production.25 Serum androgens can act directly on sebaceous gland or it may be more sensitive to effect of androgens. Sebocytes and keratinocytes have various enzymes which are capable of producing TT and DHT locally. There is increased peripheral conversion of pre-hormone (DHEA-S, androstenedione) into more potent androgenic hormones like TT and DHT.26
In our study, serum TT was raised in 6.7% patients. Raised TT has been found in 3.04%–12.8% of cases in other studies.4, 23 18.3% patients had raised serum FSH in our study. Both Sardana et al.12 and Bansal et al.17 reported deranged serum FSH in 1.6% of the patients. In our study, raised LH levels were present in only 3.3% patients. In a study from India, LH was found to be raised in 4.1% of patients.12
During the follicular phase, LH/FSH ratio is approximately 1:1. Increased LH secretion is a characteristic feature of PCOS.5 LH/FSH ratio >1 was found in 36.6% patients in our study. Serum LH: FSH >2 was found in 0.86%–7.5% of patients.4, 12 Our study reported raised LH/FSH ratio in higher percentage of patients. This may be due to lower threshold value of LH/FSH ratio taken in our study as compared to other studies.
In our study, serum TSH was raised in 15% patients. This was similar to a recent study from Nepal who found raised TSH in 15.4% of patients.23
Women with hyperprolactinemia might present with hyperandrogenic signs like hirsutism and acne. This may be due to increased DHEA-S secretion from adrenals and reduced SHBG, leading to high free TT levels.5 We found raised PRL in only 3.3% patients while it was raised in 21.6% of patients in a recent study.12
Women with PCOS are frequently overweight, have raised serum androgen levels and are insulin resistant, all of which predispose to acne.27 In our study, USG was suggestive of PCOS in only 3.3% patients. Khunger et al.4 reported PCOS in 2 (0.86%) patients in their study. However, USG pelvis was suggestive of PCOS in 25% of the patients in another study from India.12 The method used for performing pelvic USG was transabdominal in above mentioned studies which was similar to our study. For diagnosing PCOS, transvaginal ultrasound (TV-USG) is recommended which is invasive and is not performed in virgin females where a transabdominal ultrasound is preferred.28 Transabdominal ultrasound is not as sensitive as TV-USG. The other limitation of transabdominal USG in obese patients is sub-optimal visualization of ovaries.28 Measurement of antimullerian hormone(AMH) is also considered good tool for diagnosing PCOS.28 However, it was not done in our study due to unavailability of resources.
We did not find statistically significant association between acne severity and FBG, insulin and HOMA-IR index. There is only 1 study21 on association of acne severity with FBG, insulin and HOMA-IR index. In this study, no association was found between acne severity and FBG, insulin and HOMA-IR index, similar to our study.
We did not find any statistically significant association between acne severity and lipid profile. Romanska-Gocka et al.29 and da Cunha et al.6 in their studies reported no association between acne severity and lipid profile. Our results were consistent with their results. However, both these studies included patients with age ≥20 years.6, 29
We observed no association between acne severity and any of hormonal parameters (LH/ FSH/TSH/Prolactin/Testosterone/LH/FSH ratio). Association of acne severity and hormonal parameters has been done in only few studies in literature.2, 23 These studies also reported no association between acne severity and hormonal parameters which was in consonance to our study. Similarly, Bansal et al.17 found no association between acne severity and hormonal parameters (TT, PRL, TSH, LH, FSH) except LH/FSH ratio where positive association was found.
In our study, no association was found between acne severity and obesity. There is only one30 study on association between acne severity and obesity in adult female. Lu et al.30 in their study found that BMI was negatively associated with acne lesion counts in moderate-to-severe AFA.
5 CONCLUSION
Our study highlighted the importance of measuring lipid profile in AFA and calculating HOMA-IR index for measuring insulin resistance rather than simply measuring serum insulin levels. In our study, additional parameter deranged in significant number of patients was FBG. Hence, we recommend routine screening and management of lipid abnormalities, FBG and calculation of HOMA-IR index in AFA.
AUTHOR CONTRIBUTIONS
Dr. Amit Kumar Meena, Dr. Vibhu Mendiratta, and Dr. Rajeev Goyal conceptualized the study. Dr. Amit Kumar Meena collected the data, performed literature search, interpreted the data, statistically analyzed, wrote the manuscript, reviewed the manuscript, and approved the manuscript. Dr. Vibhu Mendiratta and Dr. Rajeev Goyal interpreted the data, performed the literature search, reviewed, and approved the manuscript. Dr. Kavita Bisherwal performed the literature search, prepared the manuscript, reviewed, and approved the manuscript. Dr. Vivin Prasadh and Dr. Vidya Yadav performed literature search, performed the literature search, involved in assistance in collection and interpretation of data, reviewed, and approved the manuscript.
CONFLICT OF INTEREST
None.
ETHICS STATEMENT
The study was approved by the Institutional Ethics Committee.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




