Modulation of TRPV1 function by Citrus reticulata (tangerine) fruit extract for the treatment of sensitive skin
Abstract
Background
Sensitive skin (SS) is a clinical syndrome defined by the occurrence of unpleasant sensations (such as stinging, burning, pain, pruritus, and tingling) in response to stimuli that normally should not provoke them. According to growing evidence, transient receptor potential vanilloid subtype 1 (TRPV1) has elevated expression in individuals with SS and is linked with the severity of SS symptoms. However, its pathogenesis is still unknown.
Objective
Herein, Citrus reticulata (Tangerine) fruit extract (CR) was obtained and examined for its effect on SS with a focus on TRPV1 stimulation and expression.
Methods
A recombinant hTRPV1 over-expression cell line (HaCaT-TRPV1-OE cell) was constructed to screen substances and extracts from several plants. Intracellular calcium mobilization was monitored by Flexstation 3 and a fluorescence microscope using Fluo 8 AM fluorophore. Next, immunofluorescence was used to detect the TRPV1 expression under different stimulants treated for 24 h. To investigate the relief and increased tolerance of CR to lactic acid-induced skin discomfort, clinical tests were carried out on the nasolabial folds or cheek areas.
Results
According to the obtained results, compared to HaCaT cells, HaCaT-TRPV1-OE cells showed a higher expression of TRPV1. Neuronal hyperresponsiveness in SS triggered by capsaicin (CAP), lactic acid, phenoxyethanol or nicotinamide may be through activation of TRPV1 and increased TRPV1 expression. CAP activates TRPV1 in HaCaT-TRPV1-OE cells, and more than 100 plants or chemicals were tested for their inhibitory effects before being screened for CR. CR (1%–4%) inhibited TRPV1 activation induced by CAP or phenoxyethanol or nicotinamide. Meanwhile, CR (0.25%) suppressed TRPV1 protein expression induced by phenoxyethanol or lactic acid. In vivo results showed that CR not only instantly relieved lactic acid-induced skin discomfort under 5 min but also enhanced skin tolerance to lactic acid after 7 days of continuous use.
Conclusions
Topical application of CR showed an instant and long-lasting improvement in SS by modulating the activation and expression of TRPV1. Moreover, it has been suggested that CR might act as a TRPV1 inhibitor to reduce skin irritation or sensitivity.
1 INTRODUCTION
Sensitive skin (SS) is a syndrome that involves unpleasant sensations such as stinging, burning, pain, pruritus, and tingling in response to stimuli that normally should not provoke them. These unpleasant sensations cannot be explained by lesions linked to any skin disease. The skin can be normal or accompanied by erythema. The International Forum for the Study of Itch (IFSI) states that SS can affect any part of the body, but it is more common on the face.1 According to epidemiological research, sensitive skin affects up to 70% of women and 60% of men.2 The patient's life quality is negatively impacted by the disease, which is upsetting.
The pathogenesis of SS is still under investigation. However, multiple recent investigations have identified discrepancies between the normal dermis and SS in terms of the epidermal barrier function, as well as vascular and neuronal hyper-reactivity in the normal dermis versus SS.3, 4 In addition, several studies have reported a correlation between the expression of transient receptor potential vanilloid subtype 1 (TRPV1) and the severity of SS symptoms.5, 6 TRPV1, a non-selective cation channel, is widely expressed in nociceptive neurons (C- and Aδ-fibers) and non-neuronal cells, such as keratinocytes, fibroblasts, and endothelial cells.7 It can be activated by various stimuli, including heat (>43°C), low pH (<5.9), capsaicin (CAP), phenoxyethanol, and a growing list of endogenous and exogenous compounds. Activation of TRPV1 is followed by increased permeability/influx of Na+ and Ca2+ activating linked signal transduction pathways exerting regulatory effects.7
Phytochemical studies showed that Citrus reticulata (Tangerine) fruit extract (CR) is rich in flavonoids and alkaloids8 which are responsible for CR's analgesic properties.9 Among them, naringenin and hesperetin are the most important flavanones that can potently and selectively block transient receptor potential cation channel subfamily M member 3.10 However, the regulatory mechanism of CR on pain concerning cutaneous discomfort remains elusive.
This research aims to identify a TRPV1 inhibitor with an abiological mechanism that might be used as a cosmetic active ingredient to treat SS. Against TRPV1, a high-throughput screening model (HaCaT-TRPV1-OE cell) was designed. Several cosmetic compounds with the potential to cause irritation were chosen to determine whether they activate TRPV1 and can be inhibited by TRPV1 inhibitors. We found that besides CAP, phenoxyethanol, nicotinamide, and lactic acid may be associated with the modulation of the TRPV1 receptor and CR relieves SS by regulating the activation and expression of TRPV1.
2 MATERIALS AND METHODS
2.1 Construction of HaCaT-TRPV1-OE cells
High-titer virus particles were obtained by 293 T cells (derived from ATCC) transfected with hTRPV1-pCDH-CMV-MCS-EF1-Puro (containing the Homo sapiens TRPV1 gene; GenBank: AY131289.1) or pCDH-CMV-MCS-EF1-Puro lentiviral plasmids (Invitrogen, USA). These particles were then used to infect the HaCaT cells (BeNa Biotech, China) to get the HaCaT-TRPV1-OE or HaCaT-TRPV1-NT cells.
Cells were further cultured in Dulbecco's Modified Eagle Medium (high glucose) (DMEM, Thermo Fisher Scientific, USA) supplemented with 10% fetal bovine serum (FBS, Thermo Fisher Scientific, USA) at 37°C and 5% CO2.
2.2 Preparation of Citrus reticulata fruit extract (CR)
Briefly, the selected fruits (0.5 kg) were extracted with water (5.0 kg) for 1.5 h at 90°C. The extract was enzymatically hydrolyzed with pectinase for 1 h at 45°C. Next, the extract was heated at 90°C for 30 min to eliminate proteins and then filtered with a polypropylene membrane (0.8 μm pore size). The filtrate (about 3.8 kg) was subjected to column chromatography over D101 macroporous resin (7.5 × 30 cm) and then eluted with water (1.5 kg), 30% butylene glycol (1.5 kg), and 40% butylene glycol (2 kg). To obtain CR, the pH of the 60% butylene glycol fraction was adjusted to 4.0–4.5 and UV absorption spectroscopy was used to identify the active components in CR.
2.3 Intracellular calcium measurement
HaCaT-TRPV1-OE, HaCaT-TRPV1-NT, or HaCaT cells were plated into black 96-well assay plates for 24 h at 2 × 104 per well. Then, the cells were incubated with 5 μM Fluo-8 AM dye (ATT, USA) for 30 min before addition of 4 μM CAP (St. Louis, USA), 0.4% phenoxyethanol (Phe, RaysonChem, China), 2% lactic acid (Sigma-Aldrich, Germany), 5% nicotinamide (RaysonChem, China), 5% glycolic acid (RaysonChem, China), 2% salicylic acid (RaysonChem, China) or 0.012% sodium dodecyl sulfate (SLS, Sigma-Aldrich, Germany). The change in cellular calcium influx was immediately monitored by Flexstation 3 system (excitation 485 nm, emission 525 nm) and SoftMax Pro 5.4.1 software (Molecular Devices). The pharmacological TRPV1 inhibitor ruthenium red (RR, Sigma-Aldrich, Germany) or capsazepine (CPZ, Sigma-Aldrich, Germany) was used as a positive control.
High throughput screening model: HaCaT-TRPV1-OE cells were cultured in 96-well plates and incubated for 24 h. Different concentrations of CR or other plant extracts was added to the cells alongin conjunction with 5 μM Fluo-8 AM dye and inculated for 30 min before addition of 4 μM CAP, 0.4% phenoxyethanol or 5% nicotinamide and cellular calcium influx was immediately monitored by Flexstation 3 system.
To measure the sustained calcium influx, HaCaT-TRPV1-OE cells were seeded onto coverslips in 24-well plates and incubated for 24 hours. A concentration of CR ranging from 0.03% to 0.25% was added to the cells alongin conjunction with 5 μM Fluo-8 AM dye for 30 min, and then cells were treated with either 5 μM CAP or 0.4% phenoxyethanol for 10 min before being rinsed. A fluorescence microscope was used to observe the fluorescence of the sample (excitation 485 nm, emission 525 nm).
2.4 Detection of TRPV1 protein expression
0.5 × 105 cells of HaCaT-TRPV1-OE were seeded in 24-well plates onto coverslips and allowed to grow for 24 h. Co-cultures of cells with CR (0.06%–0.25%), phenoxyethanol (Phe), or lactic acid (LA, RaysonChem, China) were maintained for 24 h. As described previously, immunofluorescence was detected using TRPV1 antibodies (Abcam, UK) and Alexa Fluor 568 labeled goat anti-rabbit IgG (Invitrogen, USA).11
2.5 Clinical research
2.5.1 Samples
Formulas for CR-containing lotions with concentrations of 0.5% and 1% are shown in Table 1. The base formula without CR was considered a placebo group (negative control). Before in vivo study, samples were tested for adverse skin reactions using a 24 h skin patch test.
| Product name | BASE (%w/w) | 0.5% CR (%w/w) | 1% CR (%w/w) |
|---|---|---|---|
| Water | Added to 100.00 | Added to 100.00 | Added to 100.00 |
| EDTA disodium | 0.02 | 0.02 | 0.02 |
| Glycerin | 3.00 | 3.00 | 3.00 |
| Butylene glycol | 2.00 | 2.00 | 2.00 |
| GPL antiseptic | 0.40 | 0.40 | 0.40 |
| CR | 0.00 | 0.50 | 1.00 |
| PEG-40 hydrogenated castor oil | 3.00 | 3.00 | 3.00 |
2.5.2 Subjects and screening procedures
The selection process for SS participants was conducted in two stages. To begin, people aged 20–45 who believed they had SS filled out a questionnaire.12 Pregnant women, people with known or suspected malignancies, those with scars or wounds at the testing site, and those with cutaneous disorders like acne, atopic dermatitis, and rosacea were not permitted to take part. A lactic acid sting test was also administered, and participants were selected if they had a total sting score of more than 3 at both 2.5 and 5 min. The lactic acid sting test was carried out exactly as described elsewhere.13 To apply lactic acid to the nasolabial folds or cheeks, a cotton swab was dipped in a 10% lactic acid solution. Discomfort on a 5-point scale was recorded within 5 min of applying lactic acid to the skin (0, no discomfort; 1, mild discomfort; 2, moderate discomfort; 3, severe discomfort; 4, extreme discomfort).
2.5.3 Instant skin soothing test
The instant skin soothing test included 28 participants (10 males and 18 females) with SS and an average age of 29 years. Initially, a 10% lactic acid solution was applied to the nasolabial folds or cheeks using a cotton swab; the sting score was recorded 2.5 min after application using the 5-point scale shown above; this score was also used as the sting score prior to the application of 1% CR (0 min). After this, 1% CR was applied to the same areas of each subject's cheeks or nasolabial folds at a dose of 20 μl/cm2 immediately, and skin discomfort scores were recorded at 2.5 and 5 min after the application of 1% CR using a 5-point scale (as indicated above).
2.5.4 Long-term skin tolerance test
Twenty-four subjects (8 males and 16 females) with SS and an average age of 28 years were included for long-term lactate tolerance testing. First, on Day 0, we performed the lactic acid stinging test by applying 10% lactic acid solution with a cotton swab to the left and right nasolabial folds or cheeks of the subjects, respectively, and recording the stinging score at 1 and 5 min after application of 10% lactic acid on a 5-point scale (as indicated above), before washing the face with warm water. Step 2 involved a half-face control application of a sample: half of the faces received base (placebo) and the other half received 0.5% CR at a dose of 20 μl/cm2 every morning and night for seven days. Third, we repeated the lactic acid stinging test in the afternoon of day 7 to get stinging scores again, as we did in steps 1 and 2.
2.5.5 Statistical analysis
Data were reported as means ± SEM based on at least three experiments. Statistical analyses were performed using GraphPad 5.0 statistical software (GraphPad, USA). For in vitro experiments, p-values were calculated using the one-way analysis of variance (ANOVA) method; while for in vivo tests, p-values were calculated using the t-test method. Differences between groups are considered significant if the p-value is less than 0.05 (p < 0.05).
Data with p ≤ 0.05 indicated a significant improvement. *indicates comparison with vehicle, *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001.
3 RESULTS
3.1 HaCaT-TRPV1-OE cells as a screening model for SS
According to previous research, TRPV1 expression is elevated in SS patients, and its levels positively correlate with disease severity.5, 6 As shown in Figure 1A, TRPV1 protein (red fluorescence) expression was higher in HaCaT-TRPV1-OE cells than in HaCaT cells. The most potent calcium influx was caused by 4 μM CAP in HaCaT-TRPV1-OE cells, which increased by almost 10-fold in comparison to the control group (the untreated group), but not in HaCaT or HaCaT-TRPV1-NT cells (Figure 1B). Moreover, chemical substances such as lactic acid, phenoxyethanol, nicotinamide, glycolic acid, salicylic acid, or sodium dodecyl sulfate (SLS) could activate TRPV1 and be tested in HaCaT-TRPV1-OE cells. Furthermore, the activating concentration of each stimulant for HaCaT-TRPV1-OE cells was within the range of the corresponding clinical concentration that elicits skin discomfort. As shown in Figure 1C, the classical antagonists of TRPV1, CPZ, and ruthenium red (RR) could block the influx of calcium into cells caused by CAP in a dose-dependent manner. This was seen with Flexsation 3 and fluorescence microscopy, respectively. Therefore, HaCaT-TRPV1-OE cells with high sensitivity and lower threshold to internal and external stimulating factors can be used as a high throughput screening model for SS.
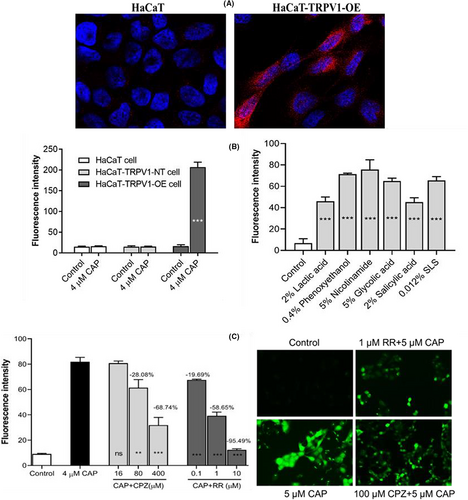
3.2 CR acts as a TRPV1 inhibitor
Hundreds of differently processed plant extracts and compounds were screened using CAP-treated HaCaT-TRPV1-OE cells to identify potent and effective inhibitors of TRPV1. Herein, a patented, specialized plant extraction method was used to produce CR. According to the obtained data, CR contained more than 0.6% alkaloids and 0.6% flavonoids.
As shown in Figure 2A, a 1%–4% concentration of CR inhibited not only the 4 μM CAP stimulated calcium influx but also that from 0.4% phenoxyethanol and 5% niacinamide in a dose-dependent manner; the maximum inhibition was >95.0%. To further clarify the CR antagonistic effect in cells against TRPV1 activation, a fluorescence microscope assay was performed to identify the continuous activation of TRPV1. Compared to the control group, both the phenoxyethanol and CAP groups had strong green fluorescence. Figure 2B shows that when CR was treated with phenoxyethanol or CAP at concentrations between 0.06% and 0.25%, the intensity of the fluorescence decreased with the dose. These results suggested that CR can inhibit both transient and continuous activation of TRPV1 receptors induced by phenoxyethanol or CAP.
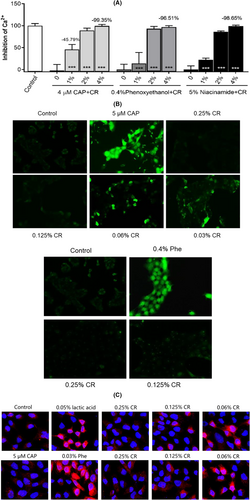
This study also evaluated the effect of CR on TRPV1 protein expression (Figure 2C). TRPV1 expression significantly increased when cells were treated for 24 h with a concentration of 0.05% lactic acid and 0.03% phenoxyethanol than the control (red fluorescence). However, this phenomenon was absent in the 5 μM CAP group. These data suggested that CAP mainly activates TRPV1, but long-term use of lactic acid and phenoxyethanol not only activates TRPV1 but also upregulates its expression, aggravating SS.
Interestingly, 0.125%–0.25% concentration of CR can significantly inhibit lactic acid or phenoxyethanol-induced upregulation of TRPV1 expression. These results indicated that CR acts as an inhibitor of TRPV1 activation and expression against different stimulants.
3.3 Topically applied CR could improve skin discomfort
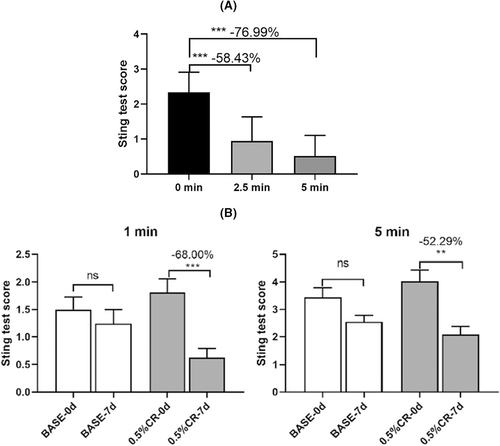
Herein, 54 participants satisfied the screening and exclusion criteria and were positive for lactate tingling following a two-step screening approach. Furthermore, 28 and 24 subjects were included in the instant skin soothing effect and long-term skin tolerance effect studies, respectively. In addition, lactic acid sting test carried out during the subject screening process have found that skin discomfort is consistently increased between 2.5 and 5 min after the application of lactic acid, if no effective substance is used to relieve this discomfort and can be prolonged more than 10 min. The results of the instant soothing effect of CR on lactic acid-induced skin discomfort are shown in Figure 3A. Compared to before application, lactic acid-induced skin discomfort was reduced by 58.43% at 2 min and 76.99% at 5 min after 1% CR. The results of the skin tolerance to lactic acid study were shown in Figure 3B. The placebo group (base) did not have a significant relieving effect against lactic acid-induced skin discomfort, whereas, patients' tolerance to lactic acid-increased after 7 days of continuous application of 0.5% CR. Additionally, the lactic acid induced skin discomfort after CR treatment reduced by 68.00% and 52.29% at 1 and 5 min, respectively, compared to before CR application. In view of the obtained data, CR not only provided immediate relief but also improved the tolerance of SS to lactic acid.
4 DISCUSSION
The diagnosis and assessment of SS mainly involve questionnaire surveys and sensory analysis, such as stinging tests with lactic acid,14 CAP, and dimethyl sulfoxide (DMSO).15 However, these strategies are associated with major challenges such as the need for a professional evaluator, long cycle, high cost, and ethical concerns. Furthermore, these methods are not suitable for large-scale screening required in the early stages of product development. Herein, a high-throughput screening model (HaCaT-TRPV1-OE cell line) for SS was successfully established and validated for product development. Moreover, CR (Shanghai JAKA Bio-Tech Co., Ltd) was found to relieve SS via inhibiting TRPV1 expression and activation.
According to a previously reported study, the role of keratinocytes in SS may not be limited to alterations of the epidermal barrier but can also be related to their sensory properties such as pain and itching.16 Additionally, keratinocytes are considered to provide only physical support for the intra-epidermal nerve fibers (IENF) which are the main factor in SS syndrome.16 Hence, keratinocytes act as intermediaries between the environment and IENF perceiving exogenous stimuli.17 Also, keratinocytes produce various skin receptors, including nociceptors, and can release a variety of substances that can activate sensory neurons, such as glutamate, acetylcholine, or ATP, further facilitating the conduction of nerve signals.18 HaCaT, a spontaneously immortalized, human keratinocyte cell line has been widely used for dermatology and differentiation studies.19 The same was used in this study.
For the examination of sensitive skin, a variety of chemicals like lactic acid, CAP, SLS, etc. are utilized as test probes.12 In addition, some personal care ingredients have the potential to induce skin discomfort and are studied for skin sensitivity, such as niacinamide, phenoxyethanol,20 glycolic acid, salicylic acid, etc. According to the results of this study, these stimuli elicit TRPV1 activation, which causes cutaneous pain by increasing calcium influx. However, additional evidence is required to confirm this finding. In addition, CAP, a canonical agonist of TRPV1,21 was used as an agonist for the high-throughput screening of TRPV1 inhibitors. Herein, hundreds of differently processed plant extracts and natural compounds were screened for their ability to block CAP-induced activation of TRPV1. According to the obtained results, CR was selected and prepared. To further study the therapeutic effects and mechanism of CR in SS, phenoxyethanol, CAP, nicotinamide, and lactic acid were selected as TRPV1 agonists.
Compared with normal skin, SS is characterized by hyper-reactivity for an exogenous stimulant, which can be attributed to high TRPV1 expression in sensitive skin.5, 6 In order to investigate the probable antagonistic molecular mechanism of CR in SS, both TRPV1 expression and TRPV1 activity was assessed in this work. TRPV1 activation studies have shown that phenoxyethanol, niacinamide, CAP, and lactic acid can all activate TRPV1 and stimulate intracellular calcium signaling, whereas CR suppresses these agonists, except for lactic acid, which increases this signal in cells. Experiments on TRPV1 protein expression revealed that phenoxyethanol and lactic acid boosted TRPV1 protein expression, whereas CAP did not. Notably, CR may decrease the production of TRPV1 protein produced by lactic acid or phenoxyethanol, except for CAP. This phenomenon is providing new insights into TRPV1 activation and expression in SS. In addition, this phenomenon may be further explained by reports about TRPV1 antagonists in clinical setbacks,22-24 which believe an increase in human core body temperature was mainly attributable to the antagonistic inhibition of both low pH and CAP activation of TRPV1. Thus, inhibition of TRPV1 activation induced by either low pH or CAP has become a new development approach. Moreover, multiple clinical studies suggest that these TRPV1 antagonists do not increase human core body temperature.22-24 Therefore, this study speculates that CR may not increase the core body temperature; however, further clinical studies are needed to confirm this concept.
Taken together, based on previous studies about the difference between normal and SS, a high-throughput screening model (HaCaT-TRPV1-OE cell line) was established to evaluate TRPV1 inhibitors. CR was identified from hundreds of samples and evaluated in CAP-induced HaCaT-TRPV1-OE cells. Furthermore, CAP, phenoxyethanol, nicotinamide, and lactic acid were used as TRPV1 activators to explore the underlying mechanism of CR on TRPV1 activation and expression. Finally, in vivo skin, soothing studies validated the immediate soothing and skin tolerance-enhancing effect of CR on sensitive skin. This study demonstrated that CR alleviates SS by reducing TRPV1 activity and expression.
ACKNOWLEDGMENTS
We would like to thank the ethics committee of Shanghai JAKA Biotech for kindly approving this clinical study and the team of Wei-Qiang Lu from East China Normal University to support our substance screening by providing Flexstation3 instruments.
FUNDING INFORMATION
This study was funded in full by Shanghai JAKA Biotech Co. Ltd. All authors are employees of Shanghai JAKA Biotech Co. Ltd.
CONFLICT OF INTEREST
The authors have no other conflict of interest to declare.
ETHCAL APPROVAL
The authors confirm that the ethical policies of the journal, as noted on the journal's author guidelines page, have been adhered to. Clinical studies have been approved by the ethics committee of Shanghai JAKA Biotech Co. Ltd. on 13th July 2022, with an approval number of JKC-2207-00020.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




