Novel conformation of hyaluronic acid with improved cosmetic efficacy
Abstract
Background
Hyaluronic acid presents a valuable cosmetic ingredient that occurs naturally. Its direct links to skin aging has led to its broad application. The aim of this study was to improve the cosmetic efficacy of high molecular weight hyaluronic acid (HMWHA) without chemical modifications and evaluate such improvements through clinical and in vitro studies.
Methods
A novel formulation of HMWHA (SCAI-HA) was prepared and investigated to comparatively assess 6 clinical and 2 in vitro parameters concerning its dermatological cosmetic efficacy and biological properties. The dermatological and cellular parameters examined in this study include skin hydration, transepidermal water loss (TEWL), skin elasticity, wrinkles, facial sagging, dermal density, cytotoxicity, and collagen synthesis.
Results
SCAI-HA exhibited the ability to improve the tested dermatological parameters (hydration, elasticity, wrinkles, and density) to magnitudes comparable to those of HMWHA. In addition, SCAI-HA showed notably improved capacities for attenuating facial sagging and TEWL and promoting cellular collagen synthesis in normal human dermal fibroblasts.
Conclusion
SCAI-HA presents a novel conformation of HMWHA with improved cosmetic efficacy in mitigating (i) facial sagging, (ii) TEWL, and promoting, and (iii) collagen synthesis. These findings denote the enhancement of SCAI-HA as a cosmetic ingredient with potential anti-aging properties.
Abbreviations
-
- FBS
-
- Fetal bovine serum
-
- FBM
-
- fibroblast basal medium
-
- GA 1000
-
- gentamicin sulfate amphotericin 1000
-
- HA
-
- Hyaluronic acid
-
- HMWHA
-
- High molecular weight hyaluronic acid
-
- LMWHA
-
- Low molecular weight hyaluronic acid
-
- NHDF
-
- Normal human dermal fibroblasts
-
- PIP
-
- procollagen type I C-peptide
-
- RC
-
- Retinacula cutis
-
- rhFGF B
-
- r-human fibroblast growth factor B
-
- TEWL
-
- Transepidermal water loss
1 INTRODUCTION
Hyaluronic acid (HA) is a naturally occurring anionic non-sulfated glycosaminoglycan with a broad physiological distribution1-3; approximately 50% of the total body HA can be found in the skin.2 The building blocks of HA are polymeric disaccharides composed of D-glucuronic acid and N-acetyl-D-glucosamine linked by alternating β-1,3 and β-1,4 glycosidic bonds.2 Numerous hydroxyl groups throughout HA's molecular structure promote the formation of stable tertiary structures4 and elaborate entangled hydrogen bonding networks in water.5, 6 Consequently, aqueous solutions of HA manifest significant viscosity in a manner dependent on its molecular weight distribution.7 As a key component of the extracellular matrix, HA presents a remarkable capacity for binding and retaining water. It is also involved in various biological processes including cell migration/proliferation, wound reparation, tissue regeneration, and matrix organization.8 Previous research indicates that the reduction of epidermal HA levels and molecular weight as key histochemical characteristics of senescent skin contributing to dehydration, loss of elasticity, and atrophy.9-11 Furthermore, the notable biocompatibility, biodegradability, mucoadhesivity, hygroscopicity, and viscoelasticity of HA make it exceedingly useful as a pharmaceutical and cosmetic substance.8 An interesting facet of HA's biological properties is its size-dependence: (i) high molecular weight HA (HMWHA; ≥ 1000 kDa) displays anti-inflammatory, anti-angiogenic, and immunosuppressive activities; (ii) low molecular weight HA (LMWHA; ≤ 50 kDa) reportedly induces inflammation, immune response, and angiogenesis; (iii) dermal penetration of HA is strongly size-dependent with reports indicating that low molecular weight HA is significantly better in permeating the skin.7, 12 Previous research regarding the size-dependent properties of HA implies the notion that the cosmetic functionality and utility of HMWHA may be superior to that of LMWHA if the limitations in dermal penetration can be overcome. In this study, a novel conformation of HMWHA (MW = ca. 1200 kDa) was prepared for the purpose of improving upon its cosmetic efficacy without the application of chemical modifications (e.g., crosslinking, covalent bonding). This conformation, referred to as SCAI-HA, was tested both clinically and in vitro. Clinical studies were performed to comparatively evaluate the conformation's effect on skin hydration, transepidermal water loss (TEWL), skin elasticity, eye wrinkles (crow's feet), facial sagging, and skin density. In vitro studies evaluated its cytotoxicity and promotional effects on cellular collagen synthesis.
2 MATERIALS AND METHODS
All reagents were purchased from commercial suppliers and used as received unless noted otherwise. Cosmetic grade HMWHA with an average molecular weight of 1200 kDa from Xi'an Lyphar Biotech (Xi'an, China) was used to prepare SCAI-HA and as the control product for this study. 1,2-hexanediol was obtained from Activon (Cheongju, South Korea). Normal human dermal fibroblasts (NHDF) cells were obtained from Lonza (Basel, Switzerland). Fetal bovine serum (FBS), r-human fibroblast growth factor B (rhFGF B), insulin, gentamicin sulfate-amphotericin B (GA 1000), fibroblast basal medium (FBM) was purchased from Lonza (Basel, Switzerland). EZ-Cytox cell viability assay kit was purchased from Daeil Biotech (Seoul, South Korea). Procollagen type I C-peptide (PIP) EIA Kit was purchased from Takara Bio.
2.1 Clinical study
A total of 22 female subjects of approximately 52.2 years of age that satisfied the purposes of the study were recruited in the 4-week trial. The study comparatively evaluated the cosmetic effects of SCAI-HA and HMWHA by monitoring the following dermatological parameters: skin hydration, transepidermal water loss (TEWL), skin elasticity, eye wrinkles (crow's feet), facial sagging, and skin density. The subjects were selected based on (i) age, (ii) appearance of eye wrinkles (crow's feet), and (iii) lack of any acute or chronic conditions including skin disorders. The following factors were considered in subject exclusion: pregnancy, psychological disorders, administration of immunosuppressants within 3 months, administration of systemic steroid or radiation therapy within 1 month, presence of facial lesions that may hinder measurements, atopic dermatitis, extreme skin sensitivity or allergies to cosmetics, pharmaceutics, and light, chemical peel, or skin treatment within 3 months. Randomized clinical studies of SCAI-HA and HMWHA was conducted with IRB approval (P2108-2373) in accordance with the Code of Ethics based on the Helsinki Declaration, applicable human testing guidelines, and relevant regulations. The clinical cosmetic efficacy studies were conducted between August 2021 and October 2021 at PNK Skin Research Center in Seoul, South Korea. All recruited subjects have provided written informed consent. SCAI-HA (experimental) and HMWHA (control) products for clinical studies were provided as clear viscous solutions with the following compositions: SCAI-HA–0.5% SCAI-HA, 2.0% 1,2-hexanediol, 97.5% water; HMWHA–0.5% sodium hyaluronate, 2.0% 1,2-hexanediol, 97.5% water. The efficacy assessments were performed as a randomized double-blind study to compare the SCAI-HA- and HMWHA-treated facial areas. A random number table was generated to indiscriminately assign the experimental and control groups on the left and right sections of the face of each subject. The subjects were trained to clean and dry their hands before the application of equivalent amounts of each solution (single pump). The products, stored at room temperature in the dark, were administered twice a day for 4 weeks. Throughout the study, participants were instructed to avoid the use of cosmetic products containing any active ingredients (e.g., whitening, anti-wrinkle, drying) that could influence the measurement of dermatological parameters. Subjects made 3 visits for clinical evaluation: before product application, after 2 weeks of product application, and 4 weeks of product application.
2.2 Instrumental analysis
To ensure the accurate assessment of the dermatological parameters, all subjects were accommodated in a controlled environment at 20–24 °C and 40–60% humidity for 30 min before instrumental analysis. Repeated measurements were taken in the same area of the face each time. All measurements and experiments were performed in triplicate and presented as their average unless noted otherwise.
2.3 Skin hydration
Hydration levels of the skin were measured using the Corneometer CM825 (Courage Khazaka electronic GmbH, Germany) on each cheek of the subjects. The corneometer assesses the level of skin hydration as a measure of the change in electric permittivity. The skin hydration levels of each side of the subjects' face were measured at 0, 2, and 4 weeks of product application. Measurements were performed in triplicate and the average was used for analysis and interpretation.
2.4 Transepidermal water loss (TEWL)
The transepidermal water loss of each subject was measured on both cheeks using a Vapometer (Delfin Technologies Ltd, Finland). TEWL was presented by a numerical value signifying the lost mass of water (g) in a specific area (m2) per hour (h) in the form of the following unit: g/m2h. The TEWL of each side of the subjects' face was measured after 0, 2, and 4 weeks of product application.
2.5 Skin elasticity
The skin elasticity of the subjects was measured using the Cutometer MPA580 (Courage Khazaka electronic GmbH, Germany) on each cheek. Cutometer applies a constant negative pressure of 450 mbar with 2 s of on-time and 2 s of off-time to monitor the corresponding changes of the skin tissue. R2 was used as a measure of the skin's ability to resist deformation and return to its original form as a parameter representing its elasticity. The skin elasticity of each side of the subjects' face was measured after 0, 2, and 4 weeks of product application.
2.6 Wrinkles (crow's feet)
Wrinkles in the crow's feet region of the subjects were evaluated using PRIMOS CR Small Field (Canfield Imaging Systems, USA) by taking images of the region of interest. These images are then analyzed to determine the Ra value as a measure of average roughness in μm. The eye wrinkles of each side of the subjects' face were assessed after 0, 2, and 4 weeks of product application.
2.7 Facial sagging
Facial sagging of the clinical subjects was evaluated by topographically imaging the sides of their face. Topographs were taken with F-ray (BEYOUNG Co., Ltd.) on each cheek of the subjects. The resultant images were then analyzed via image pro® plus (Media Cybernetics, Rockville) to determine the angles (°) representing the degree of facial sagging in each patient. Facial sagging of each side of the subjects' face was measured after 0, 2, and 4 weeks of product application.
2.8 Skin density
Skin Scanner DUB USB (TPM taberna pro medicum, Germany) was used to evaluate the skin density of subjects using dermal ultrasonic imaging. Colored two-dimensional images of the ultrasound intensity were generated at different amplitudes. These images were then analyzed to calculate the dermal density of skin tissue. Measurements were performed in areas surrounding the eyes on each side. Skin density was presented as Density (%). The skin density of each side of the subjects' face was measured after 0, 2, and 4 weeks of product application.
2.9 In vitro study
For the in vitro experiments, the products were diluted in the appropriate media to final [HA] concentrations of 0.003, 0.006, and 0.012% (w/v). The normal human dermal fibroblasts (NHDF) cell line was maintained in FBM media containing 0.1% insulin, 0.1% rhFGF B, 0.1% GA 1000, and 2% FBS. The NHDF cells were grown and maintained at 37°C in a humidified atmosphere with 5% CO2. It should be noted that the in vitro HA samples did not contain 1,2-hexanediol. Before evaluating their effect on cellular collagen synthesis, the cytotoxicity of the HA products was determined against NHDF cells.
2.10 Cellular collagen synthesis
NHDF cells were seeded onto a 24-well plate (20 000 cells per well) and incubated for 24 h. Thereafter, the cells were incubated in serum-free FBM containing the diluted HA products [i.e., SCAI-HA and HMWHA; 0.003, 0.006, 0.012% (w/v)] for 24 h. The supernatant was obtained from each well and its procollagen content was quantified using a procollagen type I C-peptide (PIP) EIA kit. The microplate reader was used to measure the absorbance at 450 nm. L-ascorbic acid (0.001%) was used as a positive control.
3 RESULTS AND DISCUSSION
3.1 Skin hydration
Acknowledged for its ability to pertain moisture, the effects of HA in both forms, SCAI-HA and HMWHA, on skin hydration were investigated. The Corneometer measures hydration levels directly on the skin based on changes in electric permittivity.13 An applied scatter field penetrates the first skin layer and the level of hydration is determined by the detected changes in the dielectric constant. Larger values denote greater hydration levels. Measurements were taken at the same facial area on both sides (Table 1). The data were analyzed by referring to the patient number and the random number table generated for assigning the experimental and control groups (SCAI-HA and HMWHA, respectively). Before product application, skin hydration levels of the SCAI-HA and HMWHA groups started at 67.2 ± 9.3 and 66.7 ± 9.0, respectively. After2 weeks, these values increased to 70.7 ± 9.0 and 70.4 ± 9.3; then 74.9 ± 8.3 and 74.2 ± 8.3 after 4 weeks of applying the corresponding HA product. Dermal application of SCAI-HA led to 5.1% and 11.4% improvements in skin hydration at week 2 and 4, respectively. Similarly, skin hydration improved by 5.6% and 11.2% from HMWHA at Weeks 2 and 4, respectively. Both SCAI-HA and HMWHA elicited statistically significant increases in skin hydration after 2 and 4 weeks of application. SCAI-HA's ability to improve skin hydration, however, was comparable to that of HMWHA.
| A.U. | % Improvement | |||
|---|---|---|---|---|
| SCAI-HA | HMWHA | SCAI-HA | HMWHA | |
| Week 0 | 67.2 ± 9.3 | 66.7 ± 9.0 | ||
| Week 2 | 70.7 ± 9.0** | 70.4 ± 9.3** | 5.1 | 5.6 |
| Week 4 | 74.9 ± 8.3** | 74.2 ± 8.3** | 11.4 | 11.2 |
- Note: Measurements were performed in triplicate with Corneometer CM825. **p < 0.05 by repeated-measures ANOVA, post hoc Bonferroni correction.
3.2 Transepidermal water loss (TEWL)
TEWL is a measure of the amount of water that passively diffuses from the dermis and epidermis through the stratum corneum to the skin surface.14 It is often considered a critical parameter of the skin barrier representing its integrity. As a dermatological parameter, TEWL is a sensitive measure that is affected by the surrounding environment and local skin properties. Kottner et al. reported that TEWL is subject to substantial intra-individual variability with the following parameters proposed as key influencing factors: (i) permeation path lengths dependent on corneocyte size and epidermal cell layer depth and (ii) quantity and composition of intercellular lipid bilayers.14-17 Therefore, it is critical to make TEWL measurements at the same position under consistent environmental conditions after a period of skin accommodation. To evaluate the effects of SCAI-HA and HMWHA on skin barrier function, the TEWL of the subjects was assessed before and after 2 and 4 weeks of product application (Table 2). Dermal application of SCAI-HA and HMWHA resulted in TEWL reduction, denoting improvements in skin barrier function: (i) SCAI-HA treatment decreased TEWL from 13.0 ± 3.2 g/m2h to 10.7 ± 2.1 and 10.2 ± 2.2 g/m2h after two and 4 weeks, respectively, (ii) HMWHA treatment decreased TEWL from 11.5 ± 2.2 g/m2h to 10.3 ± 2.1 and 9.9 ± 1.9 g/m2h after two and 4 weeks, respectively. It should be noted that SCAI-HA induced a greater decrease in TEWL at magnitudes of statistical significance (17.6 and 22.1%) after 2 and 4 weeks of application, compared with HMWHA (10.0 and 13.2%).
| TEWL (g/m2h) | % Improvement | |||
|---|---|---|---|---|
| SCAI-HA | HMWHA | SCAI-HA | HMWHA | |
| Week 0 | 13.0 ± 3.2 | 11.5 ± 2.2 | ||
| Week 2 | 10.7 ± 2.1** | 10.3 ± 2.1** | 17.6 | 10.0 |
| Week 4 | 10.2 ± 2.2** | 9.9 ± 1.9** | 22.1 | 13.2 |
- Note: Measurements were taken in triplicate and the average value is presented. **p < 0.05 by repeated measures ANOVA, post hoc Bonferroni correction.
3.3 Skin elasticity
Elasticity is a key physical parameter of the skin denoting its ability to retain its form and recover shape when stretched or deformed.18 Skin elasticity is a measure of its structural integrity and condition as skin aging and damage lead to noticeable decreases in skin elasticity.19 Reduction of skin elasticity is reportedly associated with elastin-fiber degradation, collagen fragmentation, and protein aggregation. Moreover, substantial loss of skin elasticity can result in wrinkle formation and facial sagging.20, 21 The effects of SCAI-HA and HMWHA were directly evaluated on the cheeks of the subjects using Cutometer MPA580 (Table 3). Skin elasticity was presented as a measure of R2 (Ua/Uf), the ratio of total recovery of skin on normal pressure after deformation.22 Ua is the total recovery of the skin toward its original position after a second under normal pressure; Uf is the maximal distension of the skin into the probe at the end of the vacuum period.23 Larger R2 values denote greater skin elasticity. Starting from 0.633 ± 0.056, the R2 values increased to 0.658 ± 0.058 and 0.684 ± 0.054 after 2 and 4 weeks of SCAI-HA application; HMWHA-treated groups exhibited similar magnitudes of change from 0.633 ± 0.053 to 0.659 ± 0.058 and 0.681 ± 0.048. Such improvements in R2 values were statistically significant indicating the products' ability to improve skin elasticity through dermal application. No substantial distinction was observed between the two products' ability to increase skin elasticity.
| R 2 | % Improvement | |||
|---|---|---|---|---|
| SCAI-HA | HMWHA | SCAI-HA | HMWHA | |
| Week 0 | 0.633 ± 0.056 | 0.633 ± 0.053 | ||
| Week 2 | 0.658 ± 0.058## | 0.659 ± 0.058## | 3.949 | 4.107 |
| Week 4 | 0.684 ± 0.054## | 0.681 ± 0.048## | 8.057 | 7.583 |
- Note: Measurements were made in triplicate using Cutometer MPA580. ##p < 0.025 by Friedman test, post hoc Wilcoxon signed-rank test with Bonferroni correction.
3.4 Wrinkles
Lacking a standardized classification, wrinkles or rhytides are associated with textural changes in skin caused by intrinsic and extrinsic aging.24 These features are characterized as discernible folds, ridges, or creases of the skin. The gradual loss and disorganization of collagen and elastin fibers, connective tissue that provides underlying support for skin, cause these rhytides.25 Misrepair-accumulation aging theory suggests that wrinkles develop from incorrect repairs of injured elastin and collagen fibers.26, 27 Signs of aging are often first manifested in the periorbital region in the form of crow's feet (Figure 1B).28 Therefore, the average roughness [Ra (μm); Figure 1A] of the crow's feet area was assessed as a measure of the severity of wrinkles before and after product application. Both SCAI-HA and HMWHA demonstrated the ability to attenuate wrinkles in the periorbital region to similar extents. Treatment of SCAI-HA reduced the average roughness from 18.7 ± 3.4 μm to 18.2 ± 3.5 and 17.9 ± 3.3 μm after two and 4 weeks of product application, respectively, while HMWHA reduced the average roughness from 18.7 ± 3.5 μm to 18.1 ± 3.4and 18.1 ± 3.4 μm. Although SCAI-HA exhibited slightly greater improvements in wrinkle reduction, these differences were determined to be not statistically significant.
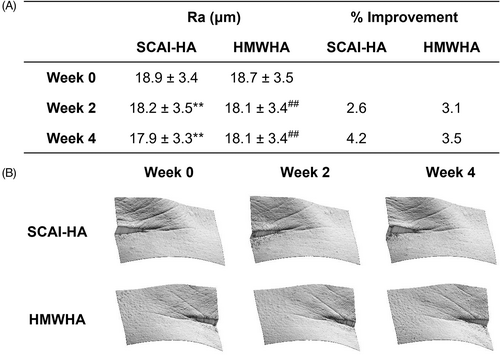
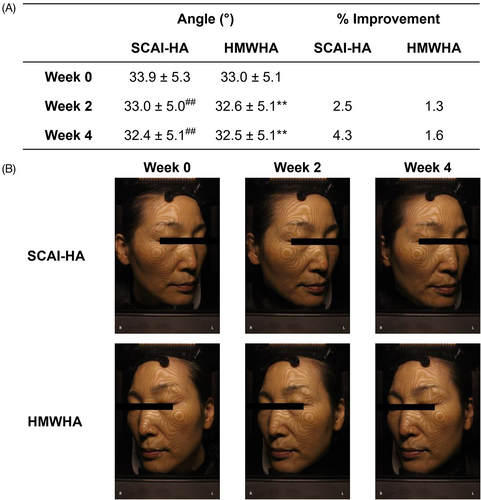
3.5 Facial sagging
Facial sagging, despite its self-explanatory terminology, is a poorly understood dermatological and morphological parameter due to its complexity.29 Relaxation of retaining facial ligaments and its consequential laxity have previously been regarded as the main cause of facial sagging.30 Recent efforts, however, aim to reframe the etiology of facial sagging by emphasizing Mendelson's theory that repetitive facial movements lead to (i) development of laxity in skin, subcutaneous tissue, superficial musculoaponeurotic system, and superficial expression muscles, and (ii) facial skeleton remodeling.31 The amalgamation of these etiological factors is hypothesized to ultimately result in facial sagging. Hence, facial sagging can serve as a dermatological parameter representing skin health at a deeper level. To evaluate the facial sagging of subjects, topographic images were taken on both sides of the subjects' faces (Figure 2B) treated with either SCAI-HA or HMWHA by forming elongated circles to connect a set of points based on absolute 3D coordinates (x, y, z).32 From these topographs, facial sagging was evaluated by an analytical method described by Saito et al. using the Image-pro® plus software.33 Facial sagging was represented as angles (°) depicting facial sagging in the cheek region (Figure 2A); larger angles denoting greater facial sagging and vice versa. Starting from an angle of 33.9 ± 5.3°, the administration of SCAI-HA resulted in a decrease in angle to 33.0 ± 5.0° and 32.4 ± 5.1° after two and 4 weeks, respectively. HMWHA also induced a reduction in angle to a lesser degree: from 33.0 ± 5.1°–32.6 ± 5.1° and 32.5 ± 5.1°. Our data indicate that both SCAI-HA and HMWHA could noticeably improve facial sagging, while SCAI-HA's ability to attenuate facial sagging was greater than that of HMWHA with statistical relevance.
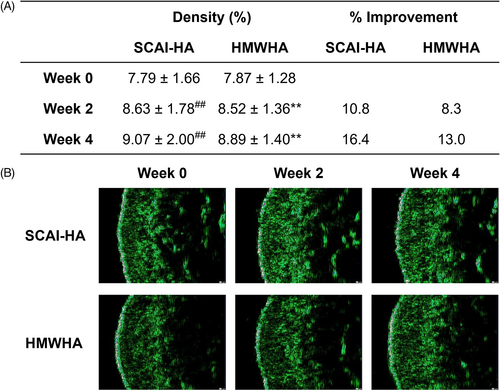
3.6 Skin density
Loss of skin density is a characteristic of intrinsic skin aging that leads to thinner skin and reduced elasticity. With age, the junction between the epidermis and dermis, composed of dermal papillae containing vessels that supply the epidermis with nutrients, undergoes deterioration where the depth and density of the papillae are reduced.34 This leads to a substantial decrease in dermal density; hence, skin density is often considered a dermatological parameter representing skin health. Herein, the dermal density of the subjects was measured via high-frequency ultrasound imaging (Figure 3).35 Before product application, the average skin densities of the subjects were 7.79 ± 1.66% and 7.87 ± 1.28% for the SCAI-HA and HMWHA-designated groups, respectively. After two and 4 weeks of SCAI-HA dermal application, the measured skin density was improved to 8.63 ± 1.78% and 9.07 ± 2.00%, respectively. Two and 4 weeks of applying HMWHA also led to improvements in skin density to 8.52 ± 1.36% and 8.89 ± 1.40%. The observed improvements in skin density were determined to be statistically significant. SCAI-HA's ability to increase skin density, however, was not determined to be substantially greater than that of HMWHA.
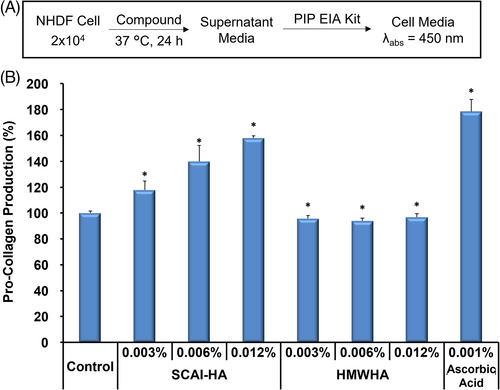
3.7 Collagen synthesis
Collagen is a major component of the extracellular matrix responsible for dermatological structure and function, which contributes to skin firmness and elasticity. Collagen type I accounts for ca. 70% of the collagen in the skin.36 Therefore, the effect of SCAI-HA and HMWHA on the cellular synthesis of Procollagen type I C-peptide (PIP) in NHDF cells was evaluated via ELISA (Figure 4). Ascorbic acid (0.001%) was used as a positive control.37 At concentrations 0.003–0.012%, SCAI-HA increased the production of PIP by 17.9%–57.9%. Oppositely, HMWHA did not noticeably alter the cellular synthesis of collagen. It should be noted that neither SCAI-HA nor HMWHA exhibited notable cytotoxicity against NHDF cells in the abovementioned range of concentrations (Figure S4).
In summary, SCAI-HA demonstrated superior dermatological properties to HMWHA in the following aspects: (i) attenuation of TEWL, (ii) reduction of facial sagging, (iii) promotion of cellular collagen synthesis. SCAI-HA also exhibited cosmetic efficacy concerning the remainder of the tested parameters to extents comparable to HMWHA. A closer examination of these experimental results can lead to the exploration of potential relationships and lack thereof among these parameters. First, despite its enhanced ability to reduce TEWL, SCAI-HA's effect on augmenting skin hydration levels was comparable to that of HMWHA. Based on the conceptual connections between skin hydration and TEWL, it may be expected that a greater reduction in TEWL would lead to a greater increase in skin hydration; greater skin barrier function, indicating an increased capacity to retain moisture in the skin, could be expected to lead to improved hydration. Our findings, however, did not support this idea: while SCAI-HA's capacity for reducing TEWL was better than that of HMWHA, their capacities for increasing skin hydration were comparable. Previous research indicates the complex relationship between skin hydration and TEWL. For instance, although a strong inverse correlation is reported between age and skin hydration,38, 39 the proportionality between age and TEWL remains controversial with numerous reports supporting the lack of correlation.40 It is possible that although the difference between SCAI-HA and HMWHA's effect on TEWL may be statistically significant, this variance is insufficient to noticeably affect skin hydration at the clinical level.
Another unexpected observation made from this study was that SCAI-HA exhibited an improved capacity for attenuating facial sagging, but not increasing skin elasticity. Skin elasticity and facial sagging are closely associated, as the skin's ability to retain its form by resisting external forces contributes to its overall elasticity. The drooping of facial features is a consequence of the deterioration of skin structure leading to the loosening of subcutaneous tissue, superficial musculoaponeurotic system, and superficial expression muscles. Remodeling of the facial skeleton resulting in decreased facial volume and fat mass have also been indicated as a key contributor to facial sagging. Considering the multifaceted etiology of facial sagging, HA's mechanism of action leading to its improved attenuation of facial sagging could stem from a number of these potential pathways including its ability to promote cellular collagen synthesis. As a key component of the extracellular matrix, collagen manifests substantial structural and functional contributions to the skin. Furthermore, its synergistic association with elastin leads to the stabilization of collagenous fibrils via intra- and intermolecular crosslinking imparting shape and firmness to skin.41, 42 In 2018, Shute et al. reported that collagen peptides significantly increased fibroblast elastin synthesis and inhibited elastin degradation.43 Furthermore, reports indicate that the density of retinacula cutis (RC), a subcutaneous network of collagenous fibers responsible for attaching the deep surface of the dermis to the underlying deep fascia, exhibited a negative correlation with facial sagging.44 In short, the increased collagen synthesis brought upon by HA leading to greater elastin-levels and RC complexity and density may present a potential dermatological pathway leading to notable improvements in facial sagging. Going a step further, SCAI-HA's enhanced ability to attenuate facial sagging could stem from SCAI-HA's increased promotional effects toward cellular collagen synthesis relative to HMWHA.
In this work, we aimed to first focus on the distinction in the cosmetic efficacy of SCAI-HA and HMWHA by assessing their short-term effects on the studied parameters; these short-term properties indicate the value of SCAI-HA. Based on the currently available data, the long-term effects of this conformation of HA can be expected to be more significant. Statistically significant differences were observed in the cosmetic efficacy of SCAI-HA and HMWHA within 4 weeks for three highlighted dermatological parameters: TEWL, facial sagging, and cellular collagen synthesis. Achieved in such a short period of time, this difference in efficacy would most likely be augmented in a longer trial. In addition, mechanistic studies regarding the improved cosmetic efficacy of SCAI-HA relative to HMWHA would be valuable in understanding the biochemical and physical properties of the novel HA conformation leading to such enhancements. A larger clinical study with a longer time frame would provide us with more reliable and valuable information regarding the long-term effects of the product. Despite these limitations, the current study sufficiently demonstrates the improved cosmetic efficacy of SCAI-HA relative to its original counterpart, HMWHA.
As for the monitoring of side effects, researchers were to take notes of any observable dermatological reactions that may have appeared following the application of our products. Through the duration of this study, however, no adverse reactions were reported. This was expected as hyaluronic acid is an API often found in cosmetic and medical products that is generally recognized as safe by the FDA.
4 CONCLUSION
As a naturally occurring biopolymer with valuable biological and chemical properties, HA presents a compound with substantial utility and exceptional bioapplicability. Such qualities have led to the broad employment of HA for various applications as a medical tool and cosmetic ingredient. In this study, we attempt to enhance HA's ability to mitigate skin aging and evaluate its cosmetic efficacy through clinical and in vitro studies. A novel conformation of HMWHA, SCAI-HA, was evaluated through clinical and in vitro experiments to test its utility as a cosmetic ingredient capable of enhancing skin hydration, elasticity, density, cellular collagen synthesis and reducing TEWL, wrinkles, facial sagging. Compared with its native counterpart HMWHA, SCAI-HA showed improved cosmetic function regarding the following dermatological parameters: (i) TEWL reduction, (ii) facial sagging mitigation, and (iii) promotion of cellular collagen synthesis. Considering the key dermatological parameters in skin aging,45 SCAI-HA could serve as a valuable anti-aging cosmetic ingredient. In-depth investigations regarding the mechanistic details behind the observed cosmetic enhancements are ongoing to better understand this technology at the molecular and cellular levels.
AUTHOR CONTRIBUTIONS
Dr. Geewoo Nam was responsible for designing the cosmetic efficacy studies, overseeing said studies, and primarily preparing the manuscript. Hye Won Lee and JiSung Jang contributed to preparing the test (SCAI-HA) and control samples. Dr. Chul Hwan Kim invented and provided the fundamental concept of altering the molecular conformation of hyaluronic acid. Dr. Kyoung-Hee Kim oversaw the production of SCAI-HA and research design.
ACKNOWLEDGMENT
This research was funded by SCAI Therapeutics®. We thank Dr. Yuna Kim for fruitful scientific discussions and insight. We would also like to acknowledge PNK Skin Research Center for conducting clinical studies.
CONFLICT OF INTEREST
There are no conflicts of interest to declare.
ETHICAL APROVAL
Randomized clinical studies of SCAI-HA and HMWHA was conducted with IRB approval (P2108-2373) in accordance with the Code of Ethics based on the Helsinki Declaration, applicable human testing guidelines, and relevant regulations. The clinical cosmetic efficacy studies were conducted between August 2021 and October 2021 at PNK Skin Research Center in Seoul, South Korea. All recruited subjects have provided written informed consent.
PHOTO CONSENT
All subjects have provided written informed consent for the use of photographs for this publication.
Open Research
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study are available in the supplementary material of this article




