Efficacy and tolerability of a hyaluronic acid-based serum and a peptide-rich cream for the face and neck in subjects with photodamaged skin
Abstract
Background
Barrier properties of the stratum corneum inhibit delivery of topical ingredients containing large molecules to desired targets in the skin. This study evaluated the efficacy and tolerability of a skincare regimen comprised of a hyaluronic acid-based serum (InF-HA) and a peptide-rich cream (InF-PEP) containing large molecular ingredients designed to improve the appearance and overall quality of skin.
Methods
This 12-week study evaluated changes from baseline in skin tone, skin texture, and lines/wrinkles (6-point grading scale) of twice-daily application of a two-part skincare regimen to the face and neck in female subjects with mild to moderate photodamage. Subject satisfaction was assessed, and Adverse Events (AEs) were captured throughout.
Results
Seventeen subjects with a mean age of 52 years completed the study. Improvements from baseline in the appearance of facial skin texture (79%), lines/wrinkles (50%), and skin tone (44%) occurred at week 12. Improvements in neck appearance from baseline were demonstrated in skin texture (68%), skin tone (48%), and lines/wrinkles (36%). No AEs occurred related to the use of study products. All subjects reported an overall improvement in the appearance of their skin and that their skin looked and felt smoother; 88% reported their skin looked more radiant, and 82% reported their skin looked firmer.
Conclusions
Application of a skincare regimen comprised of an HA-based serum and a peptide-rich cream led to substantial improvements in skin texture, skin tone, and lines/wrinkles on the face and neck over 12 weeks. Both products were well-tolerated with a high level of subject satisfaction.
1 INTRODUCTION
Skin aging is a complex process resulting in cumulative structural and physiological alterations of the extracellular matrix, which is rich in both collagen and hyaluronic acid. Hyaluronic acid (HA) and peptides are critical ingredients often incorporated into skincare formulations to help supplement and support photoaged skin. HA works by binding and retaining water molecules to hydrate skin; peptides are signaling molecules that regulate fibroblasts and support collagen.1, 2 However, while small molecule (≤500 Da maximum molecular weight) active ingredients may penetrate the stratum corneum (SC) more readily,3 delivery of large molecular actives to desired targets in the skin is often inhibited due to the effective barrier properties of the SC.
In an effort to maximize the performance of large molecular actives to the skin, a patented, comprehensive, trans-lipid formulation method (InterFuse®) has been developed to promote the efficient percutaneous transport of large and macro-molecule (>10 000 Da) actives to targets in the skin. This novel, adaptable delivery system utilizes a lipophilic coating that works concurrently with select ingredients, preserving the bioavailability and facilitating the transport of potent concentrations of key actives to targets in the skin. The lipophilic coating is comprised of a modified essential fatty acid that surrounds large molecular actives. The synergistic properties of the actives within the formulation temporarily modifies the surface tension of the SC enabling efficient permeability into the SC.
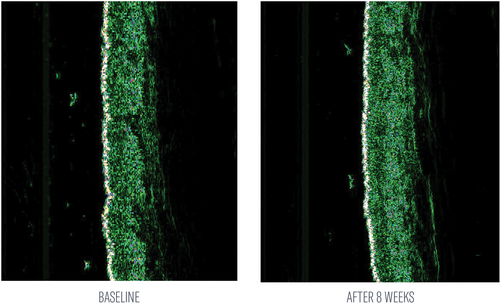
An HA-based serum, comprised of large molecular, injectable-grade HA, incorporates five types of HA into a formulation (InterFuse® Intensive Treatment LINES; InF-HA) to improve and soften the appearance of fine lines and wrinkles and maximize the hydration capacity of skin. A peptide-rich, lightweight cream (InterFuse® Treatment Cream FACE and NECK; InF-PEP) incorporates a variety of messenger peptides specifically targeting collagen types I, III, IV, VII, and XVII (Table 1). Prior studies conducted with each of these products individually demonstrated visible improvements in crow's feet, forehead wrinkles, and fine lines and wrinkles in subjects with moderate photoaged skin.4 Specifically, a study was conducted in 25 healthy female subjects, aged 30–65 years, with moderate facial photoaging in at least one of the following areas: crow's feet, forehead wrinkles, nasolabial folds, and global fine lines and wrinkles. Twice-daily treatment to targeted areas with InF-HA over 8 weeks led to improvements in the appearance of crow's feet (31%), forehead wrinkles (23%), and fine lines and wrinkles (21%). The study product was highly tolerable with no subject discontinuing product use or the study.4 A subset of subjects in this study (n = 5) applied InF-HA serum twice-daily over 8 weeks to a designated spot on the volar forearm delineated by a small, temporary tattoo. Ultrasound measurements of this area were obtained at baseline and week 8. Application of InF-HA serum demonstrated increased skin thickness and density from baseline at week 8 (Figure 1 and 2).4
| Key ingredient | Benefits | |
|---|---|---|
| InF-HA |
Hyaluronic Acid, 3000–10 000 Da Hyaluronic Acid, 10 000–100 000 Da Hyaluronic Acid, 6000 Da Hyaluronic Acid, 2 000 000 Da |
Four types of hyaluronic acid bind water to the surface of skin to improve the appearance of lines and wrinkles and hydrate skin. |
| Hyaluronic Acid, 1 600 000–2 200 000 Da | Injectable-sized, pharmaceutical-grade hyaluronic acid helps smooth the appearance of deep expression lines and wrinkles and hydrate skin. | |
| InF-PEP | Dipeptide Diaminobutyroyl Benzylamide Diacetate | Mimics activity of Walgerin-1 that is known to relax muscle, resulting in a smoothing appearance of lines and wrinkles. |
| Palmitoyl Tripeptide-5 | Supports collagen type III. | |
| Palmitoyl Dipeptide-5 Diaminobutyroyl Hydroxythreonine | Supports collagen type IV, VII, XVII. | |
| Tetradecyl Aminobutyroylvalylaminobutyric Urea Trifluoroacetate | Supports collagen type I. |
A second study conducted in 25 healthy female subjects, 30–65 years of age, demonstrated that twice-daily application of InF-PEP over 8 weeks facilitated 30%, 18%, and 15% improvements in the appearance of fine lines and wrinkles, erythema, and dyschromia, respectively, from baseline. Subjects reported high levels of satisfaction regarding improvements in the appearance of forehead lines, upper lip lines, and nasolabial folds. Mild, transient AEs were reported (dryness, n = 2). No subject discontinued use of the study product or study owing to an AE.4
Herein, we describe a study that evaluated the complementary benefits of these products. Specifically, the objective of this study was to evaluate the efficacy, tolerability, and subject satisfaction of twice-daily application of a hyaluronic acid-based serum (InF-HA) followed by twice-daily application of a peptide-rich cream (InF-PEP) in subjects with photodamaged skin of the face and neck.
2 MATERIALS AND METHODS
This single-center, open-label, clinical trial was performed by a board-certified dermatologist under Independent Review Board (IRB; Advarra IRB, Columbia, MD, USA) approval in conjunction with current Good Clinical Practices (cGCP) guidelines. The study was conducted over the course of 12 weeks in adult women, 45–65 years of age with mild to moderate photodamaged skin and laxity based on a 4-point grading scale of None (0), Mild (1), Moderate (2), and Severe (3).
Subjects were excluded if they had dermatological disorders (e.g., severe acne vulgaris/cystic acne, acne conglobate, acne fulminans, severe rosacea, and facial seborrheic dermatitis), acute illness, or autoimmune diseases. Subjects with current or recent use of any cosmetic product containing retinoid/retinol or like ingredients, AHAs, peptides, growth factors, hydroquinone, skin lightening/brightening agents, or topical/oral products were excluded, but deemed eligible following a 2-week washout period with the disqualifying ingredient or product.
Additionally, subjects were excluded if they had undergone any invasive or non-invasive skin treatment or procedure in the face or neck area in the last 6 months such as hair removal, botulinum toxin, dermal fillers, laser resurfacing, ultrasound, radio frequency, microneedling, or chemical peels which, in the investigator's opinion, would interfere with evaluations. Subjects who were pregnant, lactating, or planning a pregnancy during the study period were excluded.
Subjects applied InF-HA followed by application of InF-PEP (AM/PM) to their face and neck for 12 weeks. Digital images of the face were obtained using the Canfield VISIA-CR digital system (Canfield Scientific, Inc., Parsippany, NJ) and a high-resolution camera mounted on a tripod was used to obtain digital images of the neck, maintaining consistent distance and lighting across all images. Images were captured at baseline, week 8, and week 12. A 4-week telephone interview was conducted with each subject by a Study Coordinator to review AEs and concomitant medications, ensure adherence to the skincare regimen, and assess subject satisfaction based on their responses to pre-defined statements.
Investigator evaluations of the face and neck occurred at baseline, weeks 8 and 12 to assess visual changes from baseline in improvements in the appearance of skin tone (discoloration), skin texture (tactile roughness), and lines and wrinkles using a 6-point grading scale (0 = None, 1 = Minimal, 2 = Mild, 3 = Moderate, 4 = Moderately Severe, 5 = Severe). Global improvement was assessed at weeks 8 and 12 using a 5-point grading scale (0 = No Improvement, 1 = Minimal Improvement, 2 = Mild Improvement, 3 = Moderate Improvement, 4 = Marked Improvement).
Subjects completed a daily subject diary and self-assessment questionnaire at weeks 4 (via telephone interview), 8 and 12. AEs were captured throughout the study period. Subjects were also provided with a mineral-based sunscreen (SPF 56).
The study took place from August 2020 through December 2020. Enrolled subjects who had completed their baseline visit and were unable to adhere to the study visit schedule as a result of physical distancing or isolation measures related to COVID-19, were contacted via phone and/or video conference to review AEs/CMs, perform investigator evaluations, and obtain subject satisfaction based on the self-assessment questionnaire. Additionally, subjects were required to send to the study coordinator one set of six face and neck images (right, left, and front/center). Brief instructions regarding obtaining images were provided. The study investigator had the option of eliminating a specific parameter if the video conference or image capture did not allow for, in the investigator's opinion, accurate evaluation.
Data were summarized using descriptive statistics, which included frequency counts and percentages for categorical variables, and mean, median, standard deviation, minimum, and maximum for continuous variables.
3 RESULTS AND DISCUSSION
3.1 Demographics
Seventeen (17) subjects were enrolled and completed the study. On average, subjects were 52 years of age. Seventy percent (70%) of subjects were FST II-III and 30% of subjects were FST IV-VI. Eighty-two percent (82%) of subjects presented with moderate photodamage at baseline.
3.2 Efficacy and tolerability
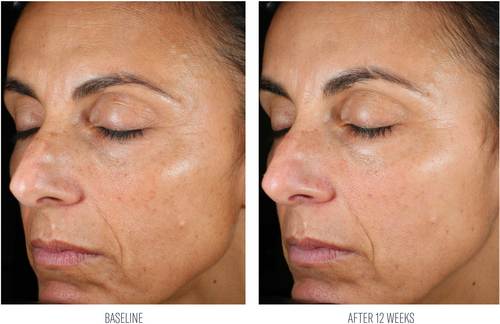
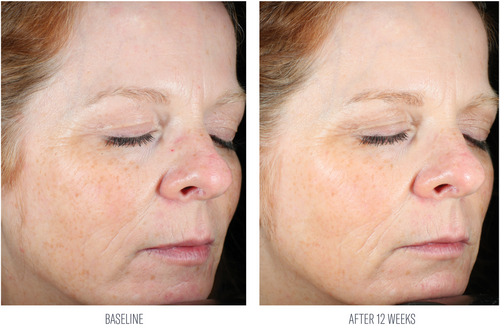
At week 8, investigator evaluations of the facial area reported an average 50% improvement from baseline in facial skin texture. After 12 weeks of use, substantial average percent improvements were demonstrated in tactile facial skin texture (79%), and the appearance of lines and wrinkles (50%) and skin tone (44%) from baseline (Table 2; Figures 3 and 4). Subjects demonstrated a 20% average increase in global improvement in facial appearance at week 12.
| Average percent improvement from baseline in the appearance of facial skin | |||
|---|---|---|---|
| Skin tone | Skin texture | Lines/Wrinkles | |
| 8 weeks | 32% | 50% | 38% |
| 12 weeks | 44% | 79% | 50% |
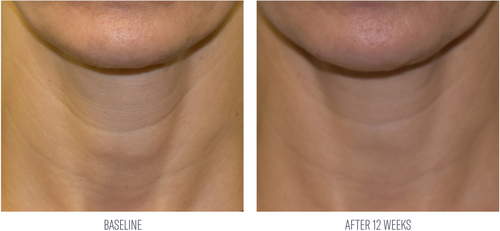
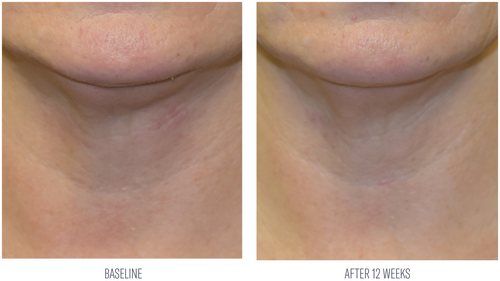
Investigator evaluations reported an average 48% improvement from baseline in tactile skin texture of the neck after 8 weeks of use. At week 12, average improvements were demonstrated in tactile neck skin texture (68%), and the appearance of skin tone (48%) and lines and wrinkles (36%) from baseline (Table 3; Figures 5 and 6). Subjects demonstrated an average 15% increase in global improvement in the appearance of their neck at week 12.
| Average percent improvement from baseline in the appearance of skin on the neck | |||
|---|---|---|---|
| Skin tone | Skin texture | Lines/Wrinkles | |
| 8 weeks | 34% | 48% | 23% |
| 12 weeks | 42% | 68% | 36% |
Subject satisfaction with the study products remained high over 12 weeks with 100% of subjects reporting an overall improvement in the appearance of their skin. As early as 4 weeks, 82% of subjects reported an overall improvement in the appearance of their skin, noting that their skin looked smoother (94%), more radiant (88%), was less red-looking (76%), and firmer looking (65%). After 12 weeks of use, 100% of subjects reported that their skin both looked and felt smoother, looked more radiant (88%) and looked firmer (82%). Ninety-four percent (94%) of subjects noted that they would continue using the study products at the conclusion of the study based on their experience. No AEs were reported during the study period that were related to the study products, and no subject discontinued the study owing to an AE.
3.3 Discussion
The desire for non- or minimally-invasive means to achieve younger looking skin of both the face and neck continues to outpace interest in surgical procedures.5 Topical approaches can address many of the main pillars of facial rejuvenation—reduction of rhytides, increasing skin firmness, and re-texturization of the skin.5 Skin aging of the neck is influenced by its unique anatomy as skin is thinner with less sebaceous glands, more vulnerable to mechanical movement and susceptible to a high level of sun exposure. Unfortunately, it is also one of the areas on the body in which skincare and sun protection is often overlooked and neglected.
Intrinsic aging causes a reduction in the proliferation of cells in the basal layer of skin, including keratinocytes, fibroblasts, and melanocytes, and a reduction in collagen and elastic fibers in the dermal layer.6 In addition, intrinsic aging leads to degeneration of both fibrous extracellular matrix components (elastin, fibrillin, and collagens) and oligosaccharides, negatively influencing the ability of skin to bind and retain water.6 Extrinsic aging, predominantly owing to environmental and ultraviolet (UV) radiation exposure, causes epidermal thickening, particularly in the stratum corneum and decreased expression of type VII collagen in keratinocytes, causing wrinkles. Increased degradation of collagen results in reduced levels of collagen type 1. Additionally, UV-exposure facilitates elastin degradation.6 Substantial reductions in dermal HA contributes to the characteristic manifestations of photoaged skin.1 Intrinsic and extrinsic aging interfere with the structure of the dermal–epidermal junction, along with the ability of nutrients and signaling molecules to move through it.7 Consequently, approaches to topical skincare aim to supplement skin with HA to bind and retain water molecules to improve hydration, and utilize peptides to help regulate fibroblasts, stimulate collagen, and improve the appearance of skin texture, firmness, lines and wrinkles, and overall skin quality.2, 7
The uncompromised stratum corneum's effective barrier properties and its ability to both prevent transepidermal water loss and penetration of exogeneous substances present challenges in the formulation of skincare and, specifically, the delivery of large molecular weight active ingredients to the epidermis.8-11 The 500 Dalton Rule notes that ingredients with a maximum 500 Da molecular weight are able to pass through the SC.3 Consequently, commonly used pharmacological or active agents used in topical dermatology are <500 Da.3 Methods employed to deliver large molecules include the addition of chemical or physical enhancers that modify permeability of the SC, chemical modification of an agent or protein, or modifications to the formulation that enable greater permeability of the molecule.12 Additionally, the use of lipid-soluble molecules employed in formulations enables efficient passage, as these molecules are more readily transported through the SC than hydrophilic compounds.3, 11
The delivery platform utilized in the products investigated in this study consists of a lipophilic coating comprised of a modified essential fatty acid (high in oleic acid) that surrounds large molecular actives. This coating works synergistically with additional ingredients within the formulation that temporarily relaxes surface tension and enhances the mobility of lipid chains in the SC to facilitate delivery to targets in the skin. Developed to facilitate binding and retention of water throughout the skin, InF-HA serum delivers five types of HA, including injectable-grade HA (1.6–2.2 million Da). The peptide-based cream, InF-PEP, includes a variety of signaling peptides purposefully designed to target a variety of different types of collagen in the skin.1, 2, 12, 13
Prior studies have examined the benefits of the two products independently,4 this study evaluated the complementary benefits of InF-HA serum and InF-PEP cream on photodamaged skin of the face and neck and demonstrated substantial benefits after 8 weeks of topical administration, with high levels of subject satisfaction and tolerability.
4 CONCLUSIONS
Twice-daily application of a hyaluronic acid-based serum and a peptide-rich cream over 12 weeks resulted in visible improvements from baseline in skin texture, skin tone, and lines and wrinkles of the face and neck areas in women with photodamaged skin. The two-part skincare system was well-tolerated, with no reports of AEs occurring related to the study products. Subjects experienced high levels of satisfaction, with all subjects reporting that their skin looked more radiant after 12 weeks of use. A complementary skincare regimen uniquely developed to facilitate the delivery of large molecular weight HA and peptides to the skin resulted in visible improvements in photodamaged skin of the face and neck over 12 weeks.
ACKNOWLEDGMENT
We would like to thank Lynne Kolton Schneider, PhD, for her editorial assistance on this manuscript.
ETHICAL APPROVAL
Advarra IRB; Approval MOD00715178.
STATEMENT OF CONSENT
All subjects have provided consent to participate in the study and to have their photographs appear in any publication stemming from the findings of the study.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




