Identification of the origin of adrenergic and cholinergic nerve fibers within the superior hypogastric plexus of the human fetus
Abstract
Nerve fibers contributing to the superior hypogastric plexus (SHP) and the hypogastric nerves (HN) are currently considered to comprise an adrenergic part of the autonomic nervous system located between vertebrae (T1 and L2), with cholinergic aspects originating from the second to fourth sacral spinal segments (S2, S3 and S4). The aim of this study was to identify the origin and the nature of the nerve fibers within the SHP and the HN, especially the cholinergic fibers, using computer-assisted anatomic dissection (CAAD). Serial histological sections were performed at the level of the lumbar spine and pelvis in five human fetuses between 14 and 30 weeks of gestation. Sections were treated with histological staining [hematoxylin-eosin (HE) and Masson's trichrome (TriM)] and with immunohistochemical methods to detect nerve fibers (anti-S100), adrenergic fibers (anti-TH), cholinergic fibers (anti-VAChT) and nitrergic fibers (anti-nNOS). The sections were then digitalized using a high-resolution scanner and the 3D images were reconstructed using winsurf software. These experiments revealed the coexistence of adrenergic and cholinergic fibers within the SHP and the HNs. One-third of these cholinergic fibers were nitrergic fibers [anti-VACHT (+)/anti-NOS (+)] and potentially pro-erectile, while the others were non-nitrergic [anti-VACHT (+)/anti-NOS (−)]. We found these cholinergic fibers arose from the lumbar nerve roots. This study described the nature of the SHP nerve fibers which gives a better understanding of the urinary and sexual dysfunctions after surgical injuries.
Introduction
The superior hypogastric plexus (SHP) may be injured through retroperitoneal surgical operations: aortoiliac reconstruction (van Schaik et al. 2001), anterior lumbar interbody fusion (Johnson & McGuire, 1981), retroperitoneal lymphadenectomy for testicular cancer (Solsona et al. 1994) and colorectal cancer surgery (Hojo et al. 1991). These injuries could cause retrograde ejaculation and anejaculation, disturbance of bladder function and in some rare cases erectile dysfunction (Havenga et al. 2000). This raises the question about the nature of nerve fibers in the SHP and their function.
The SHP originates from the autonomic central nervous system located between the T1 and L2 spinal segments that is traditionally responsible for the sympathetic innervation of the pelvic organs (Clausen et al. 2008). The SHP is a single autonomic peripheral structure located in front of the aorta at the fifth lumbar vertebra and the sacral promontory levels between the two common iliac arteries. Traditionally, this plexus arises at the level of trifurcation of the aorta by the union of the fibers of the autonomous abdominal preaortic plexus, which consists of the celiac plexus, the superior mesenteric, the inter-mesenteric and the inferior mesenteric plexus. These plexuses receive thoracic splanchnic nerves (ganglions T5–T12). The SHP also receives the first four lumbar splanchnic nerves from the lumbar sympathetic chain. It splits into two hypogastric nerves (HN) that enter the pelvis with internal iliac vessels, in front of the sacrum and deep in the perineal fascia (Awad et al. 2011).
The SHP has been thoroughly studied since the 19th century. In humans, the nerve fibers of the SHP are traditionally adrenergic (sympathetic; Latarjet & Bonnet, 1913; Delmas & Laux, 1933; Baader & Herrmann, 2003), whereas in animals [rats (Elmer, 1978) and rabbits (Sjostrand & Klinge, 1979)] the sympathetic hypogastric nerve has adrenergic and cholinergic fibers.
Traditional anatomical dissection cannot determine the nature of the nerve fibers with precision (Pick, 1970). However, immunohistochemical methods allowed the detection of the neurotransmitters in these nerve fibers (adrenergic, cholinergic, sensory, etc.; Jen et al. 1995). By using computer-assisted anatomic dissection (CAAD), which combines the advantages of sharp microscopic precision together with the advantages of spatial understanding via three-dimensional (3D) representation, we can include all the micro-nerve fibers and specify their relationship to neighboring organs (Alsaid et al. 2010). The aim of this study was to determine the nature (adrenergic, cholinergic and nitrergic) and origin of the nerve fibers within the SHP and the HN in the human fetus.
Materials and methods
Specimens
The tissues around the lumbar spine and pelvis were entirely removed between the first lumbar vertebrae and pubic arch of five normal human fetuses (four male and one female) between 14 and 30 weeks of gestation after parental consent as authorized by the ‘French Biomedical Agency’ and ‘the University of Paris-Sud’. Those fetuses were non-macerated and without either malformation or abnormality in the abdominal and pelvic cavity.
After fixing in 10% formalin for 1 day, each block was cut sagittally into 4-mm sections, which were embedded in paraffin. By using a manual microtome, a series of 5-μm-thick sections without intervals was obtained.
Histological study
- Masson's Trichrome (TriM) or Hematoxylin Eosin (HE) allow us to distinguish all the anatomic structures.
- General neuronal immunolabeling (Stefansson et al. 1982) using rabbit polyclonal antibody against S100 (code: ab15520) diluted 1 : 400 and incubated for 60 min at room temperature.
- Adrenergic nerve fiber immunolabeling (Butler-Manuel et al. 2002) using rabbit polyclonal antibody against tyrosine hydroxylase (TH; code: ab112) diluted 1 : 750 and incubated for 12 h at 4 °C.
- Cholinergic nerve fiber immunolabeling (Weihe et al. 1996) using rabbit polyclonal antibody against vesicular acetylcholine transporter (VAChT; code: V5387) diluted 1 : 2000 and incubated for 12 h at 4 °C.
- Nitrergic nerve fiber immunolabeling (Martin-Alguacil et al. 2008) using rabbit polyclonal antibody against neuronal nitric oxide synthase (nNOS; code: 160870) diluted 1 : 200 and incubated for 12 h at 4 °C. These fibers play a role in the control of vasodilatation.
An enzyme retrieval step was preferred for anti-TH and anti-VAChT. All the slides were incubated for 90 min at room temperature with biotinylated goat anti-rabbit secondary antibody (code: 401313) diluted 1 : 200 and the avidin biotin peroxidase procedure was performed using a Vector ABC Elite kit (code: SK-4100) incubated for 60 min. The DAB detection kit (code: PK-6100) was used for chromogenic detection. Negative control slides (without a primary antibody) were prepared for each antibody to confirm the specificity of the reagents.
Quantification
The adrenergic, cholinergic and nitrergic nerve fibers within the SHP (L4, L5 and S1 levels) and the spinal nerve between L3 and S3 were counted manually. One section was selected for each level.
Three-dimensional reconstruction
The serial sections marked by the different antibodies were scanned by a high optical resolution scanner (3200 dpi), and the images then aligned with Adobe photoshop image processing software. Reconstruction in 3D was completed by using winsurf software; all the structures and nerve fibers were manually outlined for all sections. All previous stages were performed manually by the authors.
Results
Third lumbar nerve level
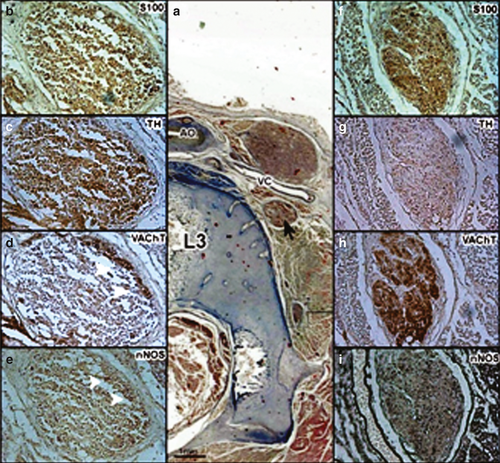
We defined a spinal nerve as the spinal root (preganglionic nerve), the ganglion of the sympathetic chain (SC) and the postganglionic nerve running along the SHP and the HNs (Fig. 1).
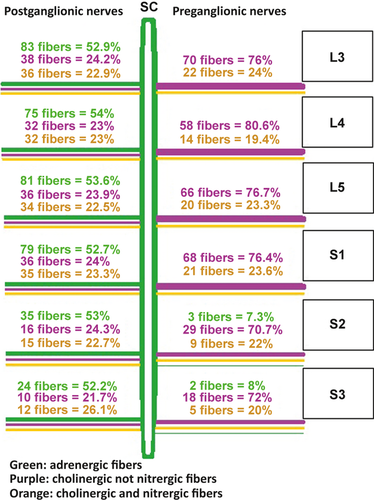
The two SCs were located at both sides of the spine, at the lateral faces of the aorta, and behind the vena cava on the right side. These SCs and the ganglions at the two sides of the aorta were strongly labeled by anti-TH (~ 83%). Some fibers (~ 28%) were marked by anti-VAChT, particularly in the periphery (Fig. 2). The SCs received the preganglionic nerve coming from the spinal cord. All the preganglionic fibers were positive for anti-VAChT and negative for anti-TH (cholinergic fibers); some of these cholinergic fibers were positive for anti-nNOS (~ 25%; Figs 1 and 2). The postganglionic fibers that left the sympathetic chains contained adrenergic (positive for anti-TH; ~ 50%) and cholinergic fibers (anti-VAChT positive; ~ 50%); 50% of these cholinergic fibers were positive for anti-nNOS (Fig. 1).
There was a large number of nerve fibers surrounding the origin of the inferior mesenteric artery forming the inferior mesenteric plexus (IMP). They were labeled in a mixed manner by the anti-TH adrenergic marker and the anti-VAChT cholinergic marker. Some fibers were labeled by the nitrergic marker anti-nNOS.
Fourth lumbar nerve level
The SHP arose at the lower part of the aorta in front of its trifurcation at the level of the intervertebral disc between the third and fourth lumbar vertebra or the fourth lumbar vertebra. The shape of the SHP was variable; in three fetuses most of the nerve fibers of the SHP were located at the front side of the aorta and in the other fetuses, the nerve fibers in the SHP dispersed around the aorta and its branches.
The immunohistochemical staining of the SHP showed the coexistence of adrenergic nerve fibers (~ 50%; anti-TH positive) and cholinergic fibers (~ 50%; anti-VAChT positive). One third of these cholinergic fibers were nitrergic fibers (~ 32%; anti-nNOS positive; Table 1, Fig. 3).
| Adrenergic fibers (n, absolute percentage) | Cholinergic fibers (n, absolute percentage) | ||
|---|---|---|---|
| Nitrergic (n, relative. percentage) | Not nitrergic (n, relative percentage) | ||
| L4 | 29 (± 5) 48.3% | 31 (± 8) 51.7% | |
| 10 (± 4) 32.2% | 21 (± 4) 67.8% | ||
| L5 | 44 (± 2) 47.8% | 48 (± 5) 52.2% | |
| 19 (± 5) 39.6% | 29 (± 5) 60.4% | ||
| S1 | 55 (± 6) 51.4% | 52 (± 6) 48.6% | |
| 19 (± 5) 36.5% | 33 (± 5) 63.5% | ||
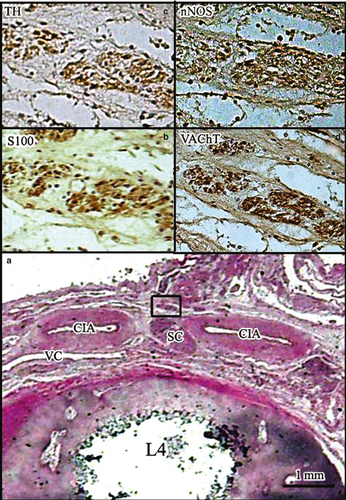
The adrenergic fibers of the SHP originated from the IMP (40–45%), the adjacent ganglions (40–45%) and the postganglionic fibers of bilateral lumbar nerve roots (10–20%), whereas the cholinergic fibers originated from the IMP (65–70%) and the fourth lumbar nerve roots (30–35%; Fig. 4).
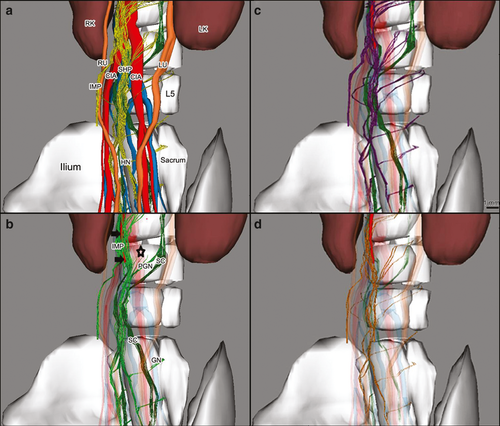
At this level, the two sympathetic chains moved closer to each other toward the median line behind the aortic trifurcation alongside the anterior surface of the fourth lumbar vertebra (Fig. 3).
Fifth lumbar nerve level
The SHP descended in front of the confluence of the two common iliac veins (CIV). In all cases, the SHP deviated from the sagittal plane to the left, and stood in front of the left common iliac artery (CIA) and the region between the two right and left CIAs.
At this level, the SHP received preganglionic nerve fibers originating from both sides of the spinal roots. The preganglionic nerve branched off into two bundles running anteromedially. One bundle passed in front of the CIA towards the SHP without connection with the sympathetic chain. The other passed behind the CIA to the sympathetic chain that lies between the SHP and the CIA. These preganglionic fibers contained only cholinergic fibers (~ 100% anti-VAChT positive, ~ 20% anti-nNOS positive and ~ 0% anti-TH positive; Figs 4 and 5).

First sacral nerve level
The SHP continued caudally between the two internal iliac arteries (IIA) and in the posterior surface of the colon at the level of the promontory.
The number of nerve fibers forming the SHP at this level was higher than the previous levels due to the lumbar nerve roots joining the SHP with the same coexistence of adrenergic and cholinergic fibers (Table 1).
At the lower part of the first sacral vertebra, the SHP separated into two oblique bundles: the right and left hypogastric nerves (HN). They ran caudally and laterally to end up at the medial aspect of the internal iliac arteries (IIA) and the posterolateral side of the colon. Some nerve fibers of the SHP followed the external iliac arteries (EIA).
All nerve fibers of the SHP and the HN were marked by anti-TH (~ 50%) or anti-VAChT (~ 50%). Some fibers were positive for both anti-VAChT and anti-nNOS (~ 20%; Table 1).
Second sacral nerve level
Both HNs descended postero-laterally parallel to the internal iliac arteries, which were in front of the presacral fascia, behind the ureters in a multi-layered fascia forming the uterosacral ligament complex (USLC). The HN supplied some nerve fibers to the distal ureter and the posterior surface of the rectum.
Third and fourth sacral nerve levels
The HNs received the pelvic splanchnic nerves (PSN) coming from the anterior sacral foramina (S2, S3 and S4). The PSN contained very short preganglionic fibers because the sympathetic chain lay directly on the anterolateral aspect of the sacral vertebral bodies near the sacral foramina and postganglionic nerve.
Immunohistochemical staining showed that preganglionic fibers of PSNs contained adrenergic and cholinergic (nitrergic and non-nitrergic) fibers. On the other hand, the postganglionic fibers were mixed (Fig. 2). The HNs also contained adrenergic (~ 50%) and cholinergic (~ 50%) fibers; ~ 35% of these cholinergic fibers were nitrergic.
The HNs ran forward along the posterolateral aspect of the rectum and then took the nerve fibers to the ureterovesical junction and the trigone of the urinary bladder. The HNs and PSNs contributed to form the inferior hypogastric plexus (pelvic plexus; IHP), which innervates the pelvis and the erectile organs. Although one female fetus was studied, there were no differences between the male and female fetuses in these sections.
Discussion
Several clinical studies (Tiusanen et al. 1995; Havenga et al. 2000) showed that lesions of the SHP or the HNs cause abnormal ejaculation (sympathetic fibers). Other authors (van Schaik et al. 2001; Liang et al. 2008) have noted that lesions of the SHP generate erectile dysfunction (parasympathetic fibers). Brindley (1988) showed that electrical stimulation of the SHP in patients with spinal cord injury facilitates erection. Finally, Whitelaw & Smithwick (1951) showed that impotence can be caused by lumbar sympathectomy. All these studies call into question the nature of the nerve fibers in the SHP and the HNs.
The SHP arises after the origin of the inferior mesenteric artery and runs caudally to the anterior surface of the aorta by the union of nerve fibers of the abdominal preaortic plexus and the four lumbar splanchnic nerves (Williams et al. 1995). The shape of the SHP is variable and the nerve fibers of this plexus is located between the two CIAs and in front of the left CIA. Correia et al. (2010) found six forms of the SHP, the dominant form in the fetus being affected (rectangular). The deviation of the SHP towards the left under the trifurcation of the aorta and between the CIAs was discovered in our study and has already been discussed in the literature (Shiozawa et al. 2010). Lu et al. (2009) determined that approach via the retroperitoneal route is performed by incision of the parietal peritoneum in front of the abdominal aorta and right CIA.
These nerve fibers of the SHP are traditionally adrenergic (sympathetic; Baader & Herrmann, 2003; Awad et al. 2011). The methodology we used allows us to determine the nature of each nerve fiber with a diameter greater than 20 μm (Alsaid et al. 2012), which cannot be evaluated in traditional anatomical studies. We found here the coexistence of adrenergic [anti-TH (+)] and cholinergic [anti-VAChT (+)] nerve fibers in the SHP and the HN. One-third of these cholinergic fibers were nitrergic [anti-nNOS (+)] (Table 1).
Considering that nitrergic fibers [anti-nNOS (+)] are parasympathetic fibers [anti-VAChT (+)] (Moszkowicz et al. 2012), we would classify all the [anti-nNOS (+)] fibers as parasympathetic fibers. There were three types of fibers within the SHP and the HNs: sympathetic [anti-TH (+)], cholinergic [anti-VAChT (+)/anti-nNOS (−)] and cholinergic [anti-VAChT (+)/anti-nNOS (+)]. Traditionally, the nitrergic fibers [anti-nNOS (+)] are vasodilator and pro-erectile but we need to clarify the function of two-thirds of the cholinergic fibers [anti-VAChT (+)/anti-nNOS (−)] in the SHP. The stained histological sections (Fig. 5) and the 3D reconstruction (Fig. 4c) showed that preganglionic fibers joined the SHP and the HNs. These preganglionic fibers could form their synapses in the ganglions of the inferior hypogastric plexus located more caudally in the pelvis. In rats, there are adrenergic and cholinergic fibers in the pelvic ganglions (Kepper & Keast, 1998).
The coexistence of adrenergic sympathetic [anti-TH (+)] and cholinergic fibers [anti-VAChT (+)/anti-nNOS (−)] and [anti-VAChT (+)/anti-nNOS (+)] in all lumbar and sacral nerves changed the traditional anatomical description that claims that lumbar nerves are responsible for the sympathetic innervation, whereas the sacral nerves (S2, S3 and S4) are responsible for the parasympathetic innervation of the pelvis (Mirilas & Skandalakis, 2010). Our results demonstrated that the lumbar and sacral autonomic nerves (between L3 and S3) were adrenergic and cholinergic: the two types of cholinergic fibers were found (nitrergic and non-nitrergic).
- The vagus nerve (X; Mauroy et al. 2003; Ruffoli et al. 2011): the cholinergic fibers of the vagus nerve could reach the SHP via the preaortic abdominal plexus innervating the digestive system up to the second third of the transverse colon. The presence of the anti-VAChT+ and anti-nNOS+ in the spinal nerves does not support this hypothesis.
- These cholinergic fibers could arise from the pelvic splanchnic nerves S2, S3 and S4 continuing within the SHP and the inferior mesenteric plexus to innervate the third distal of the colon and the rectum (Wein et al. 2007; Mirilas & Skandalakis, 2010). Their presence in the lumbar nerves does not support this hypothesis.
- More likely, as our study suggests, the aforementioned cholinergic fibers arise from the spinal nerve roots.
- Preganglionic fibers, leaving the spinal roots, form synapses with postganglionic adrenergic fibers in the sympathetic chain.
- Descending adrenergic fibers pass into the sympathetic chain (originating from the spinal cord in superior levels) and continue into the postganglionic nerve toward the SHP and the HNs.
Clinical studies (Lu et al. 2009) have taken into account the preservation of the SHP during retroperitoneal aortic surgery. But our data show the necessity of changing our interpretation of erectile dysfunction after lumbar and sacral spinal cord injuries and may be changing the methods of treatment because the pro-erectile fibers [anti-VAChT (+)/anti-nNOS (+)] are found in the lumbar nerves as well as in the sacral nerves.
However, we do not know whether these results in the human fetus would be the same in adults because we do not know whether the nature of these autonomic fibers changes during the maturation of the nervous system.
Additionally, the results of electrical stimulation of the SHP in animal experimental studies (Giuliano et al. 1995) suggesting that the SHP has only sympathetic nerves must be reinterpreted.
Finally, experimental studies are required to confirm the function of the aforementioned cholinergic fibers [anti-VAChT (+)/anti-nNOS (+)], which innervate pelvic organs and also determine the function of the cholinergic fibers [anti-VAChT (+)/anti-nNOS (−)].
Conclusions
This study provides information about the neuroanatomy of the SHP and the HN using the immunolabeling of adrenergic, cholinergic and nitrergic nerve fibers. Both adrenergic and cholinergic fibers were found within the SHP, the HN and all the spinal nerves (between L3 and S4). An initial functional characterization identified that one-third of the cholinergic fibers were nitrergic (pro-erectile).