Developmental changes in the connective tissues of the porcine recurrent laryngeal nerve
Abstract
The recurrent laryngeal nerve (RLN) branches from the vagus cranial nerve to innervate structures important for voicing and swallowing. Damage to this nerve, commonly associated with surgery or idiopathic etiologies that largely occur with aging, results in impaired voicing and swallowing (Myssiorek, 2004). Sunderland proposed a model of peripheral nerve damage whereby a nerve's ability to resist damage from stretch and compression is determined by the quantity and composition of its epineurial connective tissues (Sunderland, 1951). Thus, it would be expected that epineurium differs depending upon the forces imposed on a nerve within its anatomical setting. The purpose of this study was to investigate RLN epineurium quantity and composition with development. A porcine model (piglet vs. juvenile) was used because of the similarity between porcine and human laryngeal innervation, anatomy and function. The entire RLN was excised bilaterally, and stereological methods were used to quantify the composition of epineurial connective tissues. Compared with the piglet, the juvenile pig RLN was double the diameter. While the piglet had no differences in the percentage of epineurial collagen and adipose between proximal and distal segments of both sides of the RLN, the juvenile pig had a greater percentage of collagen in the proximal segment of both sides of the RLN and a greater percentage of adipose in the distal segment of the left RLN compared with the proximal segment. In addition, unlike the piglet, the juvenile pig had a greater number of fascicles in the proximal than distal segment of the RLN, regardless of nerve side. These findings are consistent with predicted patterns associated with the different anatomical settings of the left and right RLN, show that the RLN changes with age, and support Sunderland's model.
Introduction
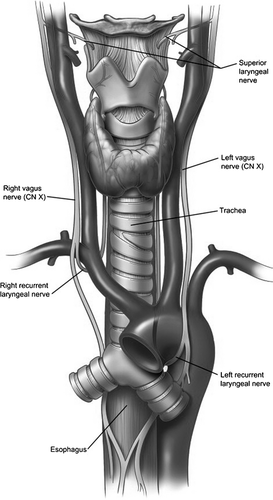
The recurrent laryngeal nerve (RLN) is a branch of the vagus nerve that provides innervation important to voicing, breathing and swallowing. The left RLN branches from the vagus nerve at the aortic arch near the heart, while the right RLN branches at the subclavian artery near the clavicle bone (Fig. 1). Damage to the RLN most typically results in unilateral vocal fold paralysis (UVP) associated with a weak, breathy voice and aspiration. Onset of UVP occurs most frequently in individuals older than 45 years, with an average age of onset of 64 years (Kelchner et al. 1999; Coyle et al. 2001). UVP is often the result of surgical trauma, idiopathic (i.e. unknown) onset or tumor (Apfelbaum et al. 2000; Loughran et al. 2002; Myssiorek, 2004). UVP occurs more often in females than males (Merati et al. 2006; Rosenthal et al. 2007).
Sunderland (1965, 1990) proposed that injury to peripheral nerves, such as the RLN, is likely related to the nerve's connective tissue composition (Fig. 2). Nerve fibers are surrounded by endoneurium, which provides a degree of tensile strength. The fibers are organized into fascicles that are surrounded by seven–15 concentric layers of collagen fibers and fibroblasts called the perineurium. The perineurium provides tensile strength and elasticity, and acts as the blood–nerve diffusion barrier (Sunderland, 1965). In general, the number and size of fascicles observed within the cross-section of a nerve is thought to indicate the degree of protection a nerve requires against stretching (Sunderland, 1990). Typically, the size and number of nerve fascicles change along the length of any given nerve as branches exit the nerve trunk toward the structures they innervate. In areas where several small fascicles are present, the nerve might demonstrate more flexibility with greater tensile strength than areas where there are fewer large fascicles (Sunderland, 1990). The former configuration may also protect against compressive forces when the smaller fascicles are surrounded by greater amounts of epineurial tissues. In this case, the epineurial tissues are thought to serve as a cushion that redirects damaging compressive forces around fascicles reducing their impact on the axons within the nerve.
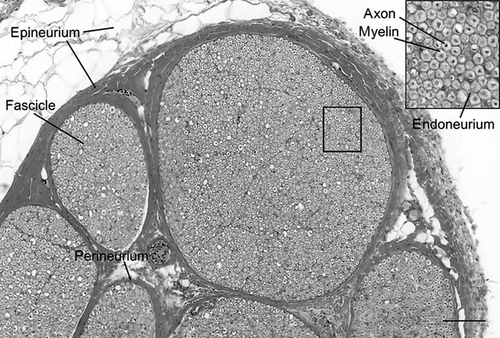
Epineurium is composed primarily of collagen, elastin and adipose tissues. It has been suggested that the collagen fibers in the epineurium provide resistance to stretch (Liu et al. 1948; Haftek, 1970), while the adipose protects against compression (Sunderland, 1945). The proportion of epineurium and its composition can vary along the length of the nerve and between individuals; the proportion of epineurium has been reported as greater where nerves cross joints and when numerous small fascicles are observed in a nerve cross-section (Sunderland, 1965). These findings indicate a protective role for the epineurium such that the amount and composition of its connective tissue may be indicative of the biomechanical forces to which a nerve is subjected within its anatomical setting.
A final level of protection may be provided via slack that is built into each peripheral nerve to accommodate stretching associated with the structural environment of a nerve. That is, an undulated pattern can be observed along the length of a nerve trunk as well as in the nerve fascicles and nerve fibers. Once stretched approximately 4% beyond its normal length, the fibers rupture (Sunderland, 1990). The nerve trunk appears to be intact on the surface until stretching compromises the perineurial tissues. At the point of perineurium compromise, the entire nerve trunk will break apart. This is an important consideration in the context of surgical retraction of the RLN, in that nerve retraction may result in injury to the nerve fibers before visible evidence of physical injury to the nerve occurs (Sunderland, 1990).
Three studies describing the connective tissues in the RLN have been completed thus far. Sunderland & Swaney (1952) found that as the right RLN approaches the larynx of humans, there is a greater amount of epineurium relative to more proximal portions of the nerve, possibly due to fewer nerve fascicles. Malmgren & Gacek (1981) studied the histology of 28 human RLNs bilaterally at a level of 0–3 cm from the inferior border of the cricoid cartilage. The cross-sectional area of the epineurium here was similar to the findings of Sunderland & Swaney (1952). Near the inferior border of the cricoid cartilage, Malmgren & Gacek (1981) found a large number of fascicles near the larynx (from one to 11), although it was not determined if the range differed between left and right RLNs. Barkmeier & Luschei (2000) compared the proportion and composition of the left RLN epineurium of 10 adult canines with the proportion and composition of the epineurium of the left flexor hallucis longus branch of the tibial nerve (FHLN). They found a greater proportion of epineurium along the length of the left RLN than in the FHLN and, consistent with Sunderland & Swaney (1952), the greatest proportion of epineurium within distal segments. Additionally, the RLN exhibited a higher proportion of adipose along its length than the tibial branch. Thus, the differing patterns observed between the FHLN and the left RLN epineurium suggest that these two nerves are exposed to different types of environmental forces within their respective anatomical settings.
While UVP is more common in adults, infants can present with UVP due to central nervous system disorders, such as Arnold–Chiari malformation (Emery & Fearon, 1984), cardiovascular anomalies (Parikh, 2004) or cardiothoracic surgery (Zbar & Smith, 1996; Daya et al. 2000; Parikh, 2004; Smith, 2006). Currently, little is known about the postnatal development of RLN epineurium. Most prior research has focused on the nerve fibers, endoneurium or perineurium development. Thus, it remains unknown whether connective tissue patterns found in adult RLN are present at birth or occur during a developmental response to environmental forces associated with its anatomical setting. As children grow into adolescence, typical causes of UVP begin to mirror those in adult studies (Parikh, 2004; Smith, 2006). Understanding how the connective tissue of the RLN changes over the course of the lifespan would help identify changes that contribute to protection of the nerve until senescence, when the occurrence of idiopathic onset of UVP increases (Yamada et al. 1983; Yumoto et al. 2002).
The purpose of this study was to define developmental changes in the proportion and composition of the connective tissues known to protect peripheral nerves, such as the RLN, from stretch and compression injuries. Thus, stereology was used to measure the epineurium and its composition in the left and right RLN. A porcine model was chosen due to recent literature demonstrating a similarity between porcine and humans for laryngeal innervation patterns, anatomy and function (Jiang et al. 2001; Stavroulaki & Birchall, 2001; Alipour & Jaiswal, 2008). We hypothesized that RLN epineurium develops differently depending upon where it resides within the neck compared with its location within the thorax.
Materials and methods
Nerve acquisition
Right and left RLNs were acquired from eight × 2–13-day-old piglet cadavers (four female and four male) and 10 × 3–4-month-old juvenile pig cadavers (five female and five male) within 3–8 h post mortem. All porcine cadavers were acquired post mortem upon conclusion of another institutionally approved research project. The RLNs were excised along their entire length and cut into segments of equal length such that four segments were acquired from the right RLN and six segments were acquired from the longer left RLN, each being approximately 2 cm in length. Segment 1 was designated as the most proximal portion of the nerve where the RLN branches from the vagus nerve. Thereafter, segment numbers increased with the progression of the nerve to its most distal portion that inserted into the posterior larynx.
Nerve preparation
Nerve segments were immersion-fixed in 4% formalin and stored in fixative for more than 24 h prior to preparation for light microscopy. Nerve specimen diameters from the piglets were 1 mm or less and juvenile pig specimens were no less than 3 mm, making it possible to fix RLN connective tissues through immersion. Previous research demonstrated that 4% formalin exhibits a fixation rate in non-perfused tissue of 2–4 mm per 24 h (Start et al. 1992). All nerve segments were prepared for light microscopy using paraffin embedding followed by cutting 10-μm-thick cross-sections. Cross-sections for each segment were stained with hematoxylin and eosin for image analysis.
Nerve image analysis
A cross-section from each nerve segment was selected for analysis based on optimum nerve section integrity and minimal artifact. Representative samples were digitized using light microscopy. Due to their smaller diameter, piglet nerves were imaged at 400 × using a Nikon T-200 bright-field microscope and Adobe Photoshop, and stored as high-resolution TIFF files. The larger juvenile pig nerves were imaged at 200 × using dmetrix software, digitalEyepiece (version 1.016, 2004–2005), and stored at the highest resolution jpg format. Boundaries were manually placed around the epineurium of each image by the supervising investigator (JBK) before a calibrated grid was overlaid on each image using a MatLab® interface for stereological point counting (Gundersen & Osterby, 1981; Gundersen & Jensen, 1987). All images were coded prior to data collection so that raters were blinded to the image being analyzed.
Four raters (EC, RV, RS and SC) were trained by the third author (NM) to code the cross-sectional nerve tissues using the following categories: collagen, adipose, blood vessel, artifact space, perineurium, fascicle and fascicle artifact space. Ratings by NM were used to calibrate measures from the other raters and the supervising investigator (JBK) until measures from practice images were within 80% or greater agreement of each other. The intra-rater reliability of coding data for the piglet data set was determined separately from the juvenile pig data. For the piglet intra-rater reliability, RS, SC and NM coded 12% (10 sections) of the sections on two occasions separated by 2 or more weeks. No intra-rater reliability was conducted for the juvenile pig data beyond training raters (EC, RV and JBK) to 80% or greater agreement of each other.
Total counts from the MatLab® program were entered into Excel spreadsheets. Total epineurium count was determined from the sum of collagen, adipose and blood vessel counts in the cross-section of each nerve section. The gross number of fascicles in each cross-sectional segment was counted, as were the perineurium tissue counts within each nerve cross-section. The total nerve cross-sectional area was determined by summing the total epineurium, perineurium and fascicle tissue counts, and determining the proportion of the total nerve cross-sectional area comprised of epineurium and perineurium. Further, the proportion of epineurium tissue comprised of adipose and collagen was determined. Transforming the tissue measures onto the same scale using a percentage of cross-sectional area allowed inter-subject comparison of measures obtained from nerves of different sizes and was consistent with the methodology of earlier studies (Sunderland, 1965, 1990; Barkmeier & Luschei, 2000).
Statistical analysis
An intra-class correlation (ICC) was completed to determine inter-rater reliability on the piglet. Segments from the proximal and distal portions of the right and left nerves were directly compared by using the average value resulting from collapsing the first two segments of the right and left RLN for each dependent variable; this value was coded as ‘proximal’. Left RLN segments 5 and 6 were coded as ‘distal’. Similarly, right RLN segments 3 and 4 were coded as ‘distal’. The coding procedure allowed both nerves to be represented by two proximal and distal segments associated with the thorax and neck, respectively, for comparisons to be done between the left and right RLN. The following three independent variables were selected for study: (i) nerve side (right vs. left); (ii) segment (proximal vs. distal for the right and left RLN); and (iii) age group (piglet vs. juvenile).
The average ICC for all variables was 0.99 between raters for the piglet (RS, NM and SC). The average ICC values were < 0.80 for all categories, except blood vessel (0.69) and artifact space (0.75). The three primary raters (EC, JBK and RV) for the juvenile data collection achieved 80% or greater inter-rater reliability on 10% of the juvenile pig tissue images for all dependent variables. Thus, data obtained among raters were considered adequate for comparison of the dependent variables of interest for this study.
A mixed-design repeated-measures analysis of variance (anova) was completed to determine differences within and between the left and right RLNs for each of the following dependent variables: number of fascicles; relative perineurium; epineurium; adipose; and collagen tissues. The within-subjects independent variables were segment (proximal vs. distal) and nerve side (left or right). The between-subjects independent variable was age (piglet vs. juvenile). An alpha level of 0.05 was used as the criterion level for significance. Post hoc testing on significant parent tests was completed using pairwise comparisons and Bonferroni's alpha adjustment to control for alpha inflation.
Results
Histology
Recurrent laryngeal nerve cross-sections revealed the three types of connective tissue typically found in a peripheral nerve. These layers consist of: epineurium, which protects the outer RLN and consists of collagen, adipose and elastic fibers; perineurium, a protective sheath consisting of fibroblasts and collagen fibers that surround bundles of nerve fibers (fascicles); and endoneurium, which surrounds nerve fibers and Schwann cells, and consists of fibroblasts and collagen fibers (Peters et al. 1970).
Morphological differences between cross-sections of piglet (Fig. 3) and juvenile pig (Fig. 4) RLN were apparent. The cross-sectional diameter of the piglet RLN was approximately half the size of the juvenile pig RLN. The quantity and complexity of the epineurium were different; piglet epineurium consisted primarily of collagen tissue along the length of the left and right RLN (Fig. 3), while the juvenile pig epineurium consisted of collagen and adipose tissue (Fig. 4). Furthermore, the pattern of fascicles and epineurium was different for proximal and distal segments of the left juvenile RLN compared with the right juvenile RLN. Proximal segments of the left juvenile RLN exhibited less adipose tissue and more collagen than did distal segments, and several nerve fascicles were contained within the center of the surrounding epineurium (Fig. 4A). In contrast, distal segments of both right and left sides of the juvenile RLN exhibited fewer nerve fascicles, and the fascicles of the left juvenile RLN were separated from each other by encircling epineurium tissues (Fig. 4B and D). There was less contrast between proximal and distal epineurium composition in the right juvenile RLN (Fig. 4C and D).
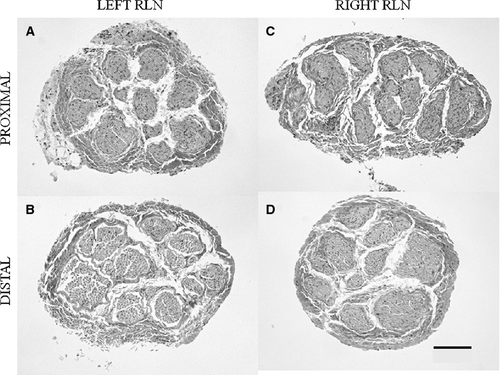
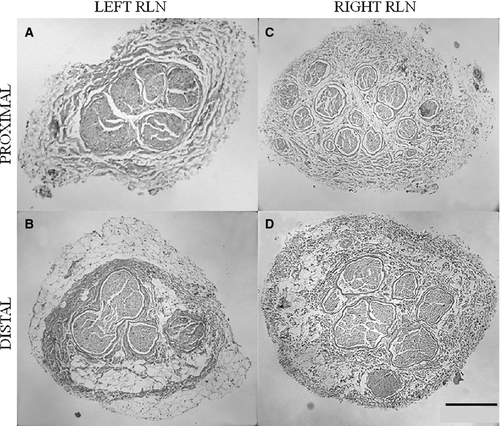
In summary, the piglet RLN tissues (Fig. 3) appeared more homogenous within and between the left and right sides than observed for the juvenile RLN (Fig. 4). The right juvenile RLN exhibited greater consistency in the amount of collagen and adipose tissue between the proximal and distal segments compared with the left juvenile RLN. The left juvenile RLN exhibited more collagen in the proximal segment than seen in the distal segment where an increased amount of adipose tissue occurred.
Stereology
Stereological data reflected what was demonstrated histologically.
Epineurium
The main effect of nerve side was found to be significant. That is, the percentage of the RLN cross-section composed of epineurium differed, on average, between the left and right RLN (F1,16 = 34.4, P < 0.0001), regardless of age. The average percentage of epineurium for the left RLN was 62.9% (± 12.6) compared with 72.5% (± 10.8) for the right RLN. Thus, a greater percentage of the right RLN was composed of epineurium, on average, than the left RLN. In addition, there was a significant main effect for age (F1,16 = 28.4, P < 0.0001), with a higher average percentage of epineurium in the juvenile RLN [74.3% (± 9.9%)] than the piglet RLN [59.5% (± 10.7%)].
Perineurium
Nerve side was significant for perineurium, regardless of age (F1,16 = 16.6, P = 0.001). The average percentage of perineurium for the left RLN was 9.1% (± 3.6%) compared with 6.6% (± 3.4%) for the right RLN. Consequently, a greater percentage of the left RLN cross-section was composed of perineurium than found for the right RLN. In addition, a significant difference was found between age groups for perineurium (F1,16 = 37.8, P < 0.0001). The average nerve cross-sectional area of the piglet RLN composed of perineurium [10.4% (± 3.0%)] was higher than for the juvenile RLN [5.8% (± 2.7%)].
Collagen
Significant main effects for the percentage of the epineurium comprised of collagen were found for nerve side (F1,16 = 9.1, P = 0.008) and segment (F1,16 = 6.5, P = 0.021). On average, the left RLN exhibited a greater percentage of collagen [88.5% (± 12.5%)] than the right RLN [80.0% (± 11.8%)]. However, ‘segment’ was also found to have a significant interaction with ‘age’ (F1,16 = 9.8, P = 0.006). Post hoc testing showed significant differences between the juvenile proximal and distal segments using a Bonferroni adjustment to the alpha level of 0.05/2 = 0.025 (Fig. 5). The proximal segment of the juvenile nerve, regardless of the side of the RLN (i.e. right vs. left RLN), had a significantly greater percentage of collagen than the distal segment (P = 0.001). No differences were found between the proximal and distal segments of the piglet RLN. In addition, between-subject testing showed a significant difference between age group (F1,16 = 18.7, P = 0.001). On average, a significantly greater percentage of collagen was found within the epineurium in the piglet nerve tissues [avg = 89.8 (± 8.7)] than the juvenile nerve tissues [79.8 (± 12.0)].
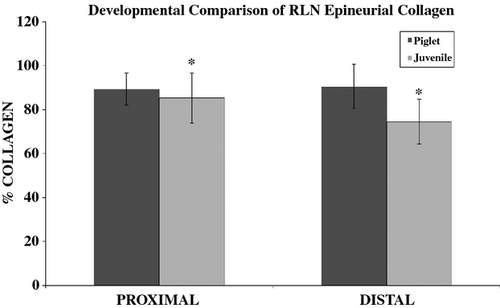
Adipose
The significant main effects of nerve side (F1,16 = 8.5, P = 0.01), segment (F1,16 = 5.4, P = 0.033) and age group (F1,16 = 41.7, P < 0.0001) were found for the percentage of adipose measured within the epineurium. Several significant interactions occurred. These included ‘nerve side × group’ (F1,16 = 5.6, P = 0.031), ‘segment × group’ (F1,16 = 13.2, P = 0.002) and ‘nerve side × segment × group’ (F1,16 = 7.1, P = 0.017). Post hoc testing of the latter interaction showed the only significant differences were between proximal and distal segments for the left (P < 0.0001) and right (P = 0.009) RLN of the juvenile age group (after Bonferroni alpha adjustment α = 0.05/4 = 0.0125). A comparison of distal and proximal segment percentage adipose showed that the distal segment of the juvenile RLN had a greater percentage adipose than in the proximal segment (Table 1).
| Age group | Nerve | Segment | Percentage adipose |
|---|---|---|---|
| Juvenile | Left RLN | Proximal | 4.5 (± 2.3) |
| Distal | 17.9 (± 10.8) | ||
| Right RLN | Proximal | 20.9 (± 10.8) | |
| Distal | 28 (± 6.5) | ||
| Piglet | Left RLN | Proximal | 5.7 (± 7.1) |
| Distal | 1.0 (± 2.7) | ||
| Right RLN | Proximal | 4.6 (± 7.4) | |
| Distal | 4.8 (± 11.4) |
- RLN, recurrent laryngeal nerve.
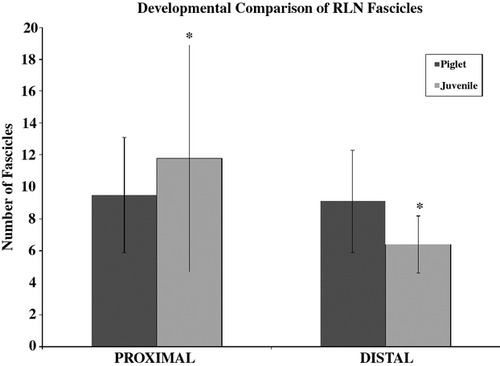
Number of fascicles
A significant main effect for segment was found (F1,16 = 8.4, P = 0.01) with a significant ‘segment × age’ interaction (F1,16 = 6.4, P = 0.023). Figure 6 displays the average number of fascicles found in the proximal (i.e. segment 1) and distal (i.e. segment 2) segments of the juvenile and piglet nerves, regardless of the left or right side. Post hoc testing found a significantly greater number of fascicles in the proximal [avg = 11.8 (± 7.1)] than distal [avg = 6.4 (± 1.8)] segment of the juvenile age group nerves. No difference was found between segments for the piglet nerves.
Discussion
Developmental changes in the connective tissues of porcine RLN were studied in relation to differences in anatomical setting between the left and right RLN proximal and distal segments. Stereology was conducted on cross-sectional nerve tissues to measure epineurium, perineurium, collagen, adipose and fascicle number because these elements are thought to protect peripheral nerves, such as the RLN, from stretch and compression injuries. Overall, this study demonstrated that piglet RLN exhibited significantly less cross-sectional epineurium, on average, than found for juvenile pig RLN. In addition, piglet connective tissues were homogenously comprised of collagen along their length with little to no adipose tissue. In contrast to piglet, juvenile pig RLN exhibited significantly more adipose tissue in the epineurium, on average, particularly in distal segments residing within the neck. Finally, piglet RLN exhibited no change in the average number of nerve fascicles from proximal to distal segments, whereas the juvenile pig RLN exhibited a significant reduction in the number of nerve fascicles from proximal to distal segments.
Given the shorter duration of exposure to forces imposed upon the piglet RLN, we hypothesized less epineurium and a similar composition of connective tissue along the nerve's length compared with findings for the juvenile pig RLN. Bright-field microscopy revealed a considerable difference in the gross cross-sectional size of the piglet and juvenile pig RLN, with the piglet RLN being almost half the size of the juvenile pig RLN. This finding concurs with a study showing that the diameter of the sciatic nerve increases with lifespan (Sladjana et al. 2008). Changes in nerve diameter during postnatal development are expected to occur due to body growth. Prior work has demonstrated that increased amounts of perineurium and epineurium are the primary contributors to nerve diameter changes (Sladjana et al. 2008; Sharma et al. 2009). Although the specific mechanism by which the nerve changes in size is unknown, it is likely related to anatomical changes in physical size of the porcine body that require lengthening of the RLN and resultant changes in environmental forces to which the nerve is exposed.
In addition to observable differences in the gross size of piglet and juvenile RLN, the quantity and complexity of the nerve connective tissue was found to differ between age groups. As predicted, significantly greater amounts of adipose were identified within juvenile RLN than found in piglet RLN. This finding is consistent with a previous observation that the amount of adipose tissue increases within the human sciatic nerve epineurium with age (Sladjana et al. 2008). Moreover, the piglet had a higher average RLN cross-sectional area composed of perineurium than the juvenile pig. The perineurium has been associated with greater resistance to stretch as opposed to compression (Sunderland, 1965). Millesi and colleagues determined that greater nerve stiffness was found within nerve sections containing several nerve fascicles, or increased amounts of perineurium (Millesi et al. 1995). Interestingly, piglet RLN was previously shown to exhibit greater stiffness (i.e. resistance to stretch) at the location of the aortic arch (i.e. proximal segment) than found for other segments of the left or right RLN (Alexander et al. 2010). The aortic arch is a large-diameter pulsating vessel with greater ranges of diameter change than exhibited by the smaller subclavian artery around which the right RLN travels within the neck (Fig. 1). Thus, it is likely that the thoracic portion of the left RLN undergoes more stretch-related forces than does its distal location within the neck. Unfortunately, biomechanical testing has not yet been completed in the juvenile pig tissues to make firm conclusions associating anatomical differences between piglet and juvenile pig RLN epineurium quantity and composition with biomechanical differences. However, Sunderland's model would propose that the difference in the proportion of perineurium between porcine age groups suggests that the initial type of force imposed on the right and left RLN at birth is initially that of stretching rather than compression.
In addition to differences between the two age groups studied, the juvenile pig RLN exhibited differences between the left and right RLN. A greater quantity of epineurium was found in the right RLN, on average, than in the left RLN of the juvenile pig. Further, the left RLN of the juvenile pig showed a significantly greater percentage of epineurium in the distal compared with the proximal segments, the latter residing within the thorax rather than in the neck region. These findings are similar to previous investigations studying RLN epineurium in adult humans and canines (Sunderland & Swaney, 1952; Malmgren & Gacek, 1981; Barkmeier & Luschei, 2000). Of particular interest is the replication of findings in the left canine RLN (Barkmeier & Luschei, 2000) with the additional comparison to the right RLN and between different age groups. Using Sunderland's model, the differences found between the canine RLN and the branch of the tibial nerve (Barkmeier & Luschei, 2000) might previously be explained as differences reflective of a cranial nerve and a spinal nerve with differing anatomical makeup due to different target musculature rather than different anatomical settings. However, the current study investigated epineurium differences within the bilateral RLN differing only in anatomical setting of the proximal segments of the right and left sides of the same nerve. As such, differences identified in this study between piglet and juvenile RLN and between the left and right juvenile RLN offer evidence supporting Sunderland's proposal that connective tissue develops differently within peripheral nerves related to the forces imposed on them within their respective anatomical settings.
In general, the developmental changes in epineurium connective tissues found between piglet and juvenile RLN age groups are consistent with predicted changes associated with anatomical setting and environmental forces. One unanticipated finding of this study was the relatively similar numbers of nerve fascicles along the entire length of the piglet RLN compared with significantly fewer nerve fascicles in distal juvenile RLN segments compared with proximal segments. No difference occurred in the average number of fascicles identified within the RLN between age groups. Given that peripheral nerves branch to innervated structures along their length, it was no surprise to find that there were fewer nerve fascicles in distal than proximal segments in the juvenile RLN. However, our results suggest a reduction in the total number of fascicles along the length of the RLN during postnatal development. Such a modification might occur as neurons reach their target sites and strengthen their participation during neuromotor development. Ontogenetic studies of nerve development reveal an extended growth and maturation period ranging from several months to years, depending upon the species (Miller & Dunmire, 1976; Saitua & Alvarez, 1988; Rosenberg et al. 1989; Wheeler & Plummer, 1989; Ceballos et al. 1999; Jeronimo et al. 2005; Lima et al. 2007; Kaplan et al. 2009). For example, Lima et al. (2007) studied the postnatal development of the RLN in rats, and reported developmental decreases in the number of myelinated fibers as well as a reduction in myelinated fiber density with increasing age. They did not find a reduction in the number of RLN fascicles during postnatal development, however, which may reflect the anatomical simplicity of the RLN in this species (Lima et al. 2007). To our knowledge, developmental patterns in the number of nerve fascicles along the length of peripheral nerves have not yet been systematically investigated.
Overall, the findings of this study support Sunderland's model regarding differences in epineurium quantity and composition in the same peripheral nerve that differs in the location of proximal segments between its left and right side. Future studies addressing developmental aspects of peripheral nerve connective tissues may elucidate whether our findings were unique or represent a general pattern across age groups and species. Of particular interest is to determine whether patterns studied within the piglet and juvenile pig are similar to those within the developing human. During human neurodevelopment, an infant progresses from being virtually immobile as a newborn to crawling and then walking. Thus, a comparison of RLN connective tissue in human infants, toddlers and adults could help determine the additional role gravitational forces play in connective tissue formation associated with body position.
Determining the degree of stretch and compression the RLN can withstand before protective layers are compromised and neurons become damaged would provide important information regarding the degree of stretch (i.e. nerve retraction) possible before compromising important protective layers during surgery. The information may also help elucidate sources of post-surgical damage to the RLN, such as forces imposed on the RLN from post-surgical edema in surrounding or intra-neural tissues, and direct future surgical approaches.
Concluding remarks
Differences in quantity and composition of the piglet and juvenile RLN lend evidence in support of Sunderland's model regarding formation of connective tissues within peripheral nerve epineurium related to the types of forces imposed on the nerve. The data acquired from this study will inform future investigations of predicted biomechanical consequences of developmental and aging changes in the connective tissues of the pig and for comparison to similar investigation of the RLN in humans. Ultimately, this will elucidate the role of epineurial connective tissues in supporting or undermining nerve protection and neural regeneration associated with UVP caused by surgical or idiopathic etiologies.
Acknowledgements
The research described was supported by Grant Number 1R01DC005422-01 from The National Institute on Deafness and Other Communication Disorders. The authors would like to thank Mark Borgstrom for his contributions to the statistical analysis, Douglas W. Cromey for sharing his digital imaging expertise, Rebecca Volk for assisting in coding data, and the researchers in the Veterinary Science and Microbiology program for contributing post-mortem juvenile pigs and piglets to this research project. Further thanks go to Steve Tinling for his assistance in finalizing histology images for this publication. None of the contributing authors has a conflict of interest to declare related to the work described in this manuscript.
Author contributions
Ellen Campbell and Robin Samlan served as the primary authors of this work in that they each made significant contributions to data collection, analysis, and interpretation of the juvenile pig and piglet portion of the research, respectively. Each also made significant contributions to drafting the manuscript. Sarah Cook made significant contributions to image acquisition and data collection of piglet nerves. Nathaniel McMullen contributed to image acquisition, data acquisition, analysis, and interpretation and contributions to drafting of the manuscript. Suzette Smiley-Jewell made contributions to manuscript revision and final drafting. The submitted work was conceived of and designed by Julie Barkmeier-Kraemer. She supervised the two primary authors on nearly all aspects of this research, including significant contributions to data analysis, interpretation and writing the manuscript.




