A deep intronic variant in MME causes autosomal recessive Charcot–Marie–Tooth neuropathy through aberrant splicing
Abstract
Background
Loss-of-function variants in MME (membrane metalloendopeptidase) are a known cause of recessive Charcot–Marie–Tooth Neuropathy (CMT). A deep intronic variant, MME c.1188+428A>G (NM_000902.5), was identified through whole genome sequencing (WGS) of two Australian families with recessive inheritance of axonal CMT using the seqr platform. MME c.1188+428A>G was detected in a homozygous state in Family 1, and in a compound heterozygous state with a known pathogenic MME variant (c.467del; p.Pro156Leufs*14) in Family 2.
Aims
We aimed to determine the pathogenicity of the MME c.1188+428A>G variant through segregation and splicing analysis.
Methods
The splicing impact of the deep intronic MME variant c.1188+428A>G was assessed using an in vitro exon-trapping assay.
Results
The exon-trapping assay demonstrated that the MME c.1188+428A>G variant created a novel splice donor site resulting in the inclusion of an 83 bp pseudoexon between MME exons 12 and 13. The incorporation of the pseudoexon into MME transcript is predicted to lead to a coding frameshift and premature termination codon (PTC) in MME exon 14 (p.Ala397ProfsTer47). This PTC is likely to result in nonsense mediated decay (NMD) of MME transcript leading to a pathogenic loss-of-function.
Interpretation
To our knowledge, this is the first report of a pathogenic deep intronic MME variant causing CMT. This is of significance as deep intronic variants are missed using whole exome sequencing screening methods. Individuals with CMT should be reassessed for deep intronic variants, with splicing impacts being considered in relation to the potential pathogenicity of variants.
1 INTRODUCTION
Charcot–Marie–Tooth neuropathy (CMT) is the most common inherited peripheral neuropathy, affecting 1/2500 individuals.1 CMT is characterized by progressive length-dependent loss of peripheral motor and sensory nerves, resulting in distal muscle weakness and sensory symptoms.2 Patients are broadly divided into subtypes based on whether nerve conduction studies (NCS) indicating demyelinating (CMT1) or axonal (CMT2) forms of the disease. Recessive loss-of-function variants in the membrane metalloendopeptidase (MME) gene have been previously reported to cause axonal Charcot–Marie–Tooth neuropathy type 2T (CMT2T; OMIM: #617017).3-11 MME encodes for neprilysin, a widely expressed membrane-bound metallopeptidase that has a key role in neuropeptide processing.12 A significant portion of patients with axonal CMT remain genetically undiagnosed,13-17 indicating that further disease-causing genes and pathogenic variants in known genes are yet to be identified.
Splicing variants are increasingly being recognized as a cause of Mendelian disease.18, 19 The precise removal of introns (non-coding regions) and inclusion of exons (coding regions) in the final mature mRNA relies on the spliceosome and auxiliary splicing factors recognizing specific sequence motifs, such as the 5′ donor splice site and 3′ acceptor splice site. Splice-altering variants can weaken or abolish recognition of the correct splice sites, or alternatively strengthen or create cryptic splice sites that mimic consensus splicing sequences.20 These variants typically lead to one or more mis-splicing events that result in the skipping of partial or complete exons, and/or the retention of partial or complete introns.21-42 Pathogenic splicing variants have been found in several CMT genes including MPZ,21, 30, 39, 40 MFN2,22, 30, 31 LRSAM1,23 IGHMBP2,24 INF2,25 MCM3AP,26 SH3TC2,30, 32, 38 GDAP1,27, 42 SBF1,28 NDRG1,37 and FGD4.29 Variants affecting canonical splice donor and acceptor sites have also been described in MME.4, 41
Exon-trapping, also known as a mini-gene assay, is an in vitro technique used to identify exons in a genomic region of interest.43 This is of particular use when relevant patient tissue is unavailable or when relevant transcripts may be unstable or degraded by nonsense mediated decay (NMD).44 The genomic region of interest is cloned into an exon-trapping vector between two known exons. This exon-trapping vector is transfected into a cell line where it is transcribed and undergoes a series of post-transcriptional processes that include pre-mRNA splicing to create mature mRNA. This mRNA consists of the ‘trapped’ exons of the genomic region of interest flanked by the known exons, which can then be Sanger sequenced to characterize the ‘trapped’ exons. Comparison of ‘trapped’ exons between wild type and variant genomic sequences can also indicate if a candidate variant affects splicing.45-51 The well-validated exon-trapping vector pSpliceExpress52 consists of known exons of the rat insulin gene, Ins2, and has been used previously to determine the splicing impacts of multiple pathogenic variants in Mendelian disease.45-51
Here we report a deep intronic variant in MME [chr3:155142758A>G (hg38); MME c.1188+428A>G], found in a recessive state in two Australian families. Exon-trapping revealed that this variant creates a novel splice donor site in MME intron 12 (NM_000902.5) which, along with a preceding existing cryptic splice acceptor site, results in the incorporation of an 83 bp pseudoexon in the MME transcript [chr3:155142675-155 142 757 (hg38); r.1188_1189ins[1188+345_1188+427]]. The coding frameshift caused by this pseudoexon leads to a PTC in exon 14 and likely NMD of the MME transcript (p.Ala397ProfsTer47), resulting in a loss of MME function.
2 MATERIALS AND METHODS
2.1 Subjects
Members of Family 1 were recruited and informed consent was obtained for this study using protocols approved by the Sydney Local Health District Human Ethics Research Committee (2019/ETH07839). Recruitment and informed consent for the proband of Family 2 was approved by the Human Research Ethics Committee of the Royal Melbourne Hospital (HREC/16/MH/251).
2.2 Variant detection
Genomic DNA for Family 1 was extracted from peripheral blood using the PureGene Kit (Qiagen) following the manufacturer's instructions. WGS for two individuals in Family 1 (V:1 and V:3) was outsourced to the Garvan Sequencing Platform. Paired-end sequencing reads of 150 base pairs were generated using the Illumina NovaSeq 6000 sequencing machine, with 30-fold average read depth.
In Family 2, genomic DNA for the proband was extracted at the Department of Diagnostic Genomics (PathWest, Perth, Australia) using the QIAsymphonySP machine and QIAsymphone® DSP DNA Midi Kit. The proband underwent WGS at the Australian Genomics Research Facility (AGRF), Melbourne, following GATK4 best-practices. Paired-end sequencing reads of 150 base pairs were generated using the Illumina NovaSeq 6000 sequencing machine, with 30-fold average read depth. Parental DNA was not available for sequencing.
WGS data processing was performed at the Centre for Population Genomics (CPG) following the DRAGEN GATK best practices pipeline. Reads were aligned to the hg38 reference genome using Dragmap (v1.3.0). Cohort-wide joint calling of single nucleotide variants (SNVs) and small insertion/deletion (indel) variants was performed using GATK HaplotypeCaller (v4.2.6.1) with ‘-dragen-mode’ enabled. Sample sex and relatedness quality checks were performed using Somalier (v0.2.15).53 Variants were annotated using VEP 105, and loaded into the web-based variant filtration platform, seqr.54 A WGS search was conducted using the seqr45 platform for low minor allele frequency (<0.01) variants in CMT-related genes, for both Family 1 and 2 [gene list- Hereditary Neuropathy_CMT_IsolatedAndComplex (Version 2.14)].55 Additionally, variants were analyzed by the CPG Automated Interpretation Pipeline (AIP, https://github.com/populationgenomics/automated-interpretation-pipeline). In silico splicing analysis was conducted using SpliceAI.56
2.3 Segregation analysis
The MME c.1188+428A>G variant in Family 1 and Family 2 was amplified using primers that spanned MME intron 12 (5′-CTCAGCCGAACCTACAAGGA-3′; 5′-GCAAATGCTGCTTCCACAT-3′) to produce a 1264 bp amplicon [chr3:155142289-155 143 552 (hg38)]. An internal sequencing primer was used (5′-CTGTGTTAAAAGTAATTTCGGGG-3′) and the amplicon was Sanger sequenced. The MME c.467del variant in Family 2 was amplified (5′-GCAGAGCCGTATGCATCACT-3′; 5′-TTCAGCTGTCCAAGAAGCACC-3′). A 717 bp amplicon was produced [chr3:155116171-155 116 887 (hg38)], which was subsequently Sanger sequenced.
2.4 Sanger sequencing
For Family 1, PCR amplicons were sent to Garvan Molecular Genetics, Garvan Institute (Sydney, Australia) for Sanger sequencing using BigDye Terminator cycle sequencing protocols. For Family 2, proband PCR amplicons were Sanger sequenced at AGRF, Perth using BigDye Terminator sequencing protocols. Sequences were visualized and analyzed using Snapgene Software version 7.1 (www.snapgene.com).
2.5 Oxford nanopore technologies (ONT) long read sequencing
High molecular weight (HMW) DNA samples of the Proband in Family 2 were transferred to the Garvan Sequencing Platform for targeted long-read sequencing analysis on ONT instruments. Prior to ONT library preparations, DNA was sheared to ~20–25 kb fragment size using a MegaRuptor 3 instrument and visualized post-shearing on an Agilent FemtoPulse.
Sequencing libraries were prepared from ~3–5 μg of HMW DNA, using native library prep kit SQK-LSK114, according to manufacturer's instructions. Each library was loaded onto a R10.4.1 flow cell and sequenced on a PromethION device with live target selection/rejection executed by the ReadFish software package.57 Detailed descriptions of software and hardware configurations used for ReadFish are provided in a previous publication.58 Samples were run for a maximum duration of 72 h, with nuclease flushes and library reloading performed at approximately 24- and 48-h timepoints for targeted sequencing runs, to maximize sequencing yield. Raw ONT sequencing data was converted to BLOW5 format59 using slow5tools (v.0.3.0)60 then base-called using Guppy (v6). Resulting FASTQ files were aligned to the hg38 reference genome using minimap2 (v2.14-r883).61 Variants were called using clair3,62 phased using Whatshap63 and visualized using the Integrative Genomics Viewer (IGV, v2.17.3).64
2.6 Cell culture
The human HeLa cervical epithelial cell line (ATCC) was maintained in Dulbecco's Modified Eagle's Medium (DMEM) (Gibco) containing 10% (v/v) fetal bovine serum (FBS) (Gibco), +100 U/mL penicillin (Gibco), and 100 μg/mL streptomycin (Gibco) at 37°C in humidified air and 5% CO2.
2.7 Cloning procedures
The region surrounding MME c.1188+428A>G [chr3:155141790-155 143 755 (hg38)] was amplified from the genomic DNA of a heterozygous carrier of the c.1188+428A>G variant, using attB adapter primers (5′-AAAAAGCAGGCTTCGCTCTTAAATGGTTGGCTT-3′; 5′-AGAAAGCTGGGTAACTAGACTCTTGGGGAAGGC-3′). The MME amplicon was then cloned into the exon-trapping pSpliceExpress vector between flanking Ins2 exons using a two-step Gateway cloning BP reaction (ThermoFisher). pSpliceExpress was a gift from Stefan Stamm (Addgene plasmid #32485).52 The pSpliceExpress-MME clones were then Sanger sequenced to verify the correct insertion of MME and to determine the c.1188+428A>G genotype of each clone (as the genomic DNA template was heterozygous for the variant).
2.8 In vitro exon-trapping
HeLa cells were grown to approximately 70% confluence in a 6-well plate. HeLa cells were separately transfected with either 2 μg of pSpliceExpress-MMEWT or 2 μg of pSpliceExpress-MMEc.1188+428A>G using Lipofectamine 3000 (ThermoFisher). RNA extraction was performed 48 h following transfection using the RNEasy Mini Kit (Qiagen), and reverse-transcribed template was prepared using the iScript cDNA Synthesis Kit (Bio-Rad). PCR amplification of cDNA was conducted using primers designed to anneal to the flanking Ins2 exons (5′-CAGCACCTTTGTGGTTCTCA-3′; 5′-CAGTGCCAAGGTCTGAAGGT-3′). The RT-PCR amplicons were size fractionated using a 1.5% w/v agarose gel. The largest amplicon for each vector was gel-purified using Isolate II PCR and Gel Kit (Bioline) for Sanger Sequencing.
2.9 In silico splicing analysis of reported MME variants
All reported MME variants in gnomAD v.4.0.0 were detected by searching the gnomAD browser for the genomic region corresponding with the MME gene; ‘3-155024 124-155183704’(hg38). The MME variants reported in gnomAD were then exported using the ‘Export Variants to CSV’ function. The consequences of the variants were described by gnomAD using the Variant Effect Predictor (VEP) annotation based on the most deleterious predicted functional effect of each variant.65 These variants were then filtered for a minor allele frequency (MAF) <0.01 and a maximum SpliceAI ∆score >0.8 (high precision splicing change prediction56) as annotated by gnomAD.
3 RESULTS
3.1 Clinical phenotypes
3.1.1 Family 1
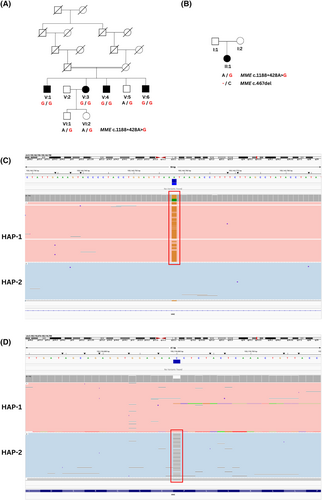
Family 1 consists of four affected siblings from a consanguineous family of European (non-Finnish) background (Figure 1A). The phenotype was consistent with a generalized sensorimotor axonal neuropathy and sensory ataxia without cerebellar signs (Table 1), and was confirmed with NCS for individuals V:1 and V:6 (Table S2). Affected individuals had previously undergone diagnostic and research whole exome sequencing with negative results.
| Family # | Family 1 | Family 2 | |||
|---|---|---|---|---|---|
| Individual | V:1 | V:3 | V:4 | V:6 | II:1 |
| Sex | M | F | M | M | F |
| Onset | Fourth decade | Childhood | Childhood | Childhood | Early-adult |
| Age at evaluation (years) | 62 | 67 | 69 | 67 | 54 |
| Presenting symptom | Gait unsteadiness | Difficulty running | Difficulty sitting on crossed legs during childhood | Difficulty running, multiple ankle sprains | Bilateral leg weakness and sensory disturbance |
| Atrophy UL/LL | Yes/yes | Yes/yes | Yes/yes | Yes/yes | No/Yes (left calf only) |
| Foot deformity | None | Pes cavus/hammer toes | - | Pes cavus | Pes cavus |
| Tone UL/LL | Normal/reduced | Normal/normal | Normal, increased | Normal/normal | |
| Shoulder abduction | 5 | 5 | 3 | 5 | 4 |
| Elbow flexion | 4 | 5 | 3 | 4 | 5 |
| Elbow extension | 4 | 5 | 3 | 4 | 5 |
| Finger abduction | 2 | 4 | 3 | 1 | 4 |
| Hip flexion | 4 | 3 | 3 | 3 | 4 |
| Knee extension | 4 | 3 | 3 | 4 | 4 |
| Knee flexion | 4 | 3 | 3 | 4 | 4 |
| Ankle dorsiflexion | 0 | 0 | 3 | 1 | 3 |
| Ankle plantarflexion | 0 | 0 | 3 | 0 | 4 |
| Deep tendon reflexes UL/LL | Absent/absent | Absent ankle | Hyperreflexia knee, absent ankle | Absent/absent | Normal/ reduced ankle reflexes |
| Plantars | Absent | - | Absent | Flexor | Flexor |
| Proprioception UL/LL | Normal/reduced to the MTP | - | - | Normal/reduced to the ankle | Normal/reduced to ankle |
| Vibration UL/LL | Normal/reduced to ankle | Absent in toes | Reduced at the knee | Reduced to wrist/reduced to knees | Reduced to mid-shin |
| Pinprick UL/LL | Normal/reduced to ankle | Normal/reduced to the mid shin | Reduced to elbows/reduced to knees | Reduced to PIP joint/reduced to knees | Reduced to mid-shin Reduced dorsum hand |
| Temperature sensation UL/LL | Normal/reduced to ankle | - | - | Reduced to wrist/reduced to above knee | Reduced to mid-shin |
| Other symptoms/signs/results | Cough | GERD and cough | Corticospinal tract signs | Nil | Fatty infiltration on left gastrocnemius on MRI |
| Gait | High steppage/ataxic | High steppage/ataxic | Steppage gait | High steppage/ataxic | High steppage |
| Romberg's sign | Positive | Positive | Positive | Mild sway | |
| Mobility aids | AFO, walking stick and mobility scooter | AFO, walker | AFO, walker, mobility scooter | Wheelchair | AFO, elbow crutch |
| CMTNS (version 2) | 31 | - | 28 | 36 | 15 |
- Abbreviations: AFO, ankle foot orthoses; CMTNS, Charcot–Marie-Tooth Neuropathy Score; GERD, gastroesophageal reflux disease; UL/LL, upper limb/lower limb.
Individual V:1
This 62-year-old man developed symptoms from age 38 years with gait unsteadiness and progressive weakness of the upper and lower limbs. The balance and weakness progressed with multiple falls, and he required walking aids (ankle foot orthoses, walking stick and mobility scooter). Additionally, he reported loss of sensation in the feet and a dry cough. Neurological examination revealed a distal pattern of weakness and sensory loss in the upper and lower limbs, absent deep tendon reflexes, unresponsive plantars, and an ataxia gait with a positive Romberg's test. NCS showed a severe, generalized, sensorimotor axonal polyneuropathy (Table S2).
Individual V:3
This 67-year-old woman presented with difficulty running at school, weakness of the ankles, and recurrent ankle sprains. She developed numbness and soreness of the feet and a slowly progressive gait disturbance with recurrent falls. She is now only just able to get around the house using a walker and fixed AFO splints. She also had a distal pattern of weakness and sensory loss.
Individual V:4
This 69-year-old man first experienced symptoms as a child with difficulty sitting on crossed legs. He then noticed weakness in the ankles and legs and sensory loss in the feet, which slowly progressed to severe walking difficulty with falls requiring a walker, mobility scooter and AFOs. On examination, he had global muscle weakness, and sensory loss to the knees and elbows, with subtle corticospinal tract signs (increased tone and hyperreflexic knee jerks).
Individual V:6
This 67-year-old man first developed symptoms in childhood with difficulty running, multiple ankle sprains and difficulty with sport. The symptoms progressed and by his mid-twenties, he developed overt distal weakness and had difficulty with balance, with multiple falls. He currently has severe weakness in the upper and lower extremities with sensory deficits and balance problems and mobilizes with a wheelchair. Neurological examination revealed global weakness in the upper and lower limbs (distal greater than proximal), absent deep tendon reflexes throughout, downgoing plantars, glove and stocking sensory loss, and a positive Romberg's sign. There were no cerebellar or extrapyramidal signs, and the head impulse test was negative. NCS revealed a severe generalized axonal sensorimotor polyneuropathy (Table S2).
3.1.2 Family 2
Family 2 consists of one affected female born to a healthy, non-consanguineous couple (Figure 1B). There is no history of neuromuscular disorders in the family. The 60-year-old proband presented with slowly progressive bilateral leg weakness and sensory disturbances. NCS showed evidence of an axonal sensorimotor neuropathy (Table S2). Neurological examination revealed mild distal upper limb weakness and moderate distal lower limb weakness, with muscle atrophy only in the left gastrocnemius muscle (Table 1). Bilateral MRI of the thighs and calves showed multifocal calf muscle T2 hyperintensity, muscle atrophy and T1 hyperintense fatty replacement of the left medial gastrocnemius muscle (Figure S1). The patient had reduced ankle reflexes and showed mild muscle cramps and muscle fatigue. Creatine kinase (CK) levels were consistently elevated (>400 U/L).
3.1.3 Genetic analysis
WGS screening using the seqr platform in Family 1 revealed a single homozygous variant in two affected individuals (V:1 and V:3), MME c.1188+428A>G (NM_000902.5). This variant was reported in dbSNP build 15566 (rs61758195), with a low MAF in gnomAD v4.0.067 (10/152120), All of Us (35/490748),68 and TOPMED69 (15/264290). No homozygous individuals were reported in gnomAD, All of Us, or TOPMED. SpliceAI predicted that MME c.1188+428A>G could create a strong novel splice donor site (Score 0.97; where a SpliceAI score above 0.8 is considered a ‘high precision’ predicted splice variant56). This in turn strengthened the SpliceAI prediction for a cryptic splice acceptor site 83 bp upstream of the novel splice donor site (Score 0.99).
Segregation analysis in Family 1 confirmed the biallelic inheritance of the MME c.1188+428A>G variant segregated with the CMT phenotype (Figure 1A). Sanger sequencing of DNA from available individuals showed that all affected individuals were homozygous for the MME c.1188+428A>G variant and unaffected individuals were carriers (Figure S2).
WGS screening using the seqr platform and AIP was conducted in the proband of Family 2 (II:1). The MME c.1188+428A>G variant was detected in a compound heterozygous state with a second MME variant (MME c.467del; p.Pro156Leufs*14), which has previously been reported as pathogenic41 (Figure 1B). Sanger sequencing validated the presence of each MME variant in the index individual (Figure S3). As parental DNA was unavailable, ONT long-read sequencing was conducted to phase the heterozygous MME variants in the proband (Figure 1C,D). ONT long-read sequencing confirmed that MME c.1188+428A>G (boxed red in Figure 1C) and c.467del (boxed red in Figure 1D) were present on alternative haplotypes (hap-1: red, hap-2: blue) and therefore were in trans in the proband.
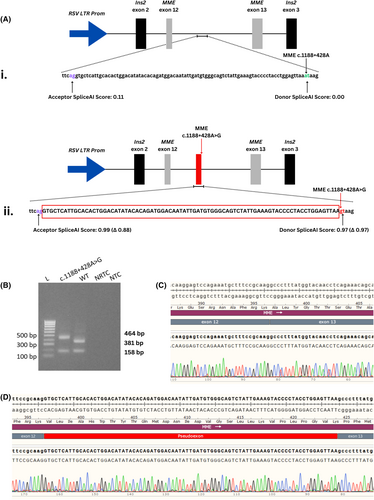
3.1.4 In vitro exon-trapping of MME-pSpliceExpress vectors
Two separate exon-trapping pSpliceExpress vectors were generated to assess the in vitro splicing impact of the MME c.1188+428A>G variant: pSpliceExpress-MMEWT (wild-type) and pSpliceExpress-MMEc.1188+428A>G (variant). A schematic of the constructs and relevant SpliceAI scores are shown in Figure 2A. RT-PCR products produced following transfection of these vectors were analyzed using gel electrophoresis (Figure 2B), which revealed a visible size difference between the wild-type (381 bp) and MME c.1188+428A>G amplicons (464 bp). The gel-purified amplicons were Sanger sequenced and the sequencing was aligned to the WT MME mRNA sequence. The sequenced products showed that the pSpliceExpress-MMEWT produced a transcript that was correctly spliced between MME exon 12 and 13 (Figure 2C). In contrast, exon-trapping of the pSpliceExpress-MMEc.1188+428A>G vector showed that an 83 bp pseudoexon had been spliced between MME exon 12 and 13 (Figure 2D). A BLAT search70 using the sequence of the trapped pseudoexon revealed alignment to the intronic region directly upstream of the MME c.1188+428A>G variant [chr3:155142675-155 142 757 (hg38)]. This suggests that the MME c.1188+428A>G variant creates a novel splice donor site leading to the aberrant inclusion of 83 bp of intronic MME sequence in the final spliced transcript, as predicted by SpliceAI. Prediction of the novel MME coding sequence caused by the introduction of the pseudoexon showed that a PTC was generated in exon 14 (p.Ala397ProfsTer47) at genomic position chr3:155144368 (hg38) (Figure S4). This PTC likely leads to NMD of the MME transcript.
3.1.5 In silico splicing analysis of MME variants
All reported MME variants in gnomAD v.4.0.0 were assessed using the integrated SpliceAI scores to determine if splicing variants in MME were a likely underrecognized cause of disease. There were 37 264 total variants reported in MME in gnomAD, of which 35 673 variants had a MAF <0.01. Of these, 88 variants had a maximum SpliceAI ∆score above 0.8 (Table S1). There were no homozygotes reported for any of the 88 MME putative splicing variants. The majority of these predicted splicing variants were predicted to directly change either the canonical splice donor sites (26/88) or canonical splice acceptor sites (30/88) of MME, including an inframe deletion (c.1317_1317+2del) and a frameshift variant (c.957+1del). An additional nine variants were predicted to affect a splicing region, including one which is also annotated as a synonymous variant (c.1188G>A; p.Lys396Lys). Nine missense variants (p.Asp209Gly, p.Ile217Ser, p.Glu282Val, p.Arg365Ile, p.Ser436Gly, p.Asp533Gly, p.Ile553Val, p.Val554Phe, p.Gln692Arg) and a synonymous variant (p.Gly417Gly) were also predicted to alter splicing. Twelve variants were annotated as ‘intron variants’, of which two could be considered ‘deep intronic’ variants (c.1188+428A>G, described in this manuscript, and c.197-9871A>G). Interestingly, further analysis using SpliceAI demonstrated that MME c.197-9871A>G was predicted to create a splice donor site (Score 0.81) and strengthen an upstream cryptic splice acceptor site (Score 0.81) in a similar manner to c.1188+428A>G, thereby possibly creating an in-frame 96 bp pseudoexon.
4 DISCUSSION
Here we report a deep intronic variant, MME c.1188+428A>G causing recessive CMT2T in two unrelated Australian families. This variant results in a pseudoexon that likely leads to NMD of the MME transcript, resulting in a loss-of-function. This is in keeping with previously reported MME variants which have broadly been characterized as ‘loss-of-function’ variants. To our knowledge, this is the first report of a pathogenic deep intronic variant in MME. Given that a significant portion of patients with axonal CMT remain genetically undiagnosed,13-17 it's possible that deep intronic variants in MME explain a portion of this diagnostic gap.
Individuals from Family 1 previously underwent both diagnostic WES and research WES, and the proband in Family 2 previously underwent targeted gene panel testing (PathWest neuro v3). These testing methods did not capture deep intronic regions and returned negative results. However, deep intronic regions have previously been shown to have a higher prevalence of variants than coding regions and canonical splice sites.71 Our findings suggest that intronic SNPs should be analyzed to determine if they impact splicing before they are dismissed as benign. Detection and functional validation of deep intronic variants has previously been shown to increase diagnostic rates in other Mendelian diseases including X-linked Alport syndrome,72 inherited retinal disorders,73, 74 and dystrophinopathy.75, 76 Pathogenic deep intronic variants, such as described here, are likely to be underreported amongst CMT-causing genes due to a lack of detection by WES and targeted gene panels, and a lack of functional investigation upon detection.
The MME c.1188+428A>G variant was reported in multiple different genetic ancestry groups in gnomAD v.4.0.0,67 including in the European (Non-Finnish) (8/68008), African/African American (1/41432), and ‘remaining' (1/2092) ancestry groups. This was also reflected in the ‘All of Us’ Research Program,68 which reported the MME c.1188+428A>G variant in the African (2/107888) and European populations (33/256804). Whilst it is possible that MME c.1188+428A>G represents a recurrent de novo variant, it is also likely that this variant has persisted at low levels in the global population. As this variant is missed by WES and may not be prioritized by variant-filtering approaches focusing on coding variants, this variant may therefore represent an underappreciated cause of recessive CMT. This is further supported by its detection in two Australian CMT families who are not known to be related.
Whilst the predicted splicing variants reported in MME are individually rare, we have described 88 variants in gnomAD that are predicted by SpliceAI to alter splicing. Nine of these variants were annotated as missense variants, and one was a synonymous change. This is of note as these ‘missense’ variants are often assumed to result in a single amino acid change, and synonymous variants are often considered functionally neutral, with their effect on splicing typically not assessed.20 Our results suggest that the discovery of any of these 88 variants in a homozygous or compound heterozygous state in an individual with CMT should prompt further functional investigation of their effect on splicing of the MME transcript.
Prior to functional validation, MME c.1188+428A>G was considered a variant of uncertain significance (VUS) according to American College of Medical Genetics and Genomics (ACMG) criteria77 (BP4, PM2, PM3, PP1), However, the functional evidence generated by the splicing assay now allows for the addition of PVS1 (null variant in a gene where loss-of-function is a known mechanism of disease) and PS3 (well-established in vitro or in vivo functional studies supportive of a damaging effect on the gene or gene product) criteria. This allows reclassification of the variant as ‘pathogenic’. Therefore, this work demonstrates the importance of functional validation to confirm the effect of candidate variants on splicing to increase diagnostic rates for those with inherited disease.
Previously described biallelic MME patients typically have a phenotype consistent with late-onset axonal neuropathy. In contrast, three of the four affected siblings in Family 1 self-described childhood onset and the proband of Family 2 self-described symptom onset in early adulthood. This may reflect an expansion of the phenotype associated with biallelic MME variants in CMT2T. It has also previously been reported that heterozygous MME variants can cause spinocerebellar ataxia type 43 (SCA43)78 as well as autosomal dominant CMT.3 However, the individuals in Family 1 who were MME c.1188+428A>G heterozygotes were all clinically assessed as neurologically normal, including an individual who is in their seventh decade. Homozygous affected individuals in Family 1 were noted to have a sensory ataxia rather than cerebellar ataxia, although MRI brain studies were not conducted. Therefore, our findings here do not support a role for MME c.1188+428A>G to cause SCA43 or autosomal dominant CMT, and further expand the phenotype of recessive CMT2T.
Understanding the specific effects of splice-affecting variants is crucial for developing potential therapeutic strategies. Antisense oligonucleotides (ASOs) that modulate splicing are an active area of research, including individualized approaches to treat rare genetic diseases.79-81 Several antisense nucleotides that modify splicing have been approved by the United States Food and Drug Administration and have resulted in marked improvements in clinical outcomes in those with genetic diseases.79, 82-91 An FDA-approved ‘n-of-1’ ASO, milasen, successfully blocked pathogenic pseudoexon inclusion in MFSD8 in a patient with neuronal ceroid lipofuscinosis type 7.91 ASOs which block pathogenic pseudoexons, such as that created by MME c.1188+428A>G, have also been described in in vivo preclinical models.79, 91-95 As such, individuals with the MME c.1188+428A>G variant may represent a form of CMT that is treatable through personalized ASO therapy and warrants further investigation.
ACKNOWLEDGMENTS
This work was funded by the Medical Research Future Fund (MRFF) Genomics Health Futures Mission (APP2007681). JMP is supported by the Australian Government Research Training Program. IWD was supported by MRF2025138 & MRF2023126. Genomic analysis was supported by the Centre for Population Genomics (Garvan Institute of Medical Research and Murdoch Children's Research Institute) and was funded in part by a National Health and Medical Research Council investigator grant (2009982) and the Medical Research Future Fund (MRFF) Genomics Health Futures Mission (2008820). Open access publishing facilitated by The University of Sydney, as part of the Wiley - The University of Sydney agreement via the Council of Australian University Librarians.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




