Clinical and genetic features of Charcot-Marie-Tooth disease 2F and hereditary motor neuropathy 2B in Japan
Abstract
Mutations in small heat shock protein beta-1 (HspB1) have been linked to Charcot-Marie-Tooth (CMT) disease type 2F and distal hereditary motor neuropathy type 2B. Only four cases with HSPB1 mutations have been reported to date in Japan. In this study between April 2007 and October 2014, we conducted gene panel sequencing in a case series of 1,030 patients with inherited peripheral neuropathies (IPNs) using DNA microarray, targeted resequencing, and whole-exome sequencing. We identified HSPB1 variants in 1.3% (13 of 1,030) of the patients with IPNs, who exhibited a male predominance. Based on neurological and electrophysiological findings, seven patients were diagnosed with CMT disease type 2F, whereas the remaining six patients were diagnosed with distal hereditary motor neuropathy type 2B. P39L, R127W, S135C, R140G, K141Q, T151I, and P182A mutations identified in 12 patients were described previously, whereas a novel K123* variant with unknown significance was found in 1 patient. Diabetes and impaired glucose tolerance were detected in 6 of the 13 patients. Our findings suggest that HSPB1 mutations result in two phenotypes of inherited neuropathies and extend the phenotypic spectrum of HSPB1-related disorders.
Introduction
Charcot-Marie-Tooth (CMT) disease is a common, clinically and genetically heterogeneous group of hereditary neuropathies. To date, more than 80 CMT disease-causing genes have been identified. Another inherited peripheral neuropathy (IPN), distal hereditary motor neuropathy (HMN), resembles CMT but is a pure motor neuron disease with no or mild sensory nervous system involvement.
The HSPB1 gene codes for heat shock protein beta-1 (HspB1, also called heat shock protein 27), which is a member of the small heat shock protein family comprising a highly conserved α-crystallin domain. HspB1 acts as a chaperone by binding misfolded or denatured proteins and preventing them from forming toxic aggregates (Dierick et al., 2005; Arrigo, 2007). Mutations in HSPB1 were shown to be associated with CMT type 2F (CMT2F, OMIM 602195) with autosomal dominant or recessive inheritance and HMN type 2B (HMN2B, OMIM 608634) with minimal sensory involvement. Since 2004, more than 40 families with CMT2F/HMN2B due to missense and rarely nonsense or frameshift mutations in HSPB1 have been reported (Evgrafov et al., 2004).
An Italian study reported the frequencies of HSPB1 mutations in HMN and CMT2 as 8% and 4%, respectively (Capponi et al., 2011). However, in Japan, only four sporadic HMN2B cases due to HSPB1 mutations have been reported thus far, and the clinical features remain poorly defined. Using DNA microarray and next-generation sequencing (NGS) technologies, we identified HSPB1 mutations in a large case series of Japanese patients and investigated the underlying clinical characteristics of CMT2F/HMN2B, including sex differences and abnormal glucose metabolism.
Materials and Methods
Patients
In this study, 1,030 patients with IPNs were enrolled. Clinical information of the patients was provided by nationwide medical facilities in Japan. The diagnostic criteria for CMT2 included progressive sensory and motor symptoms, signs of weakness and wasting, and conserved median motor nerve conduction velocities (>38 m/s) with reduced compound muscle action potential amplitudes. The diagnosis of HMN was based on distal lower and/or upper limb weakness and wasting, reduced reflexes, and pure motor axonal neuropathy confirmed by nerve conduction studies.
Genomic DNA was extracted from peripheral blood leukocytes of patients using the Gentra Puregene Blood Kit (Qiagen, Dusseldorf, Germany). Study protocols were reviewed and approved by the institutional review board of Kagoshima University. All patients and their family members who were referred to this study provided written informed consent.
Duplication/deletion mutation of PMP22 was preexcluded in all clinically suspected demyelinating CMT cases using fluorescence in situ hybridization or multiplex ligation-dependent probe amplification. In April 2007, we started mutation screening in patients with suspected CMT, hereditary neuropathy with liability to pressure palsies, or hereditary motor neuropathy who were referred to our laboratory.
DNA resequencing microarrays
Between April 2007 and April 2012, a purpose-built GeneChip® CustomSeq® Resequencing Array (Affymetrix, Santa Clara, CA, USA), which was used for screening 28 CMT and HMN disease-causing genes, was initially utilized following the protocol described previously (Zhao et al., 2012).
Targeted resequencing using Illumina Miseq
Starting in May 2012, a targeted resequencing system using the Illumina Miseq platform (Illumina, San Diego, CA, USA) was introduced for mutation screening. This system targeted a panel of 60 disease-causing or candidate genes of IPNs, and the specific design and workflow were illustrated previously (Maeda et al., 2014).
Whole-exome sequencing
Among mutation-negative cases identified by the previous screening, which was performed prior to May 2013, 398 cases that were highly suspicious for CMT were analyzed by whole-exome sequencing using HiSeq 2000 (Illumina). Sequences were aligned to the human reference genome (NCBI37/hg19) using the Burrows–Wheeler Aligner. Variant calling was performed using SAMtools and was annotated using in-house scripts.
Variant validation and interpretation
All HSPB1 variants were verified against the 1,000 Genome (http://browser.1000genomes.org/index.html), ExAC (http://exac.broadinstitute.org/), and Human Genetic Variation (http://www.genome.med.kyoto-u.ac.jp/SnpDB/) databases. Next, Sanger sequencing was used to validate the suspected variants, and a segregation analysis was performed whenever possible. Variants were interpreted according to the American College of Medical Genetics and Genomics/Association for Molecular Pathology (ACMG/AMP) guidelines published in 2015 (Richards et al., 2015).
Results
In this series, 13 unrelated patients harboring HSPB1 variants were identified. Among these, 10 patients had inherited variants with an autosomal dominant pattern, whereas 3 patients had a sporadic pattern. Clinical diagnosis was CMT2F in seven patients and HMN2B in six patients.
Clinical, genetic, and electrophysiological features of all patients with HSPB1 variants are summarized in Tables 1 and 2. The family trees of the patients were shown Figures 1, 2B and 4A. The following seven known heterozygous mutations were identified in 12 patients: T151I, P39L, R140G, R127W, S135C, K141Q, and P182A (Fig. 2A). The T151I mutation, which was found in four unrelated patients, was determined to be a mutation hot spot.
| Upper limbs | Lower limbs | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pt | Mutation | Amino acid change | Inheritance | CMT2/HMN2B | Age/sex (y.o) | Onset (y.o) | DTR† (rt/lt) | Atrophy (rt/lt) | MMT‡ (TA) | DTR | Atrophy (rt/lt) | Sensory disturbance | Other clinical findings | DM/IGT | HbA1c§,¶ |
| 1** | c.421A>C | K141Q | D | HMN2B | 69/M | 68 | ±/± | −/− | 4/4 | ±/± | +/+ | No | Mild CK elevation (488 IU/l) | No | N.D |
| 2 | c.544C>G | P182A | D | CMT2F | 49/F | 19 | +/+ | +/+ | 4/4 | ±/± | +/+ | Dysesthesia | No | N.D | |
| 3 | c.116C>T | P39L | D | CMT2F | 61/M | 57 | +/+ | −/− | 2/2 | −/− | +/+ | + | DM | 8.5% (N.D) | |
| 4 | c.116C>T | P39L | D | CMT2F | 75/M | 57 | +/+ | +/+ | 0/0 | −/− | +/+ | No | Mild CK elevation (281 IU/l) | DM | 6.8% (NGSP) |
| 5 | c.379C>T | R127W | D | HMN2B | 58/M | 55 | +/+ | N.D | 4/4 | ±/± | N.D | No | IGT | 6.9% (NGSP) | |
| 6 | c.418C>G | R140G | D | CMT2F | 70/M | 65 | ±/± | +/+ | 3/3 | −/− | +/+ | No | Mild CK elevation (426 IU/l) | IGT | 6.7% (N.D) |
| 7 | c.418C>G | R140G | D | HMN2B | 68/M | 50 | +/+ | −/− | 2/2 | +/+ | +/+ | No | Dysphagia, fasciculation. | No | N.D |
| 8 | c.404C>G | S135C | S | CMT2F | 64/M | 59 | ±/± | −/− | 0/0 | −/− | +/+ | + | Mild CK elevation (912 IU/l) | DM | 7.3% (JDS) |
| 9 | c.452C>T | T151I | D | HMN2B | 63/M | 61 | N.D | N.D | 2/2 | −/− | N.D | No | No | N.D | |
| 10 | c.452C>T | T151I | S†† | HMN2B | 61/F | 52 | +/+ | N.D | 5/0 | +/+ | N.D | No | DM | 6.1% (N.D) | |
| 11 | c.452C>T | T151I | D | CMT2F | 62/M | 51 | N.D | N.D | 1/1 | ±/± | N.D | No | No | N.D | |
| 12 | c.452C>T | T151I | D | HMN2B | 45/M | 40 | +/+ | −/− | 1/1 | −/− | +/+ | No |
PH of hyperglycemia Mild CK elevation (318 IU/l) |
No | 5.8% (NGSP) |
| 13 | c.367A>T | K123* | S | CMT2F | 61/M | 51 | +/+ | −/− | 4/4 | −/− | +/+ | + | Mild CK elevation (300–400 IU/l) | No | N.D |
- ATR, Achilles tendon reflex; CK, Creatine kinase; D, dominant; DM, diabetes mellitus; F, female; IGT, impaired glucose tolerance; JDS, Japan Diabetes Society; lt, left; M, male; MMT, manual muscle test; N.D, no data; NGSP, National Glycohemoglobin Standardization Program; OPLL, ossification of posterior longitudinal ligament; PH, past history; PTR, Patella tendon reflex; rt., right; S, sporadic; TA, tibialis anterior; y.o, years old.
- † DTR: +, normal; ±, decreased; −, absent.
- ‡ MMT: 5, normal; 4, good; 3, fair; 2, poor; 1, trace.
- § The highest HbA1c value within the data provided by the primary physician.
- ¶ Cut-off value of HbA1c for the diagnosis of DM is 6.1% in JDS criteria and 6.5% in NGSP criteria.
- ** Data of the father and brother is not available.
- †† Maeda et al. (2014).
| Median nerve | Ulnar nerve | Tibial nerve | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Motor | Sensory | Motor | Sensory | Motor | Sural nerve | |||||||
| Pt | Amp (mV) | CV (m/s) | Amp (μV) | CV (m/s) | Amp (mV) | CV (m/s) | Amp (μV) | CV (m/s) | Amp (mV) | CV (m/s) | Amp (μV) | CV (m/s) |
| 1 | 7.3 | 52 | 24.0 | 45 | 4.7 | 61 | 24.5 | 54 | 1.1 | 33 | 4.2 | 42 |
| 2 | 4.9 | 50 | 34.0 | 58 | 2.8 | 56 | 13.0 | 50 | 0.1 | N.R | 4.0 | 46 |
| 3 | 15.1 | 45 | 24.7 | 53 | 15.3 | 46 | 15.9 | 47 | 0.1 | 34 | 2.9 | 43 |
| 4 | 9.9 | 56 | 11.0 | 45 | N.D | N.D | N.R | N.R | ||||
| 5 | 10.2 | 38 | 31.3 | 9 | 18.3 | 63 | 32.9 | 53 | N.R | 2.3 | 50 | |
| 6 | N.D | W.N.L | N.D | W.N.L | N.D | N.D | 0.3 | W.N.L | N.D | |||
| 7 | 18.9 | 55 | 9.0 | 48 | 24.2 | 58 | 11.0 | 48 | N.D | N.D | ||
| 8 | 7.0 | 58 | 15.7 | 55 | 4.7 | 52 | N.D | 0.4 | 43 | 5.3 | 15 | |
| 9 | 11.0 | 63 | 32.9 | 52 | 12.3 | 58 | 23.5 | 53 | 0.2 | 44 | 7.4 | 47 |
| 10 | N.D | ≧38 | N.D | N.D | ≧38 | N.D | N.R | N.D | 55 | |||
| 11 | 4.6 | 64 | 19.3 | 56 | 8.6 | 61 | 18.0 | 57 | N.R | 2.8 | 41 | |
| 12 | 8.2 | 58 | 13.0 | 52 | 8.3 | 66 | 11.0 | 56 | N.R | 8.0 | 39 | |
| 13 | 14.4 | 41 | 7.9 | 41 | 14.9 | 45 | 11.0 | 40 | N.R | 3.1 | 34 | |
- Amp, amplitude; CV, conduction velocity; N.D, no data; N.R, not recorded; W.N.L, within normal limit.
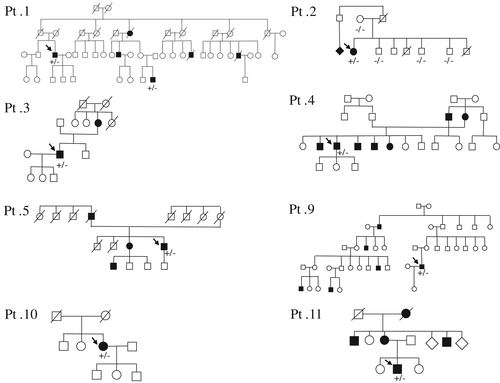
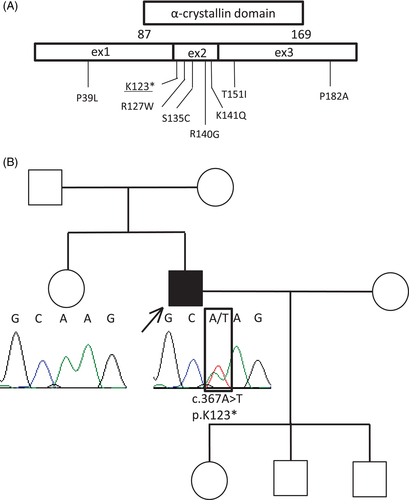
A novel heterozygous nonsense c.367A>T (K123*) variant was detected in a 61-year-old patient with sporadic CMT2F (patient 13) with asymmetrical weakness and mild sensory disturbance in the distal legs.
Among the 13 patients in our study, the mean age of onset was 53 ± 12 years, which was slightly higher than that reported previously (Tang et al., 2005). No sensory abnormalities were identified in the upper limbs of any of the patients using nerve conduction studies. The difference in the mean age of onset (51 vs. 54 years) was not different between the CMT2F and HMN2B groups. However, disease duration for patients with CMT2F (12 ± 9 years) was longer than that of patients with HMN2B (6 ± 6 years). Pathological analysis of a sural nerve biopsy performed in patient 4 showed a reduction in large myelinated fibers (3,268/mm2) and axonal degeneration without onion bulb formation (Fig. 3).

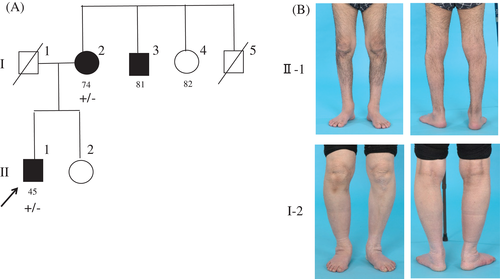
We noticed a sex imbalance in our patients, with 11 males and 2 females (Table 1). In Family 12, although harboring the same T151I mutation, the male proband exhibited a much more severe clinical phenotype than his mother with slight foot weakness (Fig. 4B). To clarify the presence of a sex difference, we reviewed the literature for patients with IPNs caused by HSPB1 mutations, which revealed a comparable male predominance (67 males vs. 26 females, despite the comparable numbers of non-affected male and female carriers (73 vs. 78, respectively; Table 3).
| Male | Female | ||||
|---|---|---|---|---|---|
| Mutation | Author (year) | Affected (n) | Non-affected (n) (carrier) | Affected (n) | Non-affected (n) (carrier) |
| R127W | Tang et al. (2005) | 8 | 13 (1) | 2 | 16 (2) |
| Dierick (2008) | 4 | 0 | 3 | 0 | |
| Benedetti (2010) | 1 | N.D | 0 | N.D | |
| Current report | 3 | 7 | 1 | 1 | |
| T151I | Dierick (2008) | 7 | 2 | 4 | 3 |
| Nishibayashi (2007) | 2 | N.D | 2 | N.D | |
| Current report | 11 | 10 | 3 | 22 (1) | |
| R140G | Houlden (2008) | 5 | 7 | 0 | 4 |
| Current report | 5 | N.D | 0 | N.D | |
| K141Q | Ikeda et al. (2009) | 3 | 10 | 0 | 5 |
| Current report | 5 | 12 | 1 | 18 | |
| S135C | Benedetti (2010) | 0 | N.D | 2 | N.D |
| Oberstadt (2016) | 2 | 1 | 3 | 0 | |
| Current report | 1 | N.D | 0 | N.D | |
| P182A | Current report | 0 | 6 | 1 | 0 |
| P39L | Houlden (2008) | 1 | 0 | 1 | 0 |
| Capponi et al. (2011) | 2 | 0 | 0 | 1 | |
| Current report | 6 | 5 | 3 | 7 | |
| K123* | Current report | 1 | 0 | 0 | 1 |
| Totala | 67 | 73 | 26 | 78 | |
- n, number; N.D, no data.
- a Total number of pedigrees in all mutations.
In our series, diabetes mellitus (DM) was found in patients 3, 4, 8, and 10 with disease durations of 6, >2, unknown, and 6 years, respectively. Impaired glucose tolerance (IGT) was identified in patients 5 and 6. Moreover, patient 12 reported a history of hyperglycemia. However, no notable clinical or electrophysiological difference was found between patients with and without DM/IGT.
Discussion
This study identified HSPB1 mutations in 13 Japanese CMT2F/HMN2B pedigrees and summarized their clinical and genetic characteristics. Overall, the clinical features of the current case series resemble those previously reported. We found HSPB1 mutations in 1.3% (13 of 1,030) of the patients clinically diagnosed with IPNs in our series, which was comparable with the frequency of the HSPB1 mutations reported in the United States (0.3% in IPNs), Taiwan (0.4% in CMT), and Korea (0.6% in CMT and HMN) (Capponi et al., 2011; DiVincenzo et al., 2014; Lin et al., 2011).
HspB1 is a 205-amino acid protein which contains a signature α-crystallin domain at residues Glu87-Pro168 flanked by the N- and C-termini. The mutant HspB1 can form intracellular aggregates, inhibit cell division, or disrupt the neurofilament network (Ackerley et al., 2006; Zhai et al., 2007). The α-crystallin domain, which has an immunoglobulin-like fold, mediates dimerization of individual protomers that subsequently assemble into larger oligomers (Baranova et al., 2011). Using DNA microarrays and NGS, we identified seven previously described HSPB1 mutations in 12 patients with CMT2F/HMN2B. Five of these mutations, P127W, S135C, R140G, K141Q, and T151I, are distributed in the center α-crystallin domain and may disrupt the assembly of HspB1. The T151I mutation, which was identified in four patients, could be attributed to a founder ancestor due to their geographical clustering.
A heterozygous nonsense variant (K123*) was discovered in the current study. Nonsense mutations are not common among the known HSPB1 mutations. As a reference, another heterozygous nonsense mutation, E175*, was previously reported (Rossor et al., 2012). We interpret this K123* variant as unknown significance on the basis of following reasons: (1) absent in any of the global or Japanese database, including 1,000 Genomes, ExAC, Human Genetic Variation Database (HGVD), and integrative Japanese Genome Variation Database (iJGVD); (2) not detected in his unaffected sister; (3) positioned in the highly conserved α-crystallin domain; (4) comparable clinical phenotype with other patients with HSPB1 mutations; and (5) could escape from nonsense-mediated mRNA decay, because of specific mechanism of the stress-responsive genes (Guo et al., 2009; Lykke-Andersen and Jensen, 2015).
The P182A mutation in patient 2, one of the three substitutions at residue P182 (others are P182L and P182S), was reported previously (Evgrafov et al., 2004; Kijima et al., 2005; Rossor et al., 2017). Except for the prominent weakness of the distal upper extremities observed in the current patient, the neurological findings and the age of onset were comparable with the previous reports.
In the current study, we also performed a meta-analysis to confirm the male predominance. The sex difference was prominent particularly in certain mutations such as R140G (10:0) and K141Q (8:1). Further, in family 12, we noted that the mother of the proband, although harboring T151I mutation, presented with relatively slight symptoms. A similar pattern was also observed in families 1 and 11; however, genetic analysis of other family members could not be conducted. These observations support the finding of an incomplete penetrance of HSPB1 mutations, particularly in females. The significance of the sex difference in disease severity requires further investigations.
Mutant HspB1 affects the binding of nuclear factors (NFs) to the anterograde motor protein kinesin, thereby reducing the anterograde transport of NFs (Holmgren et al., 2013). Two mutations in HSPB1 (p.Gln190His and p.Al204Glyfs*6) were reported recently in patients with amyotrophic lateral sclerosis (ALS) phenotype, which suggested overlap of ALS and CMT2F/HMN2B (Capponi et al., 2016). In the current study, patient 7 was suspected to have ALS because of dysphagia, fasciculation in left leg, and preserved deep tendon reflexes.
On the other hand, as with the findings in six of our patients (1.2 to 3.9 times of normal range), mild CK elevation was repetitively reported in patients with HSPB1 mutations. Recently, a new phenotype of distal myopathy was described (Lewis-Smith et al., 2016), but in another report, no myogenic change was recognized in either needle electromyography or muscle biopsy (Echaniz-Laguna et al., 2017). In the present study, needle electromyography was recorded in four patients (patients 4, 6, 12, 13) with mild CK elevation, but none of them manifested with myogenic changes. Thus, the impairment of muscle membrane integrity due to denervation from axonal loss could be the likely reason for mild CK elevation in our study.
Impaired glucose metabolism, including DM and IGT, was verified in 6 of 13 patients (7 patients if patient 12 with a history of hyperglycemia is included). In half of these six patients, sensory disturbance was identified in the clinic or with nerve conduction studies, and it was difficult to clearly attribute these abnormalities to the CMT2F/HMN2B or DM diagnosis. Patients with HSPB1 mutations were reported to be likely to have complications with glycemic abnormalities, as observed in a Japanese patient with the K141Q mutation (Ikeda et al., 2009). Induction of heat shock proteins may combat insulin resistance (McCarty, 2006), and elevated serum HspB1 levels were also reported in patients with diabetic polyneuropathy (Gruden et al., 2008). Therefore, it remains possible that the mutant HspB1 might act in concert with the hyperglycemic state to lower the onset threshold of DM or vice versa.
Acknowledgements
This study was supported in part by grants from the Nervous and Mental Disorders and Research Committee for Charcot-Marie-Tooth Disease, Neuropathy, Ataxic Disease and Applying Health and Technology of Ministry of Health, Welfare and Labor, Japan. This study was also supported by the Research Program for Conquering Intractable Disease from Japan Agency for Medical Research and Development, AMED. The authors thank the patients and their families for participating in this study and their physicians for submitting the clinical samples.




