Pregnancy and brain architecture: Associations with hormones, cognition and affect
Abstract
Sex hormones such as estradiol (E2) have long-lasting influence on brain architecture. Recent studies indicate further structural changes during hormonal transition periods including pregnancy, when women experience the greatest increase in sex hormone levels across their life span. In the present study, three groups of women (n = 44) with different levels of E2 underwent structural magnetic resonance imaging: (1) first-time pregnant women (n = 13, ‘extreme E2 group’); (2), nulliparous, naturally cycling women who received 12 mg of E2 valerate (n = 16, ‘high E2 group’); and (3) nulliparous, naturally cycling women receiving a placebo and hence low E2 (n = 15, ‘low E2 group’). Blood samples were taken to assess hormonal levels. Moreover, parameters for cognition, emotion regulation and affect were assessed. On the neuronal level, the extreme E2 compared to the high E2 group showed a reduced gray matter volume in the left putamen. However, no significant differences were found between the low vs. high E2 groups, nor between the low E2 and extreme E2 groups. Cognitive performance was reduced in the extreme E2 group, although a positive affect was increased compared to the high E2 and low E2 groups. Furthermore, regression analyses revealed several associations between cognition, subjective measures of affect, emotion regulation and gray matter volume. A volume reduction of the left putamen during pregnancy further supports the notion that the female brain is shaped by hormonal transition phases, possibly preparing women for their future roles (e.g., pregnant women for their role as mothers).
1 INTRODUCTION
Variations in estradiol (E2) and progesterone levels have been associated with gray matter changes across different hormonal transition phases in women, such as during puberty, the menstrual cycle, pregnancy and the postpartum period, as well as menopause.1 During puberty, the increase in sex hormones has been associated with a reduction of gray matter volume.2 Across the menstrual cycle differences in gray matter volume between phases with high vs. low E2 have been reported.3, 4 However, little is known about the effects during pregnancy. Pregnancy is a hormonal transition phase where women have to adapt to several physiological and psychological challenges. These include steep increases of various hormones, including E2 and progesterone. To our knowledge, only two studies so far have investigated differences in overall brain size in women pre- and post-pregnancy. Oatridge et al.5 reported a significant decrease in brain size during pregnancy in nine healthy women that was not detectable at 1 year after delivery. Hoekzema et al.,6 in contrast, reported reductions in several brain areas, including prefrontal, temporal, parietal, and midline areas, when comparing women before and after pregnancy and measuring nulliparous women as a control group. These reductions in gray matter volume in primiparous women were still observed in a follow-up measurement 2 years after delivery. Only hippocampal size increased significantly from an average of 3 months postpartum to the follow-up measurement 2 years later, although it did not reach pre-pregnancy levels. A follow-up study with the same sample additionally showed a pregnancy-related reduction in the right striatum.7 Furthermore, several studies report reductions in the pituitary gland across pregnancy.8-10 Based on these reports, structural changes during pregnancy can be assumed.
More extensive research has been conducted regarding brain architecture including different time points across the post-partum period. Here, an overall increase in brain size particularly in the prefrontal and parietal, as well as midbrain areas, was described from early to late postpartum.11 Furthermore, an increase in gray matter volume of subfields of the amygdala and of the hippocampus was observed when comparing the early to late postpartum period.12, 13 In the same sample, a reduction of the chronological brain age of approximately 5.5 years has been reported 4–6 weeks after delivery.14 These changes as a result of pregnancy and the postpartum period appear to even be visible in the brains of middle-aged women, showing less brain aging in parous compared to nulliparous women.15 The effects appear to be even stronger after multiple child births.
With the increase in sex hormones during pregnancy, women often report changes in cognitive performance16 and subjective affect.17 Women also often self-report a mild decline in cognitive functioning during pregnancy.18 However, the results from studies assessing cognitive abilities in pregnant women remain rather inconsistent as can be seen in a recent meta-analysis16: some studies found no cognitive decline,19-21 whereas others report a general decline22 or a significant effect on specific domains such as memory23, 24 or processing speed.16, 25 Also, the decline in cognitive functioning was more pronounced in the third compared to the first trimester of pregnancy.16
Overall, higher emotional sensitivity was reported in pregnant women, with some experiencing an increase in emotional dysregulation and mood swings.17, 26-28 In a behavioral emotion recognition task, especially harm and threat related stimuli, as well as negative emotions, could be reported with higher accuracy during late compared to early pregnancy in a within-subject design.26 Moreover, pregnant women were better at recognizing novel male faces and showed a higher startle-response compared to women in the postpartum period.27, 28
To specifically elucidate the effect of pregnancy vs. high E2 levels and low E2 levels on gray matter volume, cognition and subjective affect, we compared healthy first-time pregnant women (extreme E2) with young, naturally cycling women whose E2 levels were either experimentally elevated to pre-ovulatory levels (high E2) or naturally cycling women measured during the early follicular phase (low E2). To our knowledge, this neuroimaging study includes the largest sample of healthy first-time pregnant women (n = 13) measured with magnet resonance imaging (MRI). Based on previous findings,5-7, 11 we hypothesized that there would be a reduction in gray matter volume of prefrontal, parietal and midbrain areas in pregnant women (extreme E2 group), compared to the high E2 and low E2 groups. Related to a previous study investigating different menstrual cycle phases,3 we hypothesized that there would be a higher gray matter volume of the hippocampus in the high E2 compared to low E2 group. Furthermore, we used an exploratory approach to assess whether brain volume in pregnant women is associated with cognitive and affective processing. Because previous studies showed weakened emotional responses towards negative stimuli during phases with high E2 and low progesterone,29 we expected the affective ratings to be less negative under high E2 levels.
2 MATERIALS AND METHODS
2.1 Sample description
In total, 45 women between the age of 19 and 36 years were included in the present study. All women had to fulfill inclusion criteria for MRI measurements (e.g., no metal implants), and report no past or present mental or somatic disorders and no current medication intake. Pregnant women had to be in the second trimester (range 21–28 gestational weeks) and provide a copy of their second ultrasound screening to exclude any pregnancy-related complications in the mother or fetus. Naturally cycling women had to have a regular, natural menstrual cycle lasting between 26–32 days (confirmed by participants reporting the last two menstrual cycles) and no current or past pregnancies. Exclusion criteria consisted of oral contraceptive use within the last 6 months. The study received approval from the Ethics Committee of the Medical Faculty of the University of Tübingen (754/2017/BO1). Participants were recruited with a university email provider and pregnant women were additionally informed about the study through flyers that were displayed in the University Women's Hospital in Tübingen, as well as several gynecology practices in the surrounding area. All participants signed an informed consent and data protection agreement.
2.2 Procedures
Before inclusion, all women completed a screening interview for mental disorders (SCID30) to exclude women with current or past mental disorders. After the screening interview, verbal intelligence (Wortschatztest; WST31) and cognitive flexibility (Trail making test A/B; TMT-A/B32) were assessed. Participants also reported about their emotion regulation strategies (Emotion Regulation Questionnaire; ERQ33, 34). Naturally cycling women reported the onset of their menstruation and were then invited to participate in the study between day 2 and day 5 of their menstrual cycle (early follicular phase). To experimentally increase E2 levels, all naturally cycling women were invited in the laboratory 1 day before the MRI assessment (day 1), where blood was drawn and the groups either received 6 mg of estradiol valerate (Progynova® 21; Bayer Weimar GmbH und Co. KG) or placebo sugar pills. A second dose was given to participants for take home for the morning to midday on the next day (day 2). This procedure allows the experimental elevation of E2 levels at the same time as maintaining low levels of progesterone and testosterone. On day 2, all groups (pregnant women [extreme E2], E2 valerate [high E2] and placebo [low E2]) were invited to the laboratory. Before the MRI measurement, positive and negative affect (Positive and Negative Affect Schedule; PANAS35), as well as state and trait anxiety (State-trait anxiety inventory; STAI36, 37), were assessed.
2.3 Hormone assessments
In addition to changes in E2, levels of progesterone and testosterone were analyzed to ensure these levels remained low in the naturally cycling women. For analyses of E2, progesterone and testosterone, 7.5 mL of blood was drawn in serum tubes. Sex hormone analyses were conducted at the central laboratory of the University Hospital Tübingen using enzyme-linked immunoassays (ELISA). Sensitivity and range of measurement included the following values for E2 (43.6–11,010 pmol L–1), progesterone (0.67–190.8 nmol L–1) and testosterone (0.24–52.05 nmol L–1), respectively.
2.4 MRI data acquisition
Data were acquired using a 3 Tesla Prisma scanner (Siemens) at the University Hospital Tübingen. Brain structure was measured with a standard magnetization-prepared rapid gradient-echo sequence with the parameters: TR =2,300 ms, TE =4.16 ms, slice thickness =1.00 ms, voxel size =1 × 1 × 1 mm, flip angle 9°, distancing factor 50%, GRAPPA acceleration factor and sagittal orientation. Women were provided earplugs to reduce the sound intensity. To further ensure that the sound level was kept at a minimum for the unborn child, the volume of the MRI sequence was measured and reached approximately 90 dB, although, in the womb, the unborn child is further protected against noise disturbance through the amniotic fluids.
2.5 MRI analysis
Anatomical data were analyzed using SPM12 (https://www.fil.ion.ucl.ac.uk/spm/software/spm12). Voxel-based-morphometry was conducted on the T1-weighted scans, implemented via MATLAB, version R2019a (The Math Works).38 The pre-processing pipeline consisted of unified segmentation of T1 images into gray matter (GM), white matter (WM) and cerebrospinal fluid (CSF) with default settings of bias regularization 0.0001 and a bias cut-off full width at half maximum (FWHM) of 60 mm.39 A study specific template was created in the MNI space using the DARTEL (Diffeomorphic Anatomical Registration using Exponentiated Lie Algebra) toolbox as implemented in SPM12 with the GM and WM tissue probability maps.40 Later, the segmented GM tissue maps were normalized to the MNI space using the previously created template. Normalized GM images were also modulated for preserving the volume of the native GM tissue in each voxel. Finally, the modulated images were smoothed using a Gaussian kernel with a FWHM of 8 mm. In a full factorial ANOVA, gray matter volumes were compared between the groups. To control for individual differences in total intracranial volume (TIV = GM + WM + CSF), proportional scaling was applied, which calculated GM in relation to TIV. Age was included as covariate because it differed significantly between pregnant women and the control groups with older participants in the pregnant group (Table 1). Results were family-wise error (FWE)-cluster level corrected at p < .05. Brain areas were specified using the Anatomy toolbox.41
| Sample characteristics | Low E2 | High E2 | Extreme E2 | F/H/U statistic | p-value | Post-hoc |
|---|---|---|---|---|---|---|
| Age (years) | 23.9 (3.4) | 23.3 (3.1) | 29.6 (3.9) | 16.02 | < .001 |
Extreme E2> High E2 Extreme E2> Low E2 |
| Verbal intelligence (WST) | 32.3 (3.5) | 32.7 (2.5) | 30.6 (6.0) | 0.51 | .776 | |
| Processing speed (TMT-A) (s) | 19.7 (3.3) | 20.3 (4.7) | 27.0 (10.7)a | 8.17 | .017 |
Extreme E2> High E2 Extreme E2> Low E2 |
| Cognitive flexibility (TMT-B) (s) | 35.1 (9.1) | 39.6 (11.0) | 48.1 (16.8)a | 6.09 | .048 | Extreme E2> Low E2 |
| Body mass index | 22.6 (2.5) | 24.4 (4.6) | 23.4 (2.0) | 1.33 | .515 | |
| Gestational week | 23.8 (2.0) (range:21–28) | |||||
| Hormonal values | ||||||
| E2 day 1 (pmol L–1) | 192.3 (54.7) | 193.8 (79.5)b | 102 | .663 | ||
| E2 day 2 (pmol L–1) | 225.6 (117.3)c | 516.6 (188.8)c | 36,122.3 (15,315.6) | 32.86 | < .001 |
Extreme E2> High E2 Extreme E2> Low E2 |
| Progesterone day 1 (nmol L–1) | 2.0 (0.8) | 2.4 (0.7) | 86 | .270 | ||
| Progesterone day 2 (nmol L–1) | 2.3 (1.0)c | 2.2 (0.8)c | 239.2 (46.8) | 26.05 | < .001 |
Extreme E2> High E2 Extreme E2> Low E2 |
| Testosterone day 1 (nmol L–1) | 1.1 (0.3) | 1.1 (0.4) | 109 | .883 | ||
| Testosterone day 2 (nmol L–1) | 1.2 (0.5)b | 1.0 (0.4)b | 1.6 (0.7) | 5.95 | .051 | |
| Affect ratings (PANAS) | ||||||
| Positive affect | 19.3 (3.9) | 21.1 (3.9) | 24.0 (3.6) | 11.44 | .003 |
Extreme E2> High E2 Extreme E2> Low E2 |
| Negative affect | 31.8 (4.6)c | 34.0 (6.0) | 33.6 (7.96) | 0.52 | .601 | |
| State anxiety (STAI) | 35.5 (6.5) | 38.1 (5.7) | 37.8 (6.0) | 2.95 | .229 | |
| Trait anxiety (STAI) | 32.3 (5.0) | 37.0 (9.6) | 40.6 (12.0)a | 2.86 | .069 | |
| Emotion regulation (ERQ) | ||||||
| Cognitive reappraisal | 4.8 (0.8) | 5.1 (0.8) | 4.4 (0.9) | 2.63 | .084 | |
| Emotional suppression | 3.3 (1.2) | 2.9 (0.9) | 2.9 (0.8) | 0.98 | .383 | |
Note
- Data are the mean value with SD in brackets.
- Sample size: low E2 (n = 15); high E2 (n = 16); extreme E2 (n = 13).
- Abbreviations: E2, estradiol; ERQ, Emotion Regulation Questionnaire; PANAS, Positive and Negative Affect Schedule; TMT-A, trail making test A; TMT-B, trail making test B.
- a n = 12.
- b n = 15.
- c n = 14.
2.5.1 Region of interest (ROI) analysis
Besides the whole-brain approach, ROIs of brain areas that showed significant differences in gray matter volume between women nulli- and primiparous women in a previous study6 were created with the WFU Pickatlas.42 Because there is only one previous study reporting gray matter changes as a result of pregnancy, we conducted the analysis in an exploratory manner. ROIs included the bilateral hippocampus, parahippocampus, fusiform gyrus, superior temporal gyrus, precuneus, superior medial frontal gyrus, inferior and medial orbitofrontal gyrus, inferior frontal gyrus, middle frontal gyrus, and superior frontal gyrus. Masks of ROIs were then included in individual full factorial ANOVAs with group as between-subjects factor and age as covariate. Results were FWE corrected at the cluster level.
2.6 Statistical analysis
Hormonal and behavioral data were analyzed with SPSS, version 26 (IBM Corp.). p < .05 was considered statistically significant. Effect sizes for significant differences are reported in partial-eta2.
2.7 Hormones
For E2, progesterone and testosterone data, the assumption of a normal distribution was not met; thus, the non-parametric Kruskall–Wallis test was applied to assess group differences. Post-hoc tests were performed using the Mann–Whitney U test. Differences in hormonal levels at day 1 and day 2, thus before and after drug administration, in the high E2 and low E2 groups were assessed using a Wilcoxon signed rank test.
2.8 Behavioral variables
Group differences in mean values were assessed by performing univariate ANOVA. In case data were not normally distributed, the non-parametric Kruskall–Wallis test and Mann–Whitney U test were performed instead. This included data for age, positive affect (PANAS), STAI-state, WST, TMT-A, TMT-B and hormonal values (E2, progesterone, testosterone).
2.9 Regression analysis
We were further interested in possible associations between gray matter volume and hormonal levels (predictors: E2, progesterone, testosterone) and behavioral variables including cognitive performance (processing speed, cognitive flexibility), affect (positive affect), anxiety (trait anxiety) and emotion regulation (cognitive reappraisal). As a result of the scarcity of the availability of MRI data for pregnant women and no previous study testing associations between gray matter volume and behavioral or hormonal data, this was carried out in an exploratory fashion. Behavioral variables were chosen based on significant group differences. Regression analyses were performed separately for each group using SPM12 and including one of the above mentioned predictors. The results were FWE corrected at the cluster level. Later, parameter estimates were extracted from SPM12 and regression parameters were calculated using SPSS.
3 RESULTS
3.1 Sample description
Thirteen primiparous women (mean ± SD age = 29.6 ± 3.9) were measured during the second trimester of pregnancy (mean ± SD gestational weeks =23.8 ± 2.0, range 21–28). Thirty-two naturally cycling women were included for comparison during their early follicular phase (day 2 to day 5 of the menstrual cycle). One naturally cycling woman in the control group had to be excluded as a result of her hormonal levels not matching the menstrual cycle phase. Thus, the final sample size included 44 women, with thirteen pregnant women (extreme E2), 16 women receiving E2 valerate (high E2) and 15 women taking the placebo pill (low E2). Group characteristics are provided in Table 1.
3.2 Hormones
3.2.1 Baseline hormonal levels on day 1 (before drug administration)
No differences appeared for baseline hormonal values on day 1 between the high E2 and low E2 groups (all p > .269).
For the high E2 group, there was a significant increase in E2 levels from day 1 to day 2 (Z = −3.180, p = .001, r = .62), whereas, in the low E2 group, levels did not change (p = .140). For the high E2 and low E2 groups, progesterone (all p >.243) did not differ between day 1 and day 2. For testosterone, a trend (Z = −1.910, p = .056, r = .07) appeared between day 1 and day 2 (Table 1).
3.2.2 Group differences on day 2 after drug administration
For the high E2 group, there was a significant increase in E2 levels from day 1 to day 2 (Z = −3.180, p = .001, r = .62), whereas, in the low E2 group, levels did not change (p = .140). For the high E2 and low E2 groups, progesterone (all p > .243) did not differ between day 1 and day 2. For testosterone, a trend (Z = −1.910, p = .056, r = .07) appeared between day 1 and day 2 (Table 1).
When comparing the three groups, E2 levels differed significantly (H = 32.86, p < .001). As expected, E2 levels were approximately 70 times higher in the pregnant group compared to the high E2 group (U = .00, Z = −4.423, p < .001, r = .85) and 160 times higher compared to the low E2 group (U = .00, Z = −4.416, p < .001, r = .85) (Figure 1A). Moreover, E2 levels in the high E2 group were 2.3 times higher compared to the low E2 group (U = .00, Z = −3.814, p < .001, r = .72).
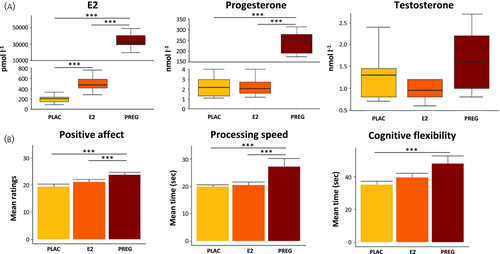
Progesterone levels differed significantly between groups (H = 26.05, p < .001) with higher values in the extreme E2 compared to the high E2 group (U = .00, Z = −4.42, p < .001, r = .85) and higher values compared to the low E2 group (U = .00, Z = −4.42, p < .001, r = .85). No difference appeared between the high E2 and low E2 groups (U = 96.5, Z = −.069, p = .946).
Testosterone values differed only at a trend level (H = 5.95, p = .051) between groups, with significantly higher testosterone values in the extreme E2 group compared to the high E2 group (U = 46, z = −2.19, p = .029, r = .42) and a trend between the extreme E2 and low E2 groups (U = 54.5, z = −1.78, p = .076, r = .34), whereas no difference appeared between the E2 and low E2 groups (p > 0.328).
3.3 Whole-brain analysis
The extreme E2 group compared to the high E2 group showed reduced gray matter volume in the left putamen (Figure 2 and Table 2). No significant differences were seen between pregnant women and the low E2 group, as well as between the high E2 and low E2 groups.
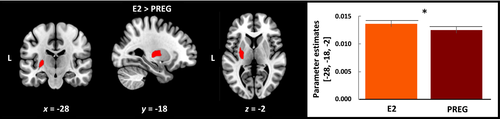
| Contrast | MNI coordinates | Region | Cluster size | t value | p value | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Whole brain analysis | |||||||
| High E2 > Extreme E2 | −28 | −18 | 6 | L putamen | 3095 | 3.31 | < .001 |
| −31 | 3 | −2 | |||||
|
Regression analyses (extreme E2) |
|||||||
| Cognitive performance | |||||||
| Cognitive flexibility (+) | 55 | −21 | 16 | L STG | 1570 | 8.57 | .002 |
| −52 | −10 | −7 | R ROL | 1758 | 6.57 | .001 | |
| Affect | |||||||
| Positive affect (–) | −8 | −78 | 24 | L cuneus | 1300 | 7.45 | .016 |
| Anxiety | |||||||
| Trait anxiety (+) | 62 | −32 | 44 | R SMG | 1501 | 6.11 | .008 |
| Emotion regulation | |||||||
| Cognitive reappraisal (+) | 9 | 9 | 32 | MCC/ACC | 2956 | 6.79 | .001 |
| 58 | 31 | 1 | R IFG | 1161 | 5.30 | .025 | |
Note
- Sample size: high E2 (n = 16), extreme E2 (n = 13).
- Abbreviations: (–), negative association; (+), positive association; IFG, inferior frontal gyrus; ITG, inferior temporal gyrus; L, left; MCC, middle cingulate cortex; MTG, middle temporal gyrus; R, right; ROL, rolandic operculum; SMG, supra marginal gyrus; STG, superior temporal gyrus.
3.4 Region of interest analysis
No significant group differences occurred for any of the a priori chosen ROIs between groups.
3.5 Behavioral variables
3.5.1 Cognitive performance
Performance on the trail making test differed significantly at the group level (TMT-A: H = 8.17, p = .017; TMT-B: H = 6.09, p = .048) with pregnant women showing a slower processing speed (TMT-A) compared to both naturally cycling groups (extreme E2 vs. high E2: U = 47.00, Z = −2.28, p = .023, r = .43; extreme E2 vs. low E2: 35.00, Z = −2.69, p = .006, r = .52) and compared to the low E2 group for cognitive flexibility (TMT-B) (extreme E2 vs. high E2: U = 62.00, Z = −1.579, p = .121; extreme E2 vs. low E2: U = 42.5, Z = −2.318, p = .019, r = .58), whereas no difference occurred between the high E2 and low E2 groups (all p > .227) (Figure 1B and Table 1; see also Supporting information, Table S1). No group difference emerged for verbal intelligence (H = .502, p = .776).
3.5.2 Subjective affect
A significant group effect for positive affect appeared (H = 16.02, p < .001), with the extreme E2 group experiencing a higher positive affect compared to the high E2 group (U = 55.00, Z = −2.16, p = .032, r = .40) and the low E2 group (U = 31.00, Z = −3.09, p = .002, r = .58) (Figure 1B). A trend appeared between the high E2 and low E2 groups (U = 74.5, Z = −1.82, p = .072, r = .33).
3.5.3 Emotion regulation
A trend for group differences for cognitive reappraisal (F2,43 = 2.63, p = .084, part-η2 = .114) was found with higher ratings in the low E2 compared to the extreme E2 group (p = .086), whereas no other differences appeared (all p > .415). No group difference emerged for emotional suppression (p > .382).
3.5.4 Anxiety
A trend for a group difference for trait anxiety occurred (F1,43 = 2.86, p = .067, part-η2 = 1.25), with a trend towards higher scores in the extreme E2 group compared to the low E2 group (p = .067), whereas no other differences appeared (all p > .463).
3.6 Regression analysis
Because group differences appeared for hormonal levels, processing speed, cognitive flexibility and positive affect, as well as for trait anxiety and cognitive reappraisal at trend level, we performed separate regression analyses using these variables as predictors for gray matter volume. As a result of the scope of the study, only results from the pregnant group for behavioral measures are reported (Figure 3 and Table 2) The results for the high E2 and low E2 groups, as well as results related to hormonal levels, are provided in the Supporting information (Table S1).
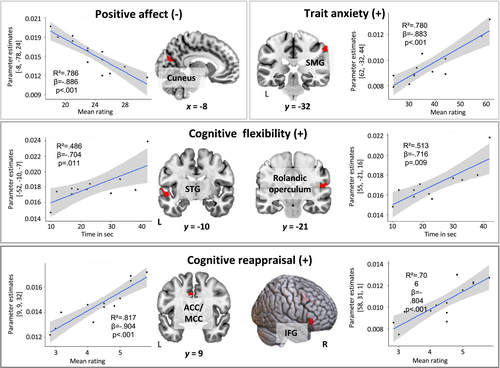
Cognitive flexibility (TMT-B) predicted gray matter volume of the left superior temporal gyrus (t13 = 8.57, FWE-cluster-corrected p = .002; r² = .486, β = −0.704, p = .011) and right rolandic operculum (t13 = 7.45, FWE-cluster corrected = .001; r² = .513, β = −0.716, p = .009). Processing speed (TMT-A) did not significantly predict gray matter volume.
Positive affect (PANAS) predicted gray matter volume of left cuneus (t13 = 7.45, FWE-cluster corrected p = .016; r² = .786, β = −0.886, p < .001).
Trait anxiety ratings (STAI-trait) predicted gray matter volume of the right supramarginal gyrus (t13 = 6.11, FWE-cluster corrected p = .008; r² = .780, β = −0.883, p < .001).
Cognitive reappraisal (ERQ) predicted gray matter volume of the right anterior- and middle cingulate cortex (ACC, MCC) (t13 = 6.79, FWE-cluster corrected p = .001; r² = .817, β = −0.904, p < .001) and the right inferior frontal gyrus (IFG) (t13 = 5.30, FWE-cluster corrected p = .025; r² = .706, β = −0.804, p < .001).
4 DISCUSSION
In the present study, we investigated gray matter volumes in healthy, first-time pregnant women (extreme E2 group) and their associations with hormones, cognition and affect. As a comparison group, we included naturally cycling women during the early follicular phase who either had experimentally increased E2 levels (high E2 group) or were taking a placebo pill (low E2 group). As expected, E2 levels differed significantly between all groups, with pregnant women having highest concentrations, followed by the high E2 group. In terms of brain structure, we found a significantly reduced volume of the left putamen in pregnant women compared to the high E2 group. We further observed a significant reduction in processing speed and cognitive flexibility, as well as higher positive affect, in pregnant women compared to both other groups. Our regression analyses showed associations between behavioral data and gray matter volume in pregnant women.
In terms of brain volume, we found reduced gray matter volume in the left putamen in pregnant women compared to women after E2 administration. This is similar to findings of a previous study that demonstrated reduced gray matter volume in the ventral striatum, consisting of the putamen and the caudate nucleus, when comparing primiparous women before and after pregnancy and a control group of nulliparous women.7 Similarly, a smaller volume of the left putamen was found in women 2 months after delivery compared to a nulliparous control group.43 Interestingly, the reduction in putamen volume is also associated with number of past childbirths.15 Together with the nucleus accumbens, the ventral striatum is part of a limbic loop that receives input from the orbital and medial prefrontal cortex and is involved in reward and emotional processing.44 Reduced gray matter size in the ventral striatum in postpartum women has been associated with higher brain activation towards cues of women's own infants.7 Similarly, a recent study also reported an increase in gray matter volume in the left caudate, a structure in close proximity to the putamen, when comparing primi-/multiparous women in the late to the early postpartum phase.45 Also, in animal studies, higher striatal activation has been associated with more adequate maternal caring behavior.46 Therefore, this anatomical reduction may prepare a woman for her future role as mother. Underlying mechanisms for structural changes in the brain during pregnancy could be versatile and may include neuroplastic changes. For example, synaptic pruning, which involves the elimination of infrequently used synapses to increase efficiency plays an important role during puberty and has been associated with a reduction in brain size.47 Notably, we did not see significant differences in gray matter volume between the pregnancy and the low E2 group. A potential explanation for the lacking difference stems from the assumption of non-linear relationships between sex hormones and brain volumes that rather fit an inverted U-shape leading to decreases in synaptogenesis and cell proliferation at very low and very high hormonal levels.48 By contrast to our first hypothesis, we saw no differences in gray matter volume in the prefrontal, parietal, or midline structures. However, because we based these hypotheses on previous studies performed during the postpartum period or comparing pre- vs. postpartum brain volumes5-7, 11 these changes may only occur later in pregnancy or close to term, as also suggested by Oatridge et al.5 Therefore, to capture the time-related changes of brain architecture during pregnancy, future studies should apply a longitudinal design with several measurement time points, both during and after pregnancy. Previous studies did not consistently assess and report hormonal levels of their participants including the nulliparous control groups. Because we only measured female participants during the early follicular phase but experimentally manipulated E2 levels, our nulliparous control sample most potentially differs in hormonal levels to previous nulliparous samples.
By contrast to our second hypothesis, we also did not find significant differences in gray matter volume of the hippocampus between the low and high E2 groups. We based this hypothesis on a study comparing women during the early and late follicular phase of the menstrual cycle.3 The lack of significant effect differences may be related to the reduced time span of exposure to high E2 levels in our study compared to the steady increase of E2 in the late follicular phase up to ovulation.
Related to cognitive performance, we observed a reduction in processing speed and cognitive flexibility in pregnant women that is in good agreement with the results of a previous study.25 Similarly, in a navigation task, pregnant women showed worse performance from an egocentric perspective compared to controls.43 Notably, these results are in contrast to animal studies showing improved spatial cognition during the second trimester.49 Fluctuations in sex hormones have often been associated with changes in mood and emotional processing.50, 51 In the present study, we observed significantly higher positive affect ratings in the pregnant compared to naturally cycling women.
Several significant regressions between behavioral measures and gray matter volume in pregnant women were revealed. A positive association between cognitive flexibility (TMT-B) and gray matter volume of the right rolandic operculum and left superior temporal gyrus was found. This is in good agreement with previous functional activation results indicating that the superior temporal gyrus is particularly involved in performing the TMT-B.52 The rolandic operculum has been associated with a wider range of cognitive, sensory and motor processes, which also involves the use of verbalization during cognitive tasks.53 Positive affect ratings were negatively associated with size of the left cuneus. Activation of the cuneus has been observed during tasks of emotion processing and cognitive empathy, which plays an is important role in understanding others.54, 55 Anxiety trait ratings were positively associated with volume in the right supramarginal gyrus. Accordingly, studies have linked anxiety personal traits with an increase in supramarginal activation towards anxiety provoking stimuli.56 Cognitive reappraisal, an emotion regulation strategy that allows reconstrual of the meaning and amount of perceived threat in an emotion eliciting situation has been associated with psychological well-being and good interpersonal relationships.57, 58 Here, we observed a positive association with gray matter volume of the right IFG and ACC/MCC. Especially coupling between these prefrontal areas with the amygdala has been associated with successful emotion regulation.59 These associations between brain structure and both cognitive and affective measures provide a first insight into changes in brain networks during pregnancy that are involved in cognition and emotion and may later be important for caretaking.
4.1 Limitations
Age differed significantly between pregnant women and the naturally cycling groups. Although we included age as a covariate, we cannot fully exclude age effects on brain volumes. The sample size was rather small; however, to the best of our knowledge, it represents the largest sample size including healthy pregnant women undergoing MRI so far. Hormonal levels in the present study were analyzed using the standard ELISA procedure. To improve sensitivity, future studies may also want to use state-of-the-art methods such as liquid chromatography coupled with tandem mass spectrometry. To obtain a better overview of time related-changes in brain structure as a result of pregnancy, future studies may want to compare the brain architecture of pregnant women during different trimesters, as well as longitudinal data, including pre-, peri- and postpartum measures. On a methodological basis, future studies may want to run the application of multivariate pattern classification to assess the reliability of the data. Moreover, another interesting aspect may be to compare brain structure between primi- and multiparous women, as well as investigate different brain networks important for cognition and emotion.
5 CONCLUSIONS
We found a reduced gray matter volume of the left putamen in pregnant women. Moreover, pregnant women not only showed reduced processing speed and cognitive flexibility, but also reported a higher positive affect. Subjective affect and cognitive performance are further related to brain volume in pregnant women, indicating possible adaptation processes. Our study emphasizes that brain plasticity continues throughout a woman's life and may be part of an adaptive process to prepare for the future role of nurturing and taking care of a baby.
ACKNOWLEDGMENTS
This study was funded by the Center for Integrative Neuroscience, Tübingen (CIN, EXC307, MRTG Pregnancy & the Brain). Open access funding enabled and organized by ProjektDEAL.
CONFLICT OF INTERESTS
The authors declare that they have no conflicts of interest.
AUTHOR CONTRIBUTIONS
Elisa Rehbein: Data curation; Formal analysis; Investigation; Visualization; Writing – original draft. Lydia Kogler: Methodology; Supervision; Validation; Writing – review & editing. Raviteja Kotikalapudi: Methodology; Software; Writing – review & editing. Anna Sattler: Data curation; Writing – review & editing. Marina Krylova: Data curation; Writing – review & editing. Karl Oliver Kagan: Writing – review & editing. Inger Sundström-Poromaa: Writing – review & editing. Birgit Derntl: Conceptualization; Funding acquisition; Project administration; Supervision; Validation; Writing – review & editing.
Open Research
DATA AVAILABILITY STATEMENT
The datasets used in the present study are available from the corresponding authors upon reasonable request.




