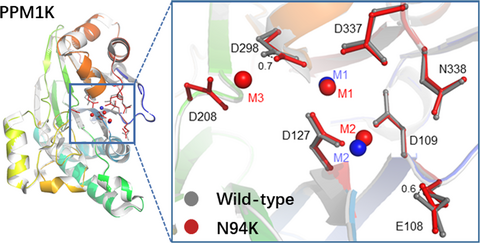
Crystal structure and catalytic activity of the PPM1K N94K mutant
Abstracts
Protein Phosphatase Mg2+/Mn2+-Dependent 1K (PPM1K),also named as PP2Cm or branched-chain α-ketoacid dehydrogenase complex phosphatase, is a member of the metal-dependent phosphatase family and an important metabolic regulator. Single nucleotide polymorphisms (SNPs) in PPM1K contributing to protein functional defects have been found to be associated with numerous human diseases, such as cardiovascular disease, maple syrup urine disease, type 2 diabetes, and neurological disease. PPM1K N94K is an identified missense mutant produced by one of the SNPs in the human PPM1K coding sequence. However, the effects of the N94K mutant on its activity and structural property have not been defined. Here, we performed a detailed enzymological study using steady-state kinetics in the presence of pNPP or phospho-peptide substrates and crystallographic analyses of the wild-type and N94K PPM1K. The PPM1K-N94K significantly impaired its Mg2+-dependent catalytic activity and structural analysis demonstrated that the N94K mutation induced a conformational change in the key residue in coordinating the Mg2+ in the active site. Specifically, three Mg2+ were located in the active site of the PPM1K N94K instead of two Mg2+ in the PPM1K wild type. Therefore, our results provide a structure basis for the metal ion-dependent PPM1K-N94K phosphatase activity.
Abbreviations used
-
- ALS
-
- amyotrophic lateral sclerosis
-
- BCAA
-
- branched-chain amino acid
-
- BCKA
-
- branched-chain α-ketoacid
-
- BCKD
-
- branched-chain α-ketoacid dehydrogenase
-
- BCKDE1α
-
- branched-chain α-ketoacid decarboxylase (E1) alpha subunit
-
- BDP
-
- branched-chain α-ketoacid dehydrogenase phosphatase
-
- BSA
-
- bovine serum albumin
-
- E. coli
-
- Escherichia coli
-
- E1b
-
- branched-chain α-ketoacid decarboxylase/dehydrogenase
-
- EDTA
-
- Ethylene-diaminetetraacetic acid
-
- HEPES
-
- 4-(2-hydroxyethyl)-1-piperazine-ethanesulfonic acid
-
- IPTG
-
- isopropyl β-D-1-thiogalactopyranoside
-
- LB
-
- Luria-Bertani broth
-
- MSUD
-
- maple syrup urine disease
-
- PCR
-
- polymerase chain reaction
-
- p-E1b
-
- phospho-E1b
-
- PEG
-
- polyethylene glycol
-
- PMSF
-
- phenylmethane sulfonyl fluoride
-
- pNPP
-
- p-nitrophenyl phosphate
-
- PP2Cm
-
- PP2C phosphatase of mitochondrial matrix residence
-
- PPM1A
-
- protein phosphatase Mg2+/Mn2+-Dependent 1A
-
- PPM1G
-
- protein phosphatase Mg2+/Mn2+-Dependent 1G
-
- PPM1K
-
- protein phosphatase Mg2+/Mn2+-Dependent 1K
-
- PPM
-
- metal-dependent protein phosphatase
-
- RRID
-
- research resource identifier
-
- SDS-PAGE
-
- sodium dodecyl sulfate–polyacrylamide gel electrophoresis
-
- TEV
-
- tobacco etch virus
-
- TRIS
-
- tris(hydroxymethyl)aminomethane
-
- WT
-
- wild type
Protein Phosphatase Mg2+/Mn2+-Dependent 1K (PPM1K), also known as branched-chain α-ketoacid dehydrogenase complex phosphatase, is a member of the metal-dependent protein phosphatase (PPM) family, and an important metabolic regulator located within the mitochondria that modulate a plethora of fundamental biological activities. PPM1K deficiency has been reported to closely correlate with type 2 diabetes, obesity, and cardiovascular and neurological disease. For instance, PPM1K gene polymorphisms that reduce PPM1K level in pancreatic β cells significantly reduced insulin secretion, and are associated with type 2 diabetes and obesity (Kettunen et al. 2012; Taneera et al. 2012; Xu et al. 2013). PPM1K expression was significantly decreased in the myocardium of the heart failure patients and inactivated PPM1K in zebrafish embryos led to aberrant neural and heart development (Lu et al. 2007).
Moreover, mounting evidence has shown that branched-chain amino acid tightly regulated by the branched-chain α-ketodehydrogenase (BCKD) has a critical function in the maintenance of correct brain function. Disorders in branched-chain amino acids homeostasis cause multiple neurological abnormalities such as toxicity and deterioration, encephalopathy, cerebral edema, inflammation, autism, epilepsy, intellectual disability, Alzheimer's disease, and seizures (Joshi et al. 2006; Novarino et al. 2012; Garcia-Cazorla et al. 2014; Conway and Hutson 2016; Scaini et al. 2017, 2018; Gruenbaum et al. 2018; Hull et al. 2018; Li et al. 2018). As PPM1K is a critical regulator of branched-chain α-ketoacid, through direct dephosphorylation of the pS293 site of the E1α subunit of the BCKD complex (Lu et al. 2009), several studies have linked PPM1K defects with neurological diseases in both rodents and humans (Cronin et al. 2009; Oyarzabal et al. 2013). Recently, a number of human PPM1K single nucleotide polymorphisms (SNPs) have been identified in the public database which have been reported to produce PPM1K defects, inducing inactive or constitutively active mutants. A frameshift mutation c.417_418TA (p.His139 fs) of PPM1K is highly associated with maple syrup urine disease that leads to severe neurological defects (Oyarzabal et al. 2013). Another SNP (rs10024717) of PPM1K is associated with sporadic amyotrophic lateral sclerosis in a genome-wide association study (Cronin et al. 2009). These studies suggest that the genetic polymorphism and activity of PPM1K play a pivotal role in the central nervous system.
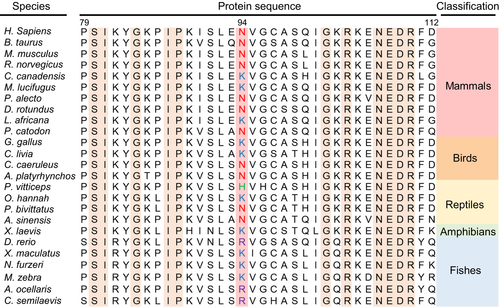
PPM1K N94K (rs17853762) is an identified missense mutant produced by one of the SNPs in the human PPM1K coding sequence (Zhou et al. 2012). Recently, in a broad survey of PPM1K homologues among different species, we found that the residue Asn94 (in H. sapiens) is prevalent among mammals, birds, and reptiles, but the Lys94 variant is less prevalent and only detected in some species, such as C. canadensis, M. lucifugus, L. africana, G. gallus, C. livia, and O. hannah. However, the Lys94 variant is prevalent among amphibians and fishes (Fig. 1), suggesting that the Asn94 may possess evolutionarily conserved but diverse functions across different species. However, the effects of the N94K mutant on its activity and structural property have not been defined.
In this study, we investigated the catalytic and structural property of the N94K mutant of PPM1K. Whereas the PPM1K-N94K mutant displays normal activity toward pNPP and a phospho-segment derived from the pS293 site of the branched-chain α-ketoacid decarboxylase (E1) alpha subunit (BCKDE1α) using Mn2+ as the catalytic ion, it significantly impaired its catalytic activity using Mg2+ as the catalytic ion. The crystallographic study demonstrated that the N94K mutation caused the conformational change in the key residue in coordinating the Mg2+ in the active site. Specifically, three Mg2+ were located in the active site instead of two Mg2+ in the wild type. Our results provide a structure basis for the metal ion-dependent PPM1K-N94K phosphatase activity.
Materials and methods
Constructs
For enzymological analysis, cDNA encoding human recombinant PPM1K (residues 30-372) with Hexahistidine (His6) and a TEV recognition site at the C-terminal was subcloned into the pET-15b vector by sequence and ligation independent cloning method, resulting in plasmid pET-15b-PPM1K30-372-TEV. Briefly, the pET-15b vector was linearized by inverse PCR using specific primers oMD-1 and oMD-2. The PPM1K (30-372) insert was prepared with primers o-MD3 and oMD-4 using PPM1K cDNA as a template to generate an amplicon with 15-bp overlapping flanking regions homologous to each end of the linearize pET-15b vector. The linearized vector and PPM1K insert were treated with T4 DNA polymerase and mixed with a 1:5 molar ratio (vector/insert). The mixture was transformed into Escherichia coli DH5α to produce pET-15b-PPM1K30-372. The TEV recognition site was introduced by PCR using pET-15b-PPM1K30-372 as the template with primers oMD-5 and oMD-6, resulting in pET-15b-PPM1K30-372-TEV. For the crystallographic study, a truncated version of PPM1K (residues 84–360) was constructed using pET-15b-PPM1K30-372 as the template by Quikchange site-directed mutagenesis (Wynn et al. 2012) using primers oMD-7 and oMD-8, resulting in plasmid pET-15b-PPM1K84-372. The N94K mutation was introduced both into pET-15b-PPM1K30-372 and pET-15b-PPM1K84-372 using primers oMD-9 and oMD-10, respectively. All constructs were verified by DNA sequencing (BioSune Company, Shanghai, China). The key resources and oligonucleotide primers used in this study are listed in Tables 1 and 2.
| Reagent or resource | Source | Identifier |
|---|---|---|
| Bacterial strains | ||
| E. coli BL21 (DE3) | Transgen Biotech (Beijing, China) | Cat# CD601 |
| E. coli DH5α | Transgen Biotech | Cat# CD201 |
| Chemicals | ||
| Para-nitrophenyl phosphate (pNPP) | Solarbio (Beijing, China) | Cat# N8150 |
| Polyethylene glycol (PEG Mol. Wt. 3350) | Solarbio | Cat# P8040 |
| BIOMOL green reagent (BML-AK111) | Enzo Life Sciences (New York, NY, USA) | Cat# 89156-426 |
| Ampicillin | Solarbio | Cat# A8180 |
| Beta-mercaptoethanol | VWR Life Science (Radnor, PA, USA) | Cat# 97064-588 |
| Triton X-100 | Sangon Biotech (Shanghai, China) | Cat# A110694-0500 |
| Glycerol | Sinopharm Chemical reagent | Cat# 10010618 |
| Acrylamide 30% | MDBio | Cat# L00529 |
| PMSF | Solarbio | Cat# P8340 |
| SDS | Sangon Biotech | Cat# A500228-0001 |
| Benzamidine | Aladdin | Cat# B100967 |
| Ni-NTA resin | Roche | Cat# 5893682001 |
| Imidazole | BBI Life Sciences | Cat# A600277-0500 |
| Coomassie brilliant blue | Solarbio | Cat# C8420 |
| EDTA | Sangon Biotech | Cat# A100105 |
| HEPES | Sangon Biotech | Cat# A100511 |
| TRIS-HCl | BBI Life Sciences | Cat# A600194 |
| MnCl2 | Sinopharm Chemical reagent | Cat# 20026118 |
| KCl | Sigma-Aldrich (St.Louis, MO, USA) | Cat# P3911 |
| MgCl2 | Aladdin | Cat# M116335 |
| IPTG | BBI Life Sciences | Cat# A600168-0100 |
| Vector | ||
| pET-15b | Novagen (EMD Millipore) | Cat# 69661-3 |
| Peptide | ||
| Phospho-peptide | China Peptides Co. Ltd (Shanghai, China) | Prod# 04010011544 |
| Columns | ||
|
Superdex 200 Increase 10/300 GL |
GE Healthcare, Life Sciences | Prod# 28990944 |
| HiTrap Q FF | GE Healthcare, Life Sciences | Prod# 17505301 |
| Software | ||
| HKL-3000 | (Minor et al. 2006) | RRID: SCR_015023 |
| CCP4 Ver.7 | (Winn et al. 2011) | RRID: SCR_014219 |
| COOT 0.8.7.1 | (Emsley et al. 2010) | RRID: SCR_014222 |
| PHENIX 1.11.1-2575 | (Afonine et al. 2012) | RRID: SCR_014224 |
| GraphPad Prism 6 | GraphPad Software, La Jolla, CA, USA | RRID: SCR_002798 |
| T-Coffee | (Notredame et al. 2000) | RRID: SCR_011818 |
| PyMol 1.6 | The PyMOL Molecular Graphics System, Schrödinger, LLC) | RRID: SCR_000305 |
| Deposited data | ||
| Coordinates and Structural factors | This study | PDB: 6AK7 |
| Instruments | ||
| Cell disrupter |
JNBIO JN-300 Plus |
Prod# 3130071 |
| PCR thermal cycler |
Bio-Rad T100™ |
S/N# 621BR28844 |
| Spectrophotometer, microplate reader | Thermo Scientific™ Multiskan™ FC (Shanghai, China) | Cat# 51119000 |
| Microspectrophotometer | Darwell, K5600 | HS Code: 9027300000 |
| Microcentrifuge | Labnet | S/N# D004343 |
|
Centrifuge (High speed) |
Thermo Scientific™ Sorvall™ RC 6 Plus |
Cat# 46910 |
| Centrifuge |
Anke DL-5200B |
Prod# 20150193 |
| Desktop shaking incubator |
Suzhou Peiying DDHZ-300 |
Factory# 09008 |
| Shaking incubator |
ZhiChu ZQZY-BS |
Prod# S2016041523 |
| Primer | Sequence (5′-3′) | Description |
|---|---|---|
| oMD-1 | TAGCTCGAGGATCCGGCTGCTAACAAAG | Forward Primer to make pET-15b-PPM1K30-372 using pET-15b as a template |
| oMD-2 | GGATTGGAAGTACAGGTTCTCATGGCTGC | Reverse Primer to make pET-15b-PPM1K30-372 using pET-15b as a template |
| oMD-3 | CCGCGCGGCAGCCATATG GACGACAGGCG | Forward Primer to make pET-15b-PPM1K30-372 using PPM1K as a template |
| oMD-4 | CAGCCGGATCCTCGAGCTAGGCCCATCGTCCAC | Reverse Primer to make pET-15b-PPM1K30-372 using PPM1K as a template |
| oMD-5 | GCAGCCATGAGAACCTGTACTTCCAATCCATGGACGACAGGC | Forward Primer to insert TEV into pET-15b-PPM1K30-372 using PPM1K as a template |
| oMD-6 | CTGTCGTCCATGGATTGGAAGTACAGGTTCTCATGGCTGC | Reverse Primer insert TEV into pET-15b-PPM1K30-372 using PPM1K as a template |
| oMD-7 | CTGTACTTCCAATCCGGCAAGCCAATTCCCAAAATCAGC | Forward Primer to make pET-15b-PPM1K84-360 using PPM1K as a template |
| oMD-8 | CGGATCCTCGAGCTATGAGAAGTTGATTTCAGAGTTC | Reverse Primer to make pET-15b-PPM1K84-360 using PPM1K as a template |
| oMD-9 | ATCAGCTTGGAAAAGGTGGGGTGCGCCTCACAGATTG | Forward Primer to make PPM1K N94K mutation |
| oMD-10 | GGCGCACCCCACCTTTTCCAAGCTGATTTTGGGAATTG | Reverse Primer to make PPM1K N94K mutation |
- All oligonucleotide primers were synthesized by TSINGKE Biological Technology (Beijing, China).
Expression and purification of PPM1K
The expression and purification of PPM1K were performed as described previously with some modifications (Wynn et al. 2012). Briefly, vectors containing wild-type and mutant PPM1K were transformed into E. coli BL21 (DE3) strain. A clone was inoculated into 200 mL of LB culture media containing 100 μg/mL ampicillin and allowed to grow overnight with shaking at 37°C. Then, 5 mL of overnight culture was transferred to 1 L of LB medium containing 50 μg/mL ampicillin and cultured at 37°C and 220 rpm until absorbance at 600 nm between 1.0 and 1.2 was reached. The expression of the protein was induced by the addition of isopropyl β-D-1-thiogalactopyranoside to a final concentration of 0.3 mM at 17°C overnight. The bacterial cells were harvested by centrifugation at 2100 g at 4°C. The cell pellets were resuspended in 50 mL of pre-cooled ‘lysis buffer’ (20 mM Tris-HCl (pH 7.9), 100 mM NaCl, 5 mM beta-mercaptoethanol, 0.625% Triton X-100, 10% glycerol, 1 mM phenylmethane sulfonyl fluoride, and 1 mM benzamidine) and crushed five times by passing through a high-pressure cell cracker at 1300 psi. The lysates were then centrifuged at 13 000 g at 4°C to remove the insoluble cell debris, and the supernatant was incubated with 10 mL of pre-equilibrated Ni-NTA resin (Roche Molecular Biochemicals, Indianapolis, IN, USA) at 4°C for 2 h with end-to-end rotation. The resin was transferred to an empty column and washed with 10-bed volume of ‘PPM1K buffer’ containing 20 mM Tris-HCl (pH 7.5), 100 mM NaCl, 5% glycerol, and 10 mM beta-mercaptoethanol. The immobilized protein was subsequently eluted with the same buffer containing 100 mM imidazole. The elution was dialyzed against PPM1K buffer to remove imidazole and loaded to a HiTrap Q Sepharose Fast flow ion exchange column (GE Healthcare, Beirut, Lebanon) equilibrated in 20 mM Tris-HCl (pH 7.5), 50 mM NaCl, 5% glycerol, and 10 mM beta-mercaptoethanol. PPM1K was eluted using 0–600 mM NaCl gradient. The concentrated fractions were further applied to a Superdex™ 200 Increase 10/300GL column (GE Healthcare, Beirut, Lebanon) equilibrated with buffer containing 20 mM Tris-HCl (pH 7.5), 5% (v/v) glycerol, 10 mM beta-mercaptoethanol, and 100 mM NaCl (for enzymological analysis) or 100 mM KCl (for crystallographic experiment). The protein purities and concentrations were evaluated at OD260/OD280 followed by Coomassie brilliant blue staining after running the protein with a range of bovine serum albumin standard protein concentrations in sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The protein purities were finally determined to be at least 95% pure.
Steady-state kinetics using pNPP as the substrate
To determine the intrinsic activities of PPM1K-WT and its N94K mutant toward pNPP, assays were performed as previously described with minor modifications (Wynn et al. 2012; Wang et al. 2014; Li et al. 2016). Briefly, all assays were performed at 37°C in 96-well plates with 0.1 mL of a reaction buffer containing 20 mM Tris-HCl (pH 7.5), 7 mM MnCl2, and 1 mM DTT (DL-Dithiothreitol). The initial rates were measured at appropriate pNPP concentrations (0.2–5 Km). The reactions were initiated by adding purified PPM1K proteins and terminated by the addition of 500 mM EDTA (pH. 8.0). Activities were assessed by monitoring the absorbance of pNP at 405 nm. The increase in absorbance at 405 nm was determined with a Multiskan -FC spectrophotometer (Thermo Fisher Scientific, Inc) against a blank reaction without enzyme. The kinetic parameters were obtained by fitting the data to the Michaelis-Menten equation using GraphPad Prism 6 software (GraphPad Software Inc., La Jolla, CA, USA). To determine the kcat and Kmetal values toward Mn2+ and Mg2+, Mn2+ and Mg2+ concentrations ranging from 0.2 to 5 Kmetal were used under saturating concentrations of pNPP.
Phosphatase activity assays using phospho-peptide as the substrate
The PPM1K-catalyzed hydrolysis of phospho-E1b peptide was determined with an inorganic phosphate assay as previously described (Wynn et al. 2012; Pan et al. 2013, 2015). The dephosphorylation reactions were performed at 37°C in 96-well plates with 60 μl of a reaction buffer containing 50 mM HEPES (pH 7.5), 7 mM MnCl2, and 100 mM KCl. The phospho-E1b peptide (NH2-Ile-Gly-His-His-Ser(P)-Thr-Ser-Asp-Asp-COOH) at appropriate concentrations was incubated with PPM1K or its N94K mutant and the reactions were halted by addition 60 μL of BIOMOL GREEN reagent (Enzo Life Science). After incubation at 37°C for 20 min, the release of phosphate was determined by the end-point reading at 620 nm using a Multiskan FC spectrophotometer (Thermo Fisher Scientific, Inc) against a blank reaction without enzyme. The kcat and Km values were obtained by fitting the data to the Michaelis-Menten equation using GraphPad Prism 6 software (GraphPad Software Inc., La Jolla, CA, USA).
Crystallization of PPM1K-N94K
The crystallization of PPM1K-N94K (84-360) was performed by vapor diffusion in hanging drops as previously described (Wynn et al. 2012; Wang et al. 2014; Xiao et al. 2014). Briefly, the protein stock solution was prepared by concentrating the PPM1K-N94K protein to 30–35 mg/mL in a buffer containing 20 mM Tris-HCl (pH 7.5), 100 mM KCl, 5% glycerol, and 10 mM β-mercaptoethanol. Crystallization was performed by mixing 3 μl of PPM1K-N94K stock solution with 3 μl of precipitating solution (19% (w/v) polyethylene glycol 3350, 0.2 M MgCl2, 0.1 M Tris-HCl, pH 8.5, and 10 mM β-mercaptoethanol. Seeding technique was used to induce nucleation in 12% (w/v) polyethylene glycol in the same condition. After 1 week of incubation at 20°C, crystals were soaked in a cryoprotectant of well solution supplemented with a dilution range of 5–20% (v/v) glycerol and flash-frozen in liquid nitrogen.
Data collection and structure determination
Diffraction data were collected at the Shanghai Synchrotron Radiation Facility (BL18UI) (Wang et al. 2014; Li et al. 2016). The crystal diffracted to 2.6 Å, and 180 frames were collected with a crystal to detector distance set at 300 mm with 1.0° oscillation steps and 5-s exposure time per frame (Winter and McAuley 2011). Data processing and reduction were performed using the HKL-3000 program suite (Minor et al. 2006). Initial phases were determined by molecular replacement using Phaser 2 in the CCP4 7 software package with the crystal structure of mitochondrial PPM1K (PDB ID: 4DA1) as a template (Winn et al. 2011; Scapin 2013). Model building and structure refinement were executed using COOT, PHENIX (Emsley et al. 2010; Afonine et al. 2012).
Data analysis
No randomization, blinding, or sample calculation was performed in this study and the study was not pre-registered. For all experiment, at least three independent experiments were performed. Both the normality and outliers of data were checked using SPSS (SPSS Inc. USA). The data were normally distributed using the Kolmogorov-Smirnov (K-S) test and the Shapiro-Wilk normality tests and there were no outliers in the dataset using Maximum-likelihood estimators (M-estimators). Values were presented as the mean ± SEM. Statistical comparisons were performed with a one-way anova test using GraphPad Prism 6 software (GraphPad Software Inc., La Jolla, CA, USA).
Sequence accession numbers
Homo sapiens PPM1K DNA and Protein sequences are available in Genbank with accession numbers NM_152542.4 and NP_689755.3, respectively. For homologous sequence alignment of PPM1K from different species, protein sequences were retrieved from GenBank under the following accession numbers: NP_689755.3 (H. sapiens), XP_005207855.1 (B. taurus), NP_780732.1 (M. musculus), NP_001101333.1 (R. norvegicus), XP_020041778.1 (C. canadensis), XP_006084667.1 (M. lucifugus), XP_015443981.1 (P. alecto), XP_024430669.1 (D. rotundus), XP_003414133.1 (L. africana), XP_007102591.1 (P. catodon), XP_420574.3 (G. gallus), XP_005509142.1 (C. livia), XP_023782073.1 (C. caeruleus), XP_005031201.1 (A. platyrhynchos), XP_020652146.1 (P. vitticeps), ETE63819.1(O. hannah), XP_007439323.1(P. bivittatus), XP_025053052.1(A. sinensis), NP_001085111.1 (X. laevis), NP_001314673.1 (D. rerio), XP_005797651.1 (X. maculatus), XP_015820947.1 (N. furzeri), XP_004549421.1 (M. zebra), XP_023147245.1 (A. ocellaris), and XP_008314609.1 (C. semilaevis).
Results
The PPM1K N94K mutant maintains Mn2+-dependent phosphatase activity toward pNPP as substrate
We expressed the catalytic domain (30-372) of wild-type and N94K PPM1K in E. coli BL21 and purified these proteins to homogeneity as previously reported (Fig. S1) (Damuni et al. 1986; Damuni and Reed 1987; Wynn et al. 2012; Zhou et al. 2012). Compared to the wild-type PPM1K, the PPM1K-N94K mutant exhibited slightly decreased activity toward small artificial substrate pNPP, using Mn2+ as the catalytic ion, with a decreased kcat (approximately 0.3-fold) (Table 3). As the catalytic metal ion can be regarded as a pseudo-substrate of metal-dependent phosphatases, we also fitted the catalytic activity of wild-type PPM1K and PPM1K-N94K mutant with different Mn2+ concentration to Michaelis-Menten equation. The PPM1K-N94K mutant displays a decreased value for both kcat (0.4-fold) and Kmetal (0.2-fold) compared to the wild-type PPM1K (Table 4), indicating a slighter tighter binding of Mn2+.
| Enzyme | pNPP | ||
|---|---|---|---|
| kcat (per s) | Km (mM) | kcat/Km (103/M/s) | |
| PPM1K-WT | 40.9 ± 1.0 | 4.2 ± 0.1 | 9.7 ± 0.7 |
| PPM1K-N94K | 29.2 ± 1.7 | 3.8 ± 0.2 | 7.7 ± 0.4 |
- The assays were performed in 20 mM Tris-HCl (pH 7.5), 7 mM MnCl2, and 1 mM DTT (DL-Dithiothreitol) at 30°C. The values of kcat and Km were derived by the fitting of the data to the Michaelis-Menten equation. For each individual assay, the pNPP concentration was adjusted to cover the range 0.2–5 Km. Data are presented as means ± SEM from three independent experiments (n = 3, n=number of independent experiments).
| Enzyme | Metals | Metal ion (Mn2+/Mg2+) | |
|---|---|---|---|
| kcat (s−1) | Kmetal (mM) | ||
| PPM1K-WT | Mn2+ | 40.9 ± 1.0 | 4.8 ± 0.2 |
| PPM1K-N94K | Mn2+ | 22.9 ± 0.04 | 3.8 ± 0.2 |
| PPM1K-WT | Mg2+ | 1.0 ± 0.02 | 50.9 ± 3.5 |
| PPM1K-N94K | Mg2+ | 0.5 ± 0.02 | 39.8 ± 3.1 |
- All assays were performed in 20 mM Tris-HCl (pH 7.5) and 1 mM DTT (DL-Dithiothreitol) with saturating levels of pNPP at 30°C and 37°C for measurement of kinetic parameters of Mn2+ and Mg2+, respectively. Data are presented as means ± SEM from three independent experiments (n = 3, n=number of independent experiments).
The PPM1K N94K mutant decreased Mg2+-dependent phosphatase activity toward pNPP as substrate
In addition to Mn2+, Fe2+, Mg2+, Co2+, and Ni2+ are able to stimulate the phosphatase activity of PPM family members (Pan et al. 2013). We then measured the catalytic activity of wild-type PPM1K and its N94K mutant toward pNPP using Mg2+ as the catalytic ion. Both wild-type and mutant PPM1K exhibited moderate catalytic activity (Table 4). Intriguingly, the PPM1K-N94K mutant significantly impaired its catalytic ability toward pNPP when using Mg2+ as the catalytic ion as its kcat value decreased approximately by twofolds compared to wild-type PPM1K (Table 4). In addition, PPM1K-N94K mutant exhibited a slight decrease in Kmetal (approximately 0.2-fold) (Table 4), indicating a higher binding affinity to Mg2+.
Crystal structure of the PPM1K-N94K mutant indicated the presence of the third Mg2+ in the active site
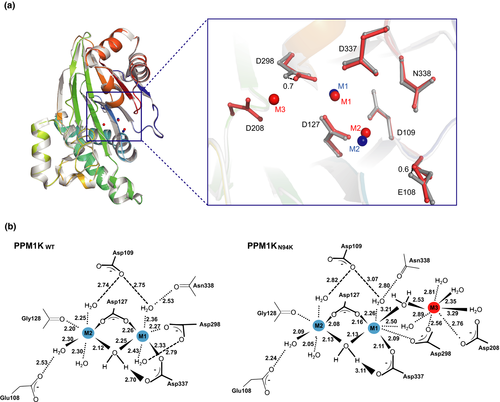
To understand the molecular mechanism underlying how N94K affect the catalytic activity of PPM1K, we expressed the truncated version of PPM1K-N94K (84-360) (Fig. S1). The successful substitution of PPM1K Asn94 with Lysine was confirmed by tandem mass spectrometry analysis (Fig. S2). The PPM1K-N94K (84-360) was crystallized in a condition similar to the wild type, diffraction quality crystals were obtained only in the presence of Mg2+ but not Mn2+. Similar to wild-type PPM1K, the space group of PPM1K-N94K is P3121 and the structure was solved at 2.6 Å resolution with a single molecule in one asymmetric unit (Table 5). Although the replacement of Asn94 with Lys did not disturb the local residue coordination in the PPM1K-N94K crystal (Fig. S3), two important metal ion–coordinated catalytic residues, including the D298 and E108, shift toward the active site by 0.7 Å and 0.6 Å, respectively (Fig. 2A). Whereas residues direct coordinate to the two conserved metal ion 1 and metal ion 2, including D127, G128, D298, and D337, are maintained, a water bridging the metal ion 1 and D298 is not visible. Moreover, the crystal structure unambiguously identified the third metal ion (M3) in the active site, which was coordinated by D208, D298, and several water molecules (Figs. 2B and S4). These observed Mg2+ coordination changes in the active site could provide a structural basis for the decreased Mg2+-dependent phosphatase activity of PPM1K-N94K compared to the wild-type PPM1K.
| PPM1K-N94K | |
|---|---|
| Data collection | |
| Space group | P3121 |
| Cell dimensions | |
| a (Å) | 122.784 |
| b (Å) | 122.784 |
| c (Å) | 61.58 |
| α (deg) | 90 |
| β (deg) | 90 |
| γ (deg) | 120 |
| Resolution |
50–2.6 (2.60 –2.69)a |
| Unique observations | 16 613 (1624) |
| Completeness (%) | 99.60 (98.72)a |
| Redundancy (σ) | 9.4 (9.8) |
| R merge | 0.3 (1.69)a |
| Structure refinement | |
| Resolution (Å) | 35.45–2.61 |
| Reflections used in refinement | 16 568 (1615) |
| Rwork/Rfreeb (%) | 0.19/22 |
| RMSD ideal bonds (Å) | 0.008 |
| RMSD ideal angles (deg) | 1.21 |
| Ramchandran plot (%) | |
| Most favored | 98.04 |
| Allowed | |
| Generously allowed | 1.96 |
| Disallowed | 3.3 |
- a Each dataset was obtained from a single crystal.
- The values in parentheses correspond to the highest resolution shell. RMSD, root-mean-square deviation.
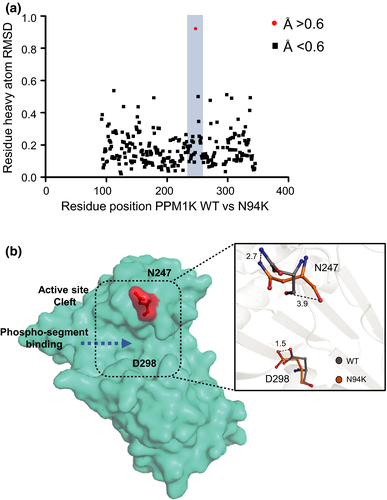
A potential structural basis for substrate specificity of PPM1K-N94K
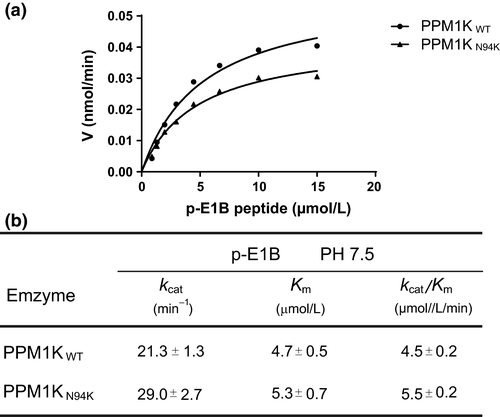
Similar to all other PPM phosphatase member, the PPM1K wild-type and PPM1K-N94K harbor a catalytic cleft, which contains residues that may contribute to their substrate specificity. In addition to D298, we identified that N247 in the catalytic cleft of N94K undergoes a larger-scale conformational change with root-mean-square deviation value of 1.0 in PPM1K-N94K mutant relative to other residues, compared to the PPM1K wild type (Fig. 3A and B). We, therefore, measured the phosphatase activities of wild-type PPM1K and N94K mutant toward a phospho-segment derived from the pS293 site of the BCKDE1α. Whereas the PPM1K-N94K only displays a slightly higher kcat/Km toward phospho-pS293- BCKDE1α segment compared with wild-type PPM1K (Fig. 4A and B) that is similar to previously published results by cellular studies (Zhou et al. 2012), it does not exclude the observed conformational change as a result of N247 that contribute to the substrate specificity of PPM1K-N94K toward another protein substrate of PPM1K.
Discussion
In this study, we measured the phosphatase activity of PPM1K-N94K mutant toward small artificial substrate pNPP and a phospho-segment derived from the pS293 site of the BCKDE1α. Whereas the Mn2+-dependent phosphatase activity of PPM1K-N94K using pNPP as the substrate was slightly decreased when compared to wild type, the catalytic activity toward phospho-segment is slightly elevated (Table 3 and Fig. 4b). These observations are similar to previously published results by cellular studies (Zhou et al. 2012). Most strikingly, we found that PPM1K exhibited moderate catalytic activity toward pNPP when using Mg2+ as the catalytic ion (Table 4), whereas all previous reports of its activity had been in the presence of Mn2+ (Wynn et al. 2012).
Mg2+ participates in many biochemical processes that are crucial for neuronal properties and synaptic plasticity (Xu et al. 2014; Nayak et al. 2018). Recent studies have demonstrated that the serum and brain magnesium levels were decreased in Alzheimer's disease (AD), cerebral injury, and other neurological abnormalities (Daher et al. 2018; Meloni et al. 2011). Therefore, it is important to understand how Mg2+ regulated activities of key enzymes with relevant functions in neurological activities. It was reported that the phosphatase activity of PPM1K is intimately related to the normal function of nerve cells (Garcia-Cazorla et al. 2014; Gruenbaum et al. 2018). Here, our studies revealed that PPM1K N94K mutant decreased Mg2+-dependent phosphatase activity, suggesting that PPM1K N94K mutant may be involved in the pathological processes of aforementioned neurological abnormalities. Future in vivo studies, such as creating a PPM1K-N94K knock in mice model, will be beneficial to test such hypothesis.
Many members of the PPM superfamily have been structurally characterized and all contain the αββα sandwich arrangement of the catalytic domain (Egloff et al. 1995; Goldberg et al. 1995; Das et al. 1996). In the active site of PPM1K, a binuclear metal ions (Mn2+ or Mg2+) bridged by the conserved D127 (corresponding to D60 in PPM1A) and a water molecular played critical roles in catalysis (Wynn et al. 2012; Pan et al. 2015). Recently, our studies have shown whereas replacing the catalytic metal ion in M1 site of PPM1A or PPM1G by cadmium abolished the phosphatase activity (Pan et al. 2013), deleting the catalytic metal in M2 site through a D38 mutation in PPM1A impaired the phosphatase activity through losing the ability in neutralization of the negative charge developed by the leaving group of the substrates (Pan et al. 2013). Moreover, some crystal structures of bacterial, plant, and human PPM phosphatases have revealed an additional, third metal ion (M3) along with two tightly bound metal ions in the active site (Pullen et al. 2004; Rantanen et al. 2007; Wehenkel et al. 2007; Schlicker et al. 2008; Su et al. 2011; Tanoue et al. 2013; Debnath et al. 2018). Studies have shown loss of M3 binding has no effect on the binding of the first metal (M1) and the second metal (M2) in the catalytic center but notably impaired the catalytic activity in Thermosynechococcus elongatus and necessity of M3 in PPM-catalyzed reactions (Su et al. 2011). Higher enzyme activity with random order, bi-substrate mechanism was observed toward phospho-peptide substrate when M3 binds a low-affinity site in presence of M1 and M2 which allows solvent access to the third metal in the catalytic domain of active site in the human phosphatases PP2Cα (PPM1A) and Wip1 (PPM1D) (Tanoue et al. 2013; Debnath et al. 2018). By determination of the PPM1K-N94K crystal structure, we identified that the PPM1K-N94K mutant accommodates a third Mg2+ in the active site, accompanied with the slight change in the coordination state of two known conserved metal ion (Figs. 2A and S4). The metal ion coordination changes in the active site could provide the possible structural basis for the decreased phosphatase activity of PPM1K-N94K toward pNPP compared to the wild-type PPM1K (Tables 3 and 4). In this study, we mainly focused on the correlation between the Mg2+ coordination changes and the decreased Mg2+-dependent activity of PPM1K-N94K. The decreased phosphatase activity of PPM1K-N94K in the presence of Mg2+ but not Mn2+ is possibly attributed to the differences in the distance between Mg2+-water and Mn2+-water dative covalent bonds because the atomic radius of manganese is lower than that of magnesium. It would be particularly important to analyze a Mn-bound structure in order to evaluate whether the same active site coordination changes are not observed in the presence of manganese. Moreover, the moderately tighter binding of Mg2+ to PPM1K-N94K may have contributed to the presence of the third metal ion (M3) in the active site in the crystal of the mutant rather than the crystal of the wild-type enzyme, which displays only two divalent ions in the active site. We also observed the conformational changes in the N247 and D298 in the catalytic cleft of the PPM1K-N94K compared with wild-type PPM1K (Fig. 3). As the N94K mutation has a minor effect on the overall structure, another explanation is that the active site conformation may be dependent on the presence/absence of Mg2+/Mn2+ ions, with different ‘states’ occurring in the PPM1K wild type or N94K mutant. Currently, all crystals of PPM1K-N94K/Mn2+ complex diffracted poorly, which impeded us to give a full interpretation of the above questions. Further studies using different constructs of PPM1K that produce better quality crystals may help in solving these problems.
Conclusion
In brief, our present study unraveled that PPM1K-N94K mutant significantly impaired its Mg2+-dependent catalytic activity while inducing a conformational change in the key residue in coordinating the Mg2+ in the active site. These observations might provide a structure basis for the metal ion-dependent PPM1K-N94K phosphatase activity and its substrate specificity.
Acknowledgments and conflict of interest disclosure
We would like to thank Xiang Ding and Mengmeng Zhang from Laboratory of Proteomics, Core Facilities for Protein Science, at the Institute of Biophysics (IBP), Chinese Academy of Sciences, for their help with the mass spectrometry analysis. This work was supported by the National Natural Science Foundation of China (31470789, 31611540337, and 81773704 to J.-P.S.; 31471102 and 31671197 to X.Y.; 31870781 to P.-J.Z.; and 31700692 to P.X.), the National Science Fund for Distinguished Young Scholars (81825022 to J.-P.S.), the Shandong Natural Science Fund for Distinguished Young Scholars (JQ201517 to J.-P.S.), the Shandong Natural Science Fund (ZR2016CQ07 to P.X.), the Key Research and Development Program of Shandong Province (2018GSF118073 to P.-J.Z.; 2018GSF118147 to P.X.), the Fundamental Research Fund of Shandong University (2016JC017 to J.-P.S.), and the China Postdoctoral Science Foundation (2015M582082 to P.X.) and the Rolling program of ‘ChangJiang Scholars and Innovative Research Team in University’ (IRT_17R68 to Y.-M.S.). The authors have no conflict of interest to declare.
Institutional approval
No institutional approval was required for this study.
Data availability
The authors declare that data supporting the finding of this study are available within the article and its supplementary information and additional data are available from the corresponding author upon reasonable request.