Adult neurogenesis and its role in brain injury and psychiatric diseases
Abstract
In the adult mammalian brain, neural stem cells (NSCs) reside in two neurogenic regions, the walls of the lateral ventricles, and the subgranular zone of the hippocampus, which generate new neurons for the olfactory bulb and dentate gyrus, respectively. These adult NSCs retain their self-renewal ability and capacity to differentiate into neurons and glia as demonstrated by in vitro studies. However, their contribution to tissue repair in disease and injury is limited, lending credence to the claim by prominent neuropathologist Ramón y Cajal that ‘once development was ended, the founts of growth and regeneration of the axons and dendrites dried up irrevocably’. However, recent progress toward understanding the fundamental biology of adult NSCs and their role in pathological conditions has provided new insight into the potential therapeutic utility of endogenous NSCs. In this short review, we highlight two topics: the altered behavior of NSCs after brain damage and the dysfunction of NSCs and oligodendrocyte precursor cells, another type of undifferentiated cell in the adult brain, in mood affective disorders.
Abbreviations used
-
- BA
-
- Brodmann area
-
- BDNF
-
- brain-derived neurotrophic factor
-
- BPD
-
- bipolar disorders
-
- CXCR-4
-
- C-X-C motif receptor 4
-
- ECM
-
- extracellular matrix
-
- FGF
-
- basic fibroblast growth factor
-
- MCP-1
-
- monocyte chemoattractant protein-1
-
- MDD
-
- major depressive disorders
-
- MMP
-
- matrix metalloproteinase
-
- NSC
-
- neural stem cell
-
- OPC
-
- oligodendrocyte precursor cell
-
- RMS
-
- rostral migratory stream
-
- SDF-1
-
- stromal cell-derived factor-1
-
- SEZ
-
- subependymal zone
-
- SVZ
-
- subventricular zone
-
- VEGF
-
- vascular endothelial growth factor
-
- V-SVZ
-
- ventricular–subventricular zone
Neural stem cells (NSCs) in the adult mammalian brain are maintained throughout life in the walls of the lateral ventricles, which supply new neurons to the olfactory bulb via the rostral migratory stream (RMS), and in the subgranular zone of the hippocampus, which produces new granule cells for the dentate gyrus (Altman and Das 1965; Gage 2000; Alvarez-Buylla and García-Verdugo 2002; Lim and Alvarez-Buylla 2016; Kaneko et al. 2017). The adult NSC/neurogenesis system is evident not only in rodents but also in primates including humans (Eriksson et al. 1998). Although this system in the adult brain is considered relatively robust, the number of NSCs gradually decreases with age and is also reduced by psychosocial and physical stress (Gould et al. 1992; Cameron et al. 1993; Schoenfeld and Gould 2012; Kempermann 2015). Conversely, the NSC population size is increased by enriched environments, exercise, learning, and by various therapeutic drug treatments, such as antidepressants and mood stabilizers (Jacobs 2002; Malberg 2004; Higashi et al. 2008; Kempermann 2015).
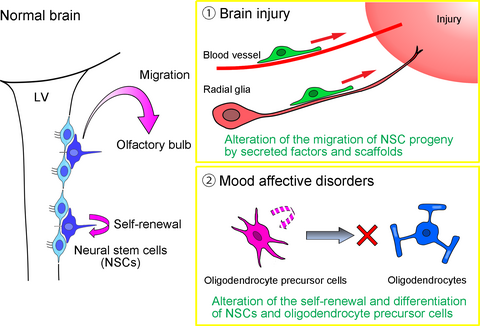
The implications arising from the presence of NSCs and ongoing neurogenesis in the postnatal and adult brain continue to inspire investigation regarding their physiological roles and potential therapeutic uses. The mobilization and fate specification of NSCs and their progeny is critical for neural repair. Therefore, to fully understand their potential, it would be useful to undertake the challenging task of elucidating the alterations that occur in the adult NSC/neurogenesis system after brain damage, in which NSCs turn to migrate toward the site of injury but are mostly blocked from actually reaching the lesion or maturing into the appropriate cells and functions (Arvidsson et al. 2002; Fais et al. 2015). Similarly, the re-defining of glial cells as active modulators of physiological and pathological processes has rendered great interest in the topic of gliogenesis. The adult mammalian brain harbors large numbers of oligodendrocyte precursor cells (OPCs), which provide mature oligodendrocytes for myelination while also retaining the potential to differentiate into other cell types (Trotter et al. 2010). Recently, OPCs in the adult brain have attracted much attention with regard to mood affective disorders (Edgar and Sibille 2012), which will be described in the latter part of this review.
Neurogenesis in the periventricular niche after brain injury
The neurogenic niche in the periventricular regions has been referred to as the ‘subependymal zone’ (SEZ, as we will refer to it here), ‘subventricular zone’ (SVZ), or more recently ventricular–subventricular zone based on the spatial arrangement and cellular morphology of NSCs (Ihrie and Alvarez-Buylla 2011; Lim and Alvarez-Buylla 2016). The SEZ is the largest NSC niche in the postnatal vertebrate brain (Paredes et al. 2016b). New immature neurons, referred to as neuroblasts, that are generated in the SEZ form chain-like clusters and migrate toward the olfactory bulb. SEZ-derived neuroblasts migrate in a saltatory manner involving the elongation of the leading process followed by cytoplasmic swelling and centrosome movement, and somal translocation (Schaar and McConnell 2005; Ota et al. 2014).
Brain injuries such as hypoxia and ischemic stroke are a leading cause of mortality and severe neurological disabilities in patients, and there are currently no available therapies to repair injured brain tissues. In rodents, after brain injury SEZ-derived neuroblasts migrate toward injured areas and differentiate into mature neurons (Arvidsson et al. 2002; Parent et al. 2002; Yamashita et al. 2006), suggesting that the SEZ could be a source of endogenous neural regeneration. To better understand the potential for regeneration of injured brain tissue, it is important to clarify the mechanisms regulating each step of neuronal regeneration, including the proliferation, migration, survival, and maturation of neuroblasts. The directional migration of neuroblasts from the SEZ to their final destination is controlled by a variety of molecular and cellular mechanisms under physiological and pathological conditions. Here, we review recent findings on the regulatory mechanisms of neuronal migration in the injured postnatal brain, including the association with migratory scaffolds, and discuss their potential use in neuronal regeneration strategies.
Neuronal migration after adult brain injury
In the injured adult brain, neuroblasts generated in the SEZ migrate toward the site of injury (Arvidsson et al. 2002; Parent et al. 2002). The directional migration of SEZ-derived neuroblasts is controlled by various guidance cues such as chemoattractants secreted from the injured area. During development, signaling between stromal cell-derived factor-1 (SDF-1) and its receptor C-X-C motif receptor 4 (CXCR-4) is involved in the directional migration of various cell types, such as cortical interneurons and hippocampal dentate granule cells (Bagri et al. 2002; Stumm et al. 2003; Tiveron et al. 2006). After ischemic stroke, SDF-1/(CXCR-4) signaling promotes neuroblast migration toward the injured regions (Ohab et al. 2006; Thored et al. 2006; Robin et al. 2006; Kojima et al. 2010). In addition, monocyte chemoattractant protein-1 (MCP-1) is produced by activated microglia and astrocytes present in the ischemic cerebral regions and attracts migrating neuroblasts which express its receptor C-C motif receptor-2 (Yan et al. 2007). Reelin, which is a secreted extracellular matrix protein, acts as a migration cue for SEZ-derived neuroblasts in the injured brain (Courtes et al. 2011). Moreover, various extracellular matrix (ECM) proteins are enriched in the migration path from the SEZ to the olfactory bulb, and some of these ECM components are involved in neuroblast migration (Saghatelyan et al. 2004; Belvindrah et al. 2007). Several ECM proteins have been shown to be increased in the damaged brain (Roll and Faissner 2014). For example, matrix metalloproteinases (MMPs), a family of proteolytic enzymes known to play a role in ECM remodeling, are up-regulated after brain injury and involved in neuroblast migration (Lee et al. 2006). Thus, it is likely that migrating neuroblasts interact with ECMs in the injured brain. Furthermore, ischemic stroke has been shown to increase the expression of vascular endothelial growth factor (VEGF), which promotes neuroblast migration as well as neurogenesis and angiogenesis (Sun et al. 2003; Thau-Zuchman et al. 2010); thus, it is possible that VEGF directly promotes injury-induced neuroblast migration.
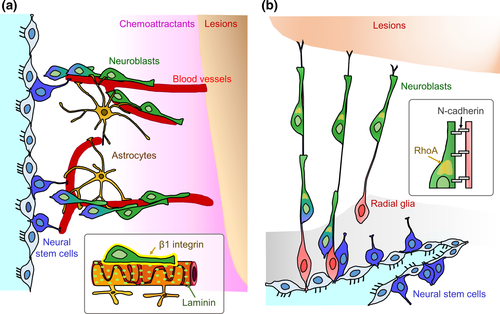
In the post-stroke brain, SEZ-derived neuroblasts migrate toward the injured areas along blood vessels which act as a scaffold (Yamashita et al. 2006; Ohab et al. 2006; Zhang et al. 2009; Kojima et al. 2010). This blood vessel-guided neuroblast migration is also observed in the RMS, olfactory bulb, and neonatal cerebral cortex (Bovetti et al. 2007; Snapyan et al. 2009; Whitman et al. 2009; Le Magueresse et al. 2012). During vascular-guided migration, neuroblasts directly contact astrocytic processes enwrapping the vessels (Yamashita et al. 2006; Whitman et al. 2009; Le Magueresse et al. 2012). Brain-derived neurotrophic factor (BDNF) is secreted from the vascular endothelial cells and promotes blood vessel-guided neuroblast migration in the ischemic brain (Grade et al. 2013), similar to the RMS (Snapyan et al. 2009). Reactive astrocytes trap BDNF using the high-affinity receptor TrkB, which decreases the amount of BDNF binding to the low-affinity receptor p75NTR on neuroblasts (Grade et al. 2013), suggesting that interactions between vascular endothelial cells and astrocytes are involved in the injury-induced neuronal migration along blood vessels. However, the mechanisms by which neuroblasts contact and use blood vessels as a migratory scaffold are not fully understood. β1-class integrins are transmembrane receptor proteins for various ECMs such as laminin. Neuroblasts generated in the SEZ express β1 integrin, which is involved in their chain migration (Jacques et al. 1998; Emsley and Hagg 2003). Our recent study demonstrated that β1 integrin is required for blood vessel-guided neuroblast migration in the post-stroke brain (Fujioka et al. 2017). We showed that specific deletion of β1 integrin in neuroblasts significantly decreased both the speed of migrating neuroblasts associated with blood vessels and the number of neuroblasts in the injured regions, suggesting that β1 integrin within neuroblasts regulates their interaction with blood vessels and is necessary for neuroblast migration toward sites of injury. Taken together, this suggests that laminin-β1 integrin signaling enables neuroblasts to appropriately adhere to laminin-rich scaffolds for efficient neuroblast migration (Fig. 1a). Approximately 30% of migrating neuroblasts within the injured brain travel along blood vessels (Thored et al. 2007; Kojima et al. 2010; Young et al. 2014). Therefore, many neuroblasts migrate independently of the blood vessel scaffold after brain injury (Young et al. 2014), which is consistent with previous observations under physiological conditions (Tavazoie et al. 2008; Nie et al. 2010). It would therefore be helpful to elucidate the mechanisms of this blood vessel-independent neuronal migration. Previous studies have suggested that neuroblast migration is supported by astrocytes and radial glia-like cells following brain injury (Szele and Chesselet 1996; Zhang et al. 2007; Saha et al. 2013). It is thus important to examine if these cells function as migratory scaffolds for SEZ-derived neuroblasts.
Neuronal migration after neonatal brain injury
In neonatal animals, neuroblasts from the SEZ migrate not only into the olfactory bulb, but also to other forebrain regions including the striatum and cortex, where they differentiate into interneurons (Inta et al. 2008; Le Magueresse et al. 2011). In humans, the neonatal SEZ has a remarkable neurogenic capacity and a large amount of neuroblasts can migrate long distances from the SEZ to the prefrontal cerebral cortex (Sanai et al. 2011; Paredes et al. 2016a). After brain injury, the neonatal SEZ supplies new mature interneurons to the injured striatum and cortex (Yang et al. 2007, 2008). Compared to the adult brain, the neurogenic niche of the neonatal brain contains a larger number of neuroblasts that migrate toward injury lesions (Covey et al. 2010). Therefore, the neonatal SEZ could be a promising source for neural regeneration after brain injury. However, the neonatal scaffolds that serve to efficiently guide the SEZ-derived neuroblasts toward injured regions have yet to be fully investigated.
We recently demonstrated that after neonatal brain injury, radial glial fibers support migration of SEZ-derived neuroblasts toward the injured area, where they differentiate into mature interneurons (Jinnou et al. 2018). Radial glia are embryonic NSCs, and neuroblasts use these radial glial fibers as scaffolds for migration (Rakic 1972; Yokota et al. 2007; Kawauchi et al. 2010). Given that radial glia differentiate into astrocytes or ependymal cells soon after birth (Tramontin et al. 2003; Kriegstein and Alvarez-Buylla 2009), the roles of radial glia during the postnatal period and in the injured brain have not been studied for an extended amount of time. Our study showed that radial glial fibers are transiently maintained after neonatal brain injury, but not after adult brain injury (Jinnou et al. 2018). Although the mechanisms of radial glial maintenance are unknown, fibroblast growth factor signaling might be involved in maintaining radial glial fibers after chronic neonatal hypoxia (Ganat et al. 2002). Induction of a constitutively active form of ErbB2 in astrocytes causes them to regain radial glial identity (Ghashghaei et al. 2007), raising the possibility that neuregulin–ErbB signaling contributes to the maintenance of radial glial fibers after neonatal brain injury. N-cadherin, a protein that regulates cell–cell adhesion, is also involved in radial glia-guided neuroblast migration during the embryonic period (Kawauchi et al. 2010). Radial glial fibers form adherens junction-like structures with migrating neuroblasts ensuring their appropriate migration to form the cortical layers (Rakic 1972; Franco et al. 2011). In our recent study, using an adenovirus vector we showed that both the specific down-regulation and inactivation of N-cadherin in radial glia affect adherens junction-like structures between neonatal radial glia and migrating neuroblasts. This disrupts the fiber-guided migration of SEZ-derived neuroblasts toward the site of injury, without changing the morphology of the radial glial fibers (Jinnou et al. 2018), suggesting that the association of radial glia with migrating neuroblasts in both the embryonic brain and in the postnatal injured brain are controlled by a common mechanism. Furthermore, we found that neonatal radial glia promote the migration speed of SEZ-derived neuroblasts by decreasing the resting-phase duration and increasing stride length. The N-cadherin-containing scaffold increases RhoA activity in the cytoplasmic swelling of migrating neuroblasts, similar to those in the RMS (Shinohara et al. 2012; Ota et al. 2014). These findings suggest that neonatal radial glia promote saltatory movement of neuroblasts via N-cadherin-mediated adhesion that promotes RhoA activation. Taken together, these studies reveal the functional significance of neonatal radial glia for endogenous neurogenesis after brain injury (Fig. 1b).
Enhancement of neuronal migration and perspectives
Although the SEZ supplies new mature neurons to the damaged brain, only about 0.2% of dead neurons can be replaced by these SEZ-derived cells (Arvidsson et al. 2002). Therefore, in order to substantially recover from neurological impairment, it is necessary to enhance the migration of neuroblasts into the injured areas. The administration of growth factors and neurotrophic factors has been shown to successfully promote SEZ cell proliferation and the recruitment of SEZ-derived neuroblasts to the injured brain (Kobayashi et al. 2006; Kolb et al. 2007). Furthermore, the transplantation of biomimetic scaffolds that release neurotrophic factors or lactate can induce sustained neurogenesis and neuronal migration (Fon et al. 2014a; Álvarez et al. 2014). Moreover, the effects of growth factors or neurotrophic factors on the recruitment of neuroblasts are enhanced by administering them with a gelatin hydrogel (Nakaguchi et al. 2012; Fon et al. 2014b).
Given that neuroblasts migrate toward the site of brain lesions using blood vessels or radial glial fibers as migratory scaffolds (Yamashita et al. 2006; Thored et al. 2007; Kojima et al. 2010; Jinnou et al. 2018), this raises the possibility of reconstructing blood vessel or radial glial scaffolds as a strategy for promoting endogenous neuronal regeneration. We have demonstrated that transplanting a laminin-rich porous sponge into the injured brain enhances the migration of neuroblasts toward the injured site without causing further post-injury inflammation (Ajioka et al. 2015). We also showed that an injectable hydrogel enriched with laminin, which self-assembles into hydrated nanofibers in vivo (Holmes et al. 2000), also promoted efficient migration of neuroblasts toward the injured striatum in adult mice (Fujioka et al. 2017). Based on the finding that N-cadherin is expressed in neonatal radial glia, we also developed a gelatin sponge conjugated with N-cadherin. This N-cadherin-containing scaffold promoted SEZ-derived neuroblast migration in the injured brain (Jinnou et al. 2018). Moreover, the transplanted N-cadherin-containing scaffold within the lesion promoted SEZ-derived neuronal maturation and successfully improved gait disturbance in neonatal mice, but not in adult mice.
In our previous study using a mouse model of neonatal brain injury, the SEZ was shown to supply new mature interneurons but not projection neurons to the injured lesion (Jinnou et al. 2018). Although interneurons represent only 10–20% of the neuronal population, they are important for regulating the activity of the glutamatergic neuronal network in the brain. We demonstrated that genetic ablation of newly generated SEZ-derived interneurons around the brain lesion decreased functional recovery by the N-cadherin-containing scaffold (Jinnou et al. 2018), suggesting that SEZ-derived neurogenesis contributes to functional recovery. In addition, cell transplantation studies showed that interneuron regeneration can promote functional recovery in the spinal cord (Bráz et al. 2012; Fandel et al. 2016). Moreover, SEZ-derived interneurons are functionally integrated into cortical circuits (Le Magueresse et al. 2011). Therefore, a promising strategy for neuronal regeneration is to boost SEZ-derived neurogenesis after brain injury. It is possible that providing an appropriate migratory scaffold in combination with additional treatments that enhance the maturation and survival of newly formed neurons in the injured brain will improve neuronal regeneration and functional recovery.
In the neonatal human brain, neuroblasts migrate to the frontal lobe at least partly along blood vessels (Paredes et al. 2016a). Radial glial cells have been identified in extremely preterm infants (Kubo et al. 2017), and post-mortem human brain studies have shown an emergence of neuroblasts around sites of injury in association with blood vessels (Jin et al. 2006). These observations suggest that neuroblast migration to lesioned sites along a scaffold may be evolutionarily conserved even in human brains. Further understanding of the mechanisms of neuronal migration under physiological and pathological conditions will contribute to the development of treatment strategies for human brain injury using endogenous NSCs.
Adult neurogenesis and psychiatric disorders
Strategies aimed at the expansion of the NSC pool and enhancement of neurogenesis, as described in the above section, could be useful not only to repair brain damage but also to restore brain dysfunction caused by deficiencies in the adult NSC/neurogenesis system. A number of studies have suggested a possible association between altered adult neurogenesis and mood affective disorders, such as major depressive disorder (MDD) and bipolar disorder (BPD). The first evidence came from studies in rodent stress models, in which decreased proliferation of neural precursor cells in the subgranular layer of the adult hippocampus was observed following psychosocial or physical stressors, and this reduced cell proliferation was restored by antidepressant treatment (Duman et al. 2001; Malberg 2004; Mirescu and Gould 2006; Hitoshi et al. 2007). The behavioral effects associated with chronic antidepressant treatment are dependent on stimulated neurogenesis in the mouse hippocampus (Santarelli et al. 2003). These effects of antidepressants on increased neural precursor cells and neurogenesis have also been demonstrated in non-human primates (Perera et al. 2007, 2011).
Lastly, investigations of neurogenesis in the hippocampus of post-morten brains from patients with psychiatric disorders (Reif et al. 2006; Boldrini et al. 2009) revealed a tendency toward reduced Ki67+ dividing cells in the dentate gyrus of patients with mood affective disorder. In contrast, antidepressant treatment strongly increased neural precursor cell proliferation in the dentate gyrus of brains from MDD patients compared with non-medicated MDD patients or healthy controls (Boldrini et al. 2009). It should be noted that several limitations are inevitable in human studies; these include the relatively small sample size and varied genetic background and medication history of the patients. In addition, Ki67 positivity only indicates the potential for cells to divide but does not provide information about continuous proliferation or cell divisions that occurred shortly before death.
The molecular mechanisms underlying the effects of antidepressants on neural precursor cells and neurogenesis are not fully understood. However, data suggest that these processes are likely mediated by the augmentation of serotonin, as blockade of serotonin activity suppresses cell proliferation in the SEZ and dentate gyrus of the hippocampus (Brezun and Daszuta 1999) and antidepressants themselves exhibit little effect on cultured NSCs from the adult SEZ (Hitoshi et al. 2007). In contrast, mood-stabilizing drugs, which are prescribed to patients with BPD, directly enhance the self-renewal of NSCs at therapeutically relevant concentrations in cerebrospinal fluid (Higashi et al. 2008). Administration of mood stabilizers to adult mice expands the pool of NSCs in the SEZ and DG and activates neurogenesis over a couple of weeks, which may explain why it takes several weeks to obtain full clinical benefits of these drugs in BPD patients (Boku et al. 2009; Chen et al. 2000; Higashi et al. 2008). Interestingly, treatment of NSCs with mood stabilizers activates Notch signaling, which plays a critical role in the self-renewal of NSCs (Hitoshi et al. 2002; Higashi et al. 2008).
Oligodendrogenesis and psychiatric disorders
While the NSCs present in the two neurogenic regions of the adult mammalian brain, the SEZ and dentate gyrus, supply new neurons to the olfactory bulb and granular layer, respectively, oligodendrocytes are produced by OPCs (Crawford et al. 2014, Dawson et al. 2003). Most OPCs are only generated from NSCs at the lateral and medial dorsoventral boundary during a narrow temporal window, from embryonic day 16 to postnatal day 10 in mice (Naruse et al. 2016). Therefore, OPCs represent an intermediate stage of progenitors in oligodendrogenesis, which are distributed and persist in the adult cortex as Olig2+/NG2+ cells. In pathological conditions, such as demyelination, SEZ-resident NSCs yield new OPCs that migrate to lesion sites, differentiate into mature oligodendrocytes, and reinforce remyelination (Menn et al. 2006). The density of oligodendrocytes and the area of myelination in the cortex increase with age in human and non-human primates (Miller et al. 2012; Peters and Sethares 2004; Vostrikov and Uranova 2011), suggesting that OPCs in the adult brain continuously provide myelinating oligodendrocytes and play essential roles in acquiring and preserving higher brain functions.
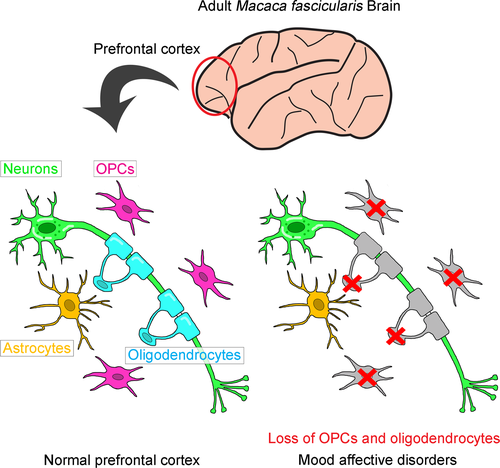
Prefrontal and anterior cingulate cortices are thought to be tightly involved in mood formation and maintenance because gray matter volume, metabolism, and blood flow in these regions are altered in patients with MDD and/or BPD (Drevets et al. 2008). Histochemical assessments using post-mortem brains from patients with mood affective disorder have identified a reduction in oligodendrocyte density in the dorsolateral prefrontal cortex (Brodmann area [BA] 9), subgenual prefrontal and anterior cingulate (BA24), and orbitofrontal (BA47) cortices (Ongur et al. 1998; Rajkowska et al. 1999; Cotter et al. 2001, 2002; Vostrikov et al. 2007). Reduced oligodendrocyte density in these regions has also been observed with aging (Vostrikov and Uranova 2011). Furthermore, comprehensive gene expression analyses have demonstrated the down-regulation of oligodendrocyte- and myelin-associated gene expression in the prefrontal cortex (BA9 and 46) of patients with mood affective disorders (Tkachev et al. 2003; Klempan et al. 2009; Kim and Webster 2010). We have also quantified the total number of NeuN+ neurons and Olig2+ oligodendrocytes in these brain regions by flow cytometry and found that oligodendrocyte-lineage cells were decreased in the frontopolar cortex (BA10) of MDD and BPD patients (Hayashi et al. 2011, 2012). Fluorescence intensity analysis of Olig2 immunostaining enabled us to separately count Olig2strong OPCs and Olig2weak mature oligodendrocytes to show that both populations are reduced, suggesting that oligodendrogenesis in patients is impaired. Since these abnormalities are disease-specific and brain region-specific, dysfunction of oligodendrocyte-lineage cells should play a pathognomonic role in mood affective disorders. Patients with MDD or BPD may develop vulnerabilities in their NSC-OPC-oligodendrogenesis system during the perinatal period through childhood/adolescence, although the time when most OPCs are generated from NSCs in humans is currently unknown.
Immune system and psychiatric disorders
Chronic inflammation has recently received much attention as a risk factor or mediator in the pathogenesis of mood affective disorders, especially MDD (Haroon et al. 2012). Inflammatory markers or cytokines, such as interleukin-6 and tumor necrosis factor α, are often elevated in the peripheral blood of MDD patients (O'Donovan et al. 2013; Wium-Andersen et al. 2013). Moreover, chronic psychosocial stress in rodents not only induces depression-like behavioral changes but also activates the hypothalamic–pituitary–adrenal axis and augments the secretion of glucocorticoids, a class of immune modulators (Kubera et al. 2011). Glucocorticoids have been shown to suppress the self-renewal of NSCs derived from the SEZ and the proliferation of OPCs in the white and gray matter of the adult mouse brain (Gould et al. 1992; Cameron et al. 1993; Alonso 2000; Hitoshi et al. 2007). As expected, reduced proliferation of OPCs and subsequent loss of myelin fibers was observed in the frontal cortex of rodent MDD models following chronic stress and this reduction was rescued by treatment with antidepressants or infusion of fibroblast growth factor-2 to the lateral ventricles, which has potential antidepressant properties (Banasr et al. 2007; Czeh et al. 2007; Elsayed et al. 2012; Lehmann et al. 2017). Interestingly, the ability of antidepressants to stimulate OPC proliferation appears to be brain region-specific (Kodama et al. 2004), suggesting that OPCs localized within regions associated with mood affective disorder are sensitive to chronic psychosocial stress but are also responsive to treatment. The molecular mechanisms of how oligodendrocyte-lineage cell dysfunction leads to altered and persistent mood change remain to be clarified, and this poses a challenging task partly because of the lack of appropriate animal models for mood affective disorders. Although MDD patients frequently experience stressful events as a symptomatic trigger, psychosocial stressors used to establish rodent MDD models are often too strong or even life-threatening, and these chronic stress models could actually represent post-traumatic stress disorder rather than MDD.
Interferon-α is a proinflammatory cytokine used to treat patients with chronic viral hepatitis and several kinds of cancer (Tagliaferri et al. 2005; Deutsch and Hadziyannis 2008). Following mild systemic symptoms such as fatigue and anorexia during the first few weeks of interferon-α treatment, a wide variety of adverse psychiatric effects emerge after long-term therapy in humans (Kovacs et al. 2016). Depressive behavioral changes are the most frequent and serious adverse symptoms, affecting up to 45% of patients receiving interferon-α treatment, and occasionally result in the cessation of therapy (Bonaccorso et al. 2001; Lieb et al. 2006). Based on these clinical observations, a mouse model of MDD using long-term administration of interferon-α was developed in which depression-like behavioral abnormalities and attenuated adult NSC neurogenesis were evident (Zheng et al. 2014). At therapeutically relevant concentrations, interferon-α directly acts on adult NSCs and diminishes their self-renewal capability, resulting in reductions in the NSC population and subsequent neurogenesis after chronic interferon-α treatment. This strategy can also be applied to non-human primates, such as marmosets or crab-eating monkeys (Macaca fascicularis). Macaques have very similar brain structures and higher brain functions comparable to humans, thus an interferon-α-induced macaque model of MDD should be the most relevant tool for investigating the pathogenesis of MDD (Fig. 2).
Conclusion
Recent progress in understanding the fundamental biology of adult NSCs under physiological and pathological conditions has provided clues for developing strategies to activate endogenous NSCs and reinforce neurogenesis and gliogenesis in the adult brain. This could eventually lead to the development of new therapies not only for brain injury and neurological diseases but also for psychiatric disorders.
Author contributions
Y.H., H.J., K.S., and S.H. wrote the manuscript.
Acknowledgments and conflict of interest disclosure
We apologize to colleagues whose work we could not cite because of space limitations. Work in the Hitoshi laboratory is supported by MEXT KAKENHI (16H04671and 16K14578), JSPS KAKENHI (26870282, 17K17215), The Fugaku Trust for Medical Research, the Brain Science Project of the Center for Novel Science Initiatives (CNSI), National Institutes of Natural Sciences (NINS) (BS261007, BS281002), and Grant-in-Aid for Research at Shiga University of Medical Science. Work in the Sawamoto laboratory is supported by MEXT KAKENHI (17H05512 and 17H05750), JSPS KAKENHI (17H01392, 17K18007 and JP16H06280), JSPS Program for Advancing Strategic International Networks to Accelerate the Circulation of Talented Researchers (S2704), and Grant-in-Aid for Research at Nagoya City University. The authors have no conflict of interest to declare.