Reduced glutathione biosynthesis in Drosophila melanogaster causes neuronal defects linked to copper deficiency
Abstract
Glutathione (GSH) is a tripeptide often considered to be the master antioxidant in cells. GSH plays an integral role in cellular redox regulation and is also known to have a role in mammalian copper homeostasis. In vitro evidence suggests that GSH is involved in copper uptake, sequestration and efflux. This study was undertaken to further investigate the roles that GSH plays in neuronal copper homeostasis in vivo, using the model organism Drosophila melanogaster. RNA interference-mediated knockdown of the Glutamate-cysteine ligase catalytic subunit gene (Gclc) that encodes the rate-limiting enzyme in GSH biosynthesis was utilised to genetically deplete GSH levels. When Gclc was knocked down in all neurons, this caused lethality, which was partially rescued by copper supplementation and was exacerbated by additional knockdown of the copper uptake transporter Ctr1A, or over-expression of the copper efflux transporter ATP7. Furthermore, when Gclc was knocked down in a subset of neuropeptide-producing cells, this resulted in adult progeny with unexpanded wings, a phenotype previously associated with copper dyshomeostasis. In these cells, Gclc suppression caused a decrease in axon branching, a phenotype further enhanced by ATP7 over-expression. Therefore, we conclude that GSH may play an important role in regulating neuronal copper levels and that reduction in GSH may lead to functional copper deficiency in neurons in vivo.
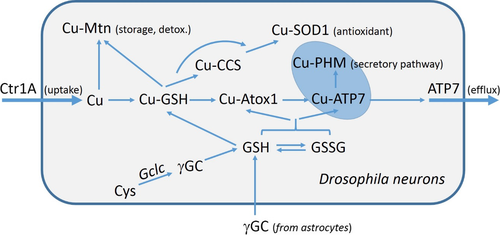
We provide genetic evidence that glutathione (GSH) levels influence Cu content or distribution in vivo, in Drosophila neurons. GSH could be required for binding Cu imported by Ctr1A and distributing it to chaperones, such as Mtn, CCS and Atox1. Alternatively, GSH could modify the copper-binding and transport activities of Atox1 and the ATP7 efflux protein via glutathionylation of copper-binding cysteines.
Abbreviations used
-
- BCS
-
- bathocuproine disulfonate
-
- CCAP
-
- crustacean cardioactive peptide
-
- CNS
-
- central nervous system
-
- HPEH
-
- hours post egg hatching
-
- PAM
-
- peptidylglycine α-amidating monoxygenase
-
- PBS
-
- phosphate-buffered saline
-
- PHM
-
- peptidylglycine alpha-hydroxylating monooxygenase
-
- RNAi
-
- RNA interference
-
- ROS
-
- reactive oxygen species
-
- TGN
-
- trans Golgi network
Glutathione (GSH) is a tripeptide (γ-glutamyl-cysteinyl-glycine) that is well known as a major antioxidant in cells and is critical for protecting cells against reactive oxygen species (ROS). GSH is abundant in the cytosol (at concentrations ranging from 1 to 10 mM) and exists both in a reduced (GSH) and an oxidised (GSSG) form. The molar ratio of GSSG/GSH is typically at 1 : 100, which corresponds to a cellular reduction potential in the range from −210 to −240 mV (Brose et al. 2014), creating highly reduced conditions within the cytosol.
The GSSG/GSH couple constitutes a redox buffer that is a key determinant and indicator of the redox status of cells. Hence, under normal cellular conditions, GSH is able to counteract the effects of a wide range of ROS (Winterbourn and Metodiewa 1994) including non-organic molecules, such as the superoxide anion, hydrogen peroxide and hydroxyl radicals, and organic alkolyl and peroxyl radicals. GSH functions in conjunction with other cellular antioxidants such as superoxide dismutase and catalase to prevent lipid peroxidation, protein modification or DNA strand breaks, all of which are severely damaging to cells (Sies 1991; Halliwell 1999).
There is growing evidence that GSH has an important role in the detoxification of ROS in the brain. The mammalian brain consumes 20% of total body oxygen, but represents only 2% of total body weight (Raichle and Gusnard 2002), rendering it susceptible to significant ROS generation. GSH deficiency in adult rats, induced by inhibition of γ-glutamyl-cysteine synthetase with buthionine sulfoximine treatment, resulted in mitochondrial damage in the brain (Jain et al. 1991). Neurodegenerative diseases, such as Alzheimer's (Retz et al. 1998; Behl 1999; Markesbery and Carney 1999), Parkinson's (Jenner 1998; Jenner and Olanow 1998; Jellinger 1999) and Huntington's disease (Browne et al. 1999), have been strongly associated with increased levels of oxidative stress, and decreased GSH levels are considered to be a biomarker for these diseases. For instance, in Parkinson's patients, GSH levels are significantly decreased in the affected substantia nigra (Sofic et al. 1992; Sian et al. 1994) and this decline is likely to be a significant factor in the localised increase in oxidative stress that arises prior to the progressive degeneration of these neurons.
GSH plays a central role in intracellular redox regulation, mainly through its interaction with protein thiols. Glutathionylation (protein-SSG formation) is the reversible, post-translational addition of GSH to the cysteine residues of proteins via disulphide bonds (Mieyal and Chock 2012; Lillig and Berndt 2013). Modification of protein thiols through thiol disulphide exchange with GSH is emerging as a central mechanism for redox regulation of protein function and signalling, comparable to protein phosphorylation (Mieyal and Chock 2012). This modification also serves to protect protein thiols from irreversible oxidative modifications that would alter protein function or stability.
The role of copper in neuronal function has been of interest since the discovery of copper transporting ATPase 1 (ATP7A) as the gene that is mutated in the rare X-linked disorder Menkes disease (Chelly et al. 1993; Mercer et al. 1993; Vulpe et al. 1993). Menkes patients suffer severe neurological abnormalities (Tumer and Moller 2010; Kaler 2011; Kodama et al. 2012) that are directly linked to a lack of copper delivery to the brain. This central nervous system (CNS) copper deficiency leads to reduced function of copper-dependent enzymes, such as cytochrome c oxidase (Maehara et al. 1983), peptidylglycine α-amidating monoxygenase (Klinman 2006) and dopamine-β-hydroxylase (Klinman 2006), as well as disruption of other copper-dependent processes, leading to neurodegeneration (Hodgkinson et al. 2015).
There is also a clear link between copper dysregulation and neurodegenerative diseases. A pertinent example is in the brains of AD patients, where copper is mislocalised and enriched in extracellular senile plaques, leaving surrounding tissue and cells deficient in copper (Lovell et al. 1998). Copper also has clear toxic effects particularly when present in excess, as evident from the severe neurological symptoms associated with a toxic accumulation of copper in the brains of Wilson disease patients (Ala et al. 2007; Litwin et al. 2013). There is mounting evidence that correct distribution of copper within neurons is critical to neuronal function (Schlief et al. 2005, 2006; Hodgkinson et al. 2015).
GSH has been shown to have a multifaceted role in mammalian copper homeostasis. It can buffer free Cuaq+ concentrations towards femtomolar levels (Xiao et al. 2011) and may serve as an initial copper acceptor upon entry of copper into cells. Early kinetics studies of 67Cu uptake suggested that GSH bound 67Cu before it was complexed with metallothioneins (Freedman and Peisach 1989; Freedman et al. 1989). In subsequent studies, a role for GSH as a potential physiological Cu(I) carrier was proposed based on the observation that metallothionein could acquire copper from Cu(I)-GSH (Ferreira et al. 1993b). Pharmacological depletion of GSH has been shown to decrease the initial rate of 64Cu uptake in HEK293T cells (Maryon et al. 2013).
GSH also plays a role in copper delivery to SOD1 in the absence of copper chaperone for SOD1 (CCS) (Carroll et al. 2004). Based on the in vitro copper-binding affinities of a representative set of key proteins in the copper transport pathway, a model was proposed whereby copper is transported along an affinity gradient from GSH, present in millimolar concentrations and with low affinity for copper, to the copper chaperones, present at micromolar levels and with much higher affinity for copper, and finally to target proteins with still higher copper affinities (Banci et al. 2010; Xiao et al. 2011).
GSH has also been linked with copper efflux through modification of cysteine thiols in the metal-binding domains of the mammalian copper transport proteins ATP7A and ATP7B (Lim et al. 2006; Singleton et al. 2010). However, more recent studies showed that the redox state of protein thiols of two copper-binding proteins, Atox1 (the metallochaperone for copper delivery to the copper-ATPases ATP7A/7B) and the antioxidant protein glutaredoxin1 (Grx1), is controlled by the molar ratio of GSSG/GSH, so that under normal cellular conditions, their protein thiols remain predominantly in their reduced form (Hatori et al. 2012; Brose et al. 2014). Depending on the GSSG/GSH balance, these thiols can undergo reversible oxidation to form an intramolecular disulphide bond, which disrupts copper binding and delivery to the secretory pathway. GSH alone can reduce the disulphide bond and restore copper-binding capacity, but this may be catalysed by enzymes such as Grx1 to accommodate rapid changes in the cellular environment (Hatori et al. 2012; Brose et al. 2014). A similar mechanism may apply to the metal-binding site thiols of the copper-ATPases based on the structural similarity between their metal-binding domains and Atox1.
The exact function of GSH in neuronal copper homeostasis has yet to be fully elucidated. Given the clear role of GSH in intracellular copper regulation, loss of neuronal GSH may perturb normal trafficking of copper in neurons and could therefore be a critical step in the progression of copper-related neurodegenerative diseases. In Drosophila melanogaster, the requirement for GSH in redox regulation is similar to that in mammals (Orr et al. 2013). Therefore, the fly is an excellent model for studying the impact of tissue-specific GSH depletion. Here, we show that neuronal RNA interference (RNAi)-mediated knockdown of the Glutamate-cysteine ligase catalytic subunit gene (Gclc), which encodes the rate-limiting enzyme in GSH biosynthesis, causes copper-associated neuronal defects. We propose that GSH has a fundamental role in neuronal copper homeostasis and that perturbation of GSH biosynthesis may be an important step in the pathogenic pathways leading to copper-related neurodegenerative diseases.
Materials and methods
Drosophila stocks
The following fly stocks were used: w1118 (BL (Bloomington Drosophila Stock Centre, Bloomington, IN, USA) 3605); UAS-GFP (BL32184, P(10XUAS-mCD8:GFP)attP2); ELAV-GAL4 (BL458, P(GawB)elavC155); double balancer (w; IF/CyO; MKRS/TM6β, gift from G. Hime, University of Melbourne, Australia); CCAP-GAL4 (yw[*]; P(w[+mC]=CCAP-GAL4.P)16); GMR-GAL4 (BL9146); Pannier (PNR)-GAL4 (BL3039); mex-GAL4; TUB-GAL4 and ACTIN-GAL4. Over-expression lines used include UAS-ATP7FLAG, UAS-Ctr1AFLAG and UAS-Ctr1BFLAG which have been described previously (Norgate et al. 2006; Binks et al. 2010) and RNAi lines obtained from the Vienna Drosophila RNAi Centre included V33512 (Gclc), V8315 (ATP7), V46757 (Ctr1A) and V5805 (Ctr1B). All transgenic Drosophila experiments carried out in this research were performed with the approval of the Monash University Institutional Biosafety Committee.
Drosophila maintenance
All Drosophila stocks and crosses were maintained at 25°C on standard medium consisting of potassium tartrate, calcium chloride, agar, yeast, dextrose, raw sugar and semolina. Standard medium was supplemented with either 500-μM bathocuproine disulfonate (BCS; Sigma-Aldrich, Castle Hill, NSW, Australia) to make copper-deficient food medium, copper sulphate (CuSO4_5H2O; Sigma-Aldrich) to make copper-supplemented medium, zinc chloride (ZnCl2; Sigma-Aldrich) to make zinc-supplemented medium or ferric chloride (FeCl3; Sigma-Aldrich) to make iron-supplemented medium.
Drosophila survival experiments
In all survival experiments, crosses were set up in cages with 50 virgin females and 20–25 males. To assess the survival of larvae to pupariation, multiple replicates of 50 first instar larvae were picked onto basal media or media supplemented with copper or BCS. The number of pupae was counted and survival of each genotype was calculated as percentage of expected based on predicted Mendelian ratios.
For adult survival experiments, replicates of 50 first instar larvae were placed onto basal media or media supplemented with copper, BCS, zinc or iron. Successful adult survival was classed as the ability of the adults to eclose from their pupal cases. For pupal and adult survival assays, n ≥ 3 for each food type.
Drosophila larval survival assay
To assess the survival rate of larvae, 10 first instar larvae of the appropriate genotype were picked onto apple juice agar plates supplemented with a small amount of yeast paste and survival of larvae was monitored every 24 h for 96 h (approximate time to pupation). Assessment of whether a larva was ‘living’ was based on a simple poke test with a larvae picker; if there was no detectable movement, then the larvae was assessed as being dead. For each genotype, n ≥ 3.
Microscopy
For phenotypic analysis, adult flies were partially dissected, then mounted directly onto plasticine and monitored with a Leica (Leica, North Ryde, NSW, Australia) MZ6 stereomicroscope. All images were recorded with Leica DC300 digital camera using Leica Application Suite software.
For localisation analysis, brains from wandering third instar larvae were dissected in phosphate-buffered saline then mounted onto glass slides in Permafluor (Thermo Fisher Scientific, Scoresby, Vic., Australia). Fluorescence imaging was performed using a Zeiss (North Ryde, NSW, Australia) Axio Imager fluorescent microscope. Image processing was completed using Adobe Photoshop 6.0 (San Jose, CA, USA) and quantitation analysis was done using Fiji Image J software.
Quantitation of axon branching density involved using the freehand drawing tool in Image J to outline the axon branching region and measurement of both the area and the integral density of the fluorescence. At least three CNS were quantified for each genotype. For neuronal area quantitation, again the freehand drawing tool was used to trace around crustacean cardioactive peptide (CCAP) neurons, with the area of at least five neurons measured per CNS. For each genotype, ≥ 3 CNS were quantified. In each case, quantitation was performed on randomly presented, de-identified images by a second researcher.
Quantitation of RNAi-knockdown efficacy
mRNA was extracted using TRIsure Reagent (Bioline, Alexandria, NSW, Australia) from 10 dissected third instar larval midguts from: (i) mex>w1118 and (ii) mex>GclcRNAi. mex-GAL4 was chosen as Gclc is predicted to be highly expressed in the larval midgut (dos Santos et al. 2015) and also midgut dissections provide a much purer RNA sample. cDNA was generated using the Tetro cDNA synthesis kit (Bioline). Gclc transcript levels were determined by semi-quantitative RT-PCR using equal concentrations of cDNA from mex>w1118 and mex>GclcRNAi midgut cDNA. PCR reactions were assembled using GoTaq® Green Master Mix (Promega, Alexandria, NSW, Australia). Housekeeping gene RP49 was used as an endogenous control in both experiments. Band intensity quantitation was completed using Image J Gels utility. Oligonucleotide sequences are available upon request.
Statistical analysis
Statistical analyses were conducted with Prism 6 (GraphPad Software Inc., San Diego, CA, USA). Quantitative data are presented as the mean with standard error of the mean (SEM) and p < 0.05 was considered to be statistically significant. Standard T-test and Tukey's multiple comparison tests were used to determine significance.
Results
Knockdown of Gclc in all neurons causes lethality which can be partially rescued by copper supplementation
To test the in vivo effect of GSH depletion in Drosophila, a UAS-RNAi line-targeting Gclc, which encodes the rate-limiting enzyme in GSH biosynthesis, was used in conjunction with a number of ubiquitous and tissue-specific GAL4 lines. Semi-quantitative RT-PCR analysis showed that Gclc knockdown in the larval midgut using mex-GAL4 reduced Gclc mRNA levels to ~ 50% of control levels (Figure S1). Strong ubiquitous knockdown of Gclc with both TUB-GAL4 and ACTIN-GAL4 caused a fully penetrant lethality. There was no obvious phenotype when Gclc was knocked down using an eye-specific driver (GMR-GAL4) or PNR-GAL4, which drives expression in dorsal midline of the adult thorax and abdomen (Figure S2).
Knockdown of Gclc in all Drosophila neurons using ELAV-GAL4 resulted in developmental arrest at the pupal stage or earlier. When quantified under density-controlled conditions, only 19 (± 4) % of ELAV>GclcRNAi flies reached pupation, compared with 90 (± 3) % of control flies (Fig. 1a), indicating that the majority of neuronal Gclc-knockdown flies were dying during larval development. The rare ELAV>GclcRNAi adult flies that managed to eclose displayed a sunken thorax with a cleft (Fig. 1c) and unexpanded wings (Fig. 1d), in contrast to the normal wings and thorax of control flies (Fig. 1b).
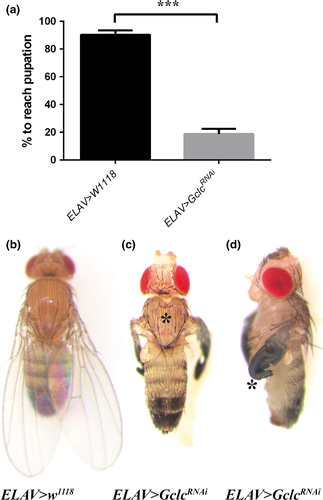
As GSH is known to regulate intracellular copper levels (Ferreira et al. 1993a; Singleton et al. 2010), we investigated the effect of dietary copper levels on the survival of flies with reduced neuronal Gclc activity. Copper supplementation significantly increased the pupation rate of ELAV>GclcRNAi flies compared to those raised on normal or copper-depleted media (Fig. 2a). Copper supplementation also partially rescued the phenotypes of rare surviving ELAV>GclcRNAi adult flies, with some of the surviving adults appearing phenotypically normal on a high-copper diet (Fig. 2b) compared to the normal food diet where all survivors showed unexpanded wing defects.
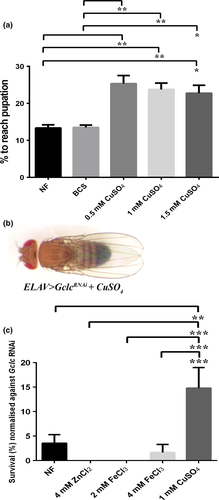
To test whether other metals were also able to restore neuronal Gclc-knockdown defects or whether this effect was copper specific, ELAV>GclcRNAi survival to adulthood was determined under copper, zinc and iron supplementation. Neither zinc nor iron was able to rescue flies above the 3.5 (± 2) % adult survival rate on normal food (NF), whereas with copper supplementation the survival rate of ELAV>GclcRNAi flies increased significantly to 15 (± 4) % (Fig. 2c).
Genetic manipulation of copper transporters modifies phenotypes resulting from neuronal-specific Gclc knockdown
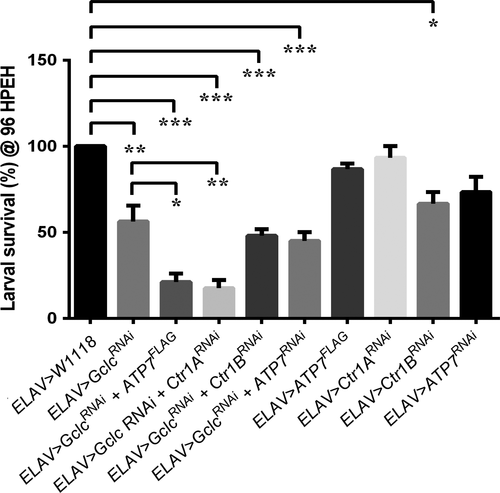
To further investigate the link between GSH and copper levels in neurons, targeted manipulation of the key copper transporters Ctr1A, Ctr1B and ATP7 was conducted in combination with Gclc knockdown. The survival of larvae of various genotypes at 96 h post egg hatching was compared. Only 56 (± 9) % of ELAV>GclcRNAi larvae were still alive compared to 100% of controls (Fig. 3). Combining ELAV>GclcRNAi with ATP7 over-expression or Ctr1A knockdown further reduced the survival rate of ELAV>GclcRNAi larvae to 21 (± 4) % and 17.5 (± 5) %, respectively. There was no difference in the survival rate of ELAV>ATP7FLAG, ELAV>Ctr1ARNAi and ELAV>ATP7RNAi larvae compared to controls.
Targeted suppression of Gclc in neuropeptide-producing cells causes an unexpanded wing phenotype
Drosophila wing expansion is controlled by a cascade of hormones, including ecdysis triggering hormone, eclosion hormone (EH) and CCAP (Jiang et al. 2000; Davis et al. 2007). To determine if GSH plays a critical role in CCAP-producing cells, CCAP-GAL4 (Luan et al. 2006) was employed to reduce GSH levels specifically in these cells. Gclc knockdown with CCAP-GAL4 resulted in a fully penetrant unexpanded wing phenotype, with 100% of eclosed adults having abnormal wings compared to wild-type controls which all had fully expanded wings (Fig. 4a). There was no alteration to the frequency of unexpanded wings with either BCS or copper supplementation (Fig. 4a). Typically, CCAP>GclcRNAi flies emerged with a softened, clefted thoracic cuticle (Fig. 4c) in conjunction with unexpanded wings (Fig. 4d), whereas CCAP-GAL4 control flies were phenotypically normal (Fig. 4b).
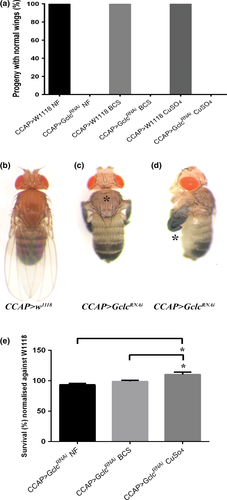
We then investigated whether the survival of CCAP>GclcRNAi flies was affected by changes in dietary copper levels. When normalised against survival of control CCAP>w1118 flies on each food type, there was a small, yet significant increase in survival of CCAP>GclcRNAi flies raised on copper-supplemented media compared to those raised on normal or copper-deficient media (Fig. 4e). Therefore, copper supplementation did not rescue the unexpanded wing phenotype but did partially increase survival of CCAP>GclcRNAi flies.
Gclc knockdown causes decreased branching of CCAP neuron axons, a phenotype exacerbated by over-expression of ATP7
To determine whether Gclc knockdown causes any alteration to the morphology or number of CCAP neurons, these cells were visualised by expression of mCD8-GFP (which localises to the outer plasma membrane) under CCAP-GAL4 control. CCAP-GAL4 drives expression in a small number of neurons in the ventral nerve cord and two neurons in each brain lobe of the third instar larval CNS; axonal projections extend along the middle of the ventral nerve cord to the brain lobes (Fig. 5a).
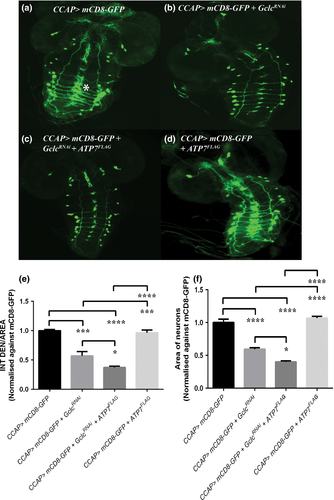
Gclc knockdown in CCAP neurons resulted in significant reduction in the density of axon branching when compared with CCAP>w1118 flies (Fig. 5b and e). There was no apparent decrease in the number of CCAP neuronal cell bodies in CCAP>GclcRNAi larval brains. While expression of ATP7FLAG alone under CCAP-GAL4 had no effect on cell number or branching (Fig. 5d and e), co-expression of ATP7FLAG exacerbated the reduction in branching caused by Gclc knockdown (Fig. 5c and e).
While there was no reduction in the number of CCAP cell bodies in any of the genotypes analysed, the area of CCAP neurons was found to be significantly smaller in CCAP>GclcRNAi larvae (Fig. 5b and f) compared to CCAP>w1118 animals (Fig. 5a and f). The area of CCAP neurons was further reduced with co-expression of ATP7FLAG (Fig. 5c and f), which alone had no effect on the size of CCAP neurons (Fig. 5d and f). Considering that genetically induced copper deficiency causes further morphological changes to CCAP neurons, this indicates that proper distribution of copper is critical to maintaining neuronal integrity and GSH may have an important role in this process.
Discussion
Copper is critical for neuronal function. It is a co-factor for important neuropeptides (Maehara et al. 1983; Klinman 2006), but has also been shown to play peptide-independent roles in neurodevelopment, synaptogenesis and axon extension (El Meskini et al. 2007), as well as being required for the modulation of neurotransmitter receptor activity and synaptic transmission (D'Ambrosi and Rossi 2015). Evidence is growing for a role for GSH as a key player in intracellular copper trafficking and homeostatic mechanisms (Ferreira et al. 1993a; Singleton et al. 2010; Hatori et al. 2012; Brose et al. 2014). However, the role of GSH in in vivo neuronal copper regulation has not been well defined. In this study, we provide genetic evidence suggesting that targeted reduction in GSH synthesis in neurons by RNAi-mediated knockdown of Gclc caused a functional copper deficiency within neurons that may result from either: (i) decreased copper uptake and/or (ii) abnormal copper distribution within neurons.
The lethality observed with neuronal knockdown of Gclc was found to primarily occur during larval development, as indicated by the low proportion of ELAV>GclcRNAi flies that reached pupation. This indicates that sufficient GSH synthesis is required for Drosophila larval brain development and/or function, although the extent of GSH reduction has not been quantified in this instance. Previously it was shown that over-expression of Gclc using neuronal-specific GAL4 drivers increased the mean and maximum life span of Drosophila by up to 50%, whereas global over-expression had no positive effect on survival times (Orr et al. 2005). In rats, GSH levels gradually decline with ageing, particularly in the brain (Watson et al. 2014), and this could be a result of decreased Gclc synthesis (Liu and Choi 2000).
The neuronal defects caused by targeted suppression of Gclc were partially rescued by copper supplementation, with regard to both increased pupation rate, and a reduction in the incidence of unexpanded wings in adult survivors. These results suggest that perturbed GSH synthesis may in turn cause neuronal copper dysregulation, although we have not measured copper content or distribution in Gclc-knockdown neurons; owing to the small size of Drosophila tissues, quantifying copper levels is challenging and our previous work with synchrotron x-ray fluorescence showed that only substantial manipulation of copper transporters leads to demonstrable changes in copper content (Lye et al. 2011). Zinc or iron supplementation did not improve the survival rate of ELAV>GclcRNAi flies, suggesting that the developmental lethality of these flies is specifically linked to dysfunctional copper homeostasis in neurons.
The fact that copper supplementation improved, but did not fully restore the survival of GSH-depleted flies could simply indicate that supplementation was insufficient to overcome a copper deficit. Alternatively, copper deficiency may contribute to the lethality but is not the sole cause of death; loss of GSH might activate additional copper-independent neuronal defects and/or cell death mechanisms. One possibility is that GSH and copper may act synergistically in protecting neurons from N-methyl-d-aspartate (NMDA) receptor-mediated excitotoxicity and that extra exogenous copper may help compensate for reduced GSH at the synapse. Acute treatment of hippocampal neurons with copper was found to have a protective effect against NMDAR excitotoxicity (Schlief et al. 2005) and the copper efflux activity of ATP7A within neurons controls the synaptic release of a pool of copper which can modulate NMDA receptor activity (Schlief et al. 2005, 2006). GSH may also act as an antagonist of NMDA receptors by regulating the NMDA receptor response to glutamate (Kohr et al. 1994) through redox modulation (Sullivan et al. 1994). Alternatively, decreased GSH levels within neurons may lead to oxidative stress, the effect of which is compounded by copper deficiency.
The phenotypes described for surviving ELAV>GclcRNAi and CCAP>GclcRNAi adult flies have been associated with the bursicon pathway, which is required for post-eclosion events, such as wing expansion and cuticle hardening. Bursicon null mutant flies have a softened, clefted thorax and unexpanded wings (Park et al. 2003; Luan et al. 2006; Davis et al. 2007; Peabody et al. 2008). The bursicon pathway involves a cascade of three hormones which are all regulated by peptidylglycine alpha-hydroxylating monooxygenase (PHM), the Drosophila equivalent of the mammalian copper-dependent neuropeptide peptidylglycine α-amidating monoxygenase (Jiang et al. 2000). The requirement for GSH for correct CCAP cell function is consistent with the notion that GSH is a key mediator of copper uptake or distribution within neurons. The unexpanded wings of CCAP>GclcRNAi flies could be caused by insufficient copper delivery to PHM. The inability of dietary copper supplementation to rescue this phenotype is reminiscent of our previous finding that exogenous copper could not reverse the effects of genetically induced copper depletion of CCAP cells (Hwang et al. 2014).
Ablation of CCAP neurons reduces the overall adult viability of Drosophila (Luan et al. 2006), reflecting additional roles for these neurons in processes such as pupal head eversion. Moderate-to-high levels of dietary copper supplementation also reduce developmental survival in a dose-dependent manner. Interestingly, with respect to survival to adulthood, we found that knockdown of Gclc actually protects flies against copper toxicity; CCAP>GclcRNAi animals show higher survival compared with CCAP-GAL4-only controls on copper-supplemented media. One possible implication of this result is that dietary copper-induced mortality is mediated, in part at least, by copper toxicity in CCAP cells. GSH depletion may lead to lower CCAP cell copper levels, protecting developing animals against copper toxicity. This observation is in contrast with an earlier in vitro study where following GSH depletion, primary cortical neurons were exquisitely sensitive to trace amounts of extracellular copper; in this instance, the neurotoxic effects involved the generation of Cu (I) and ensuing free radical–mediated oxidative stress (White and Cappai 2003).
ATP7 over-expression and Ctr1A knockdown both caused an exacerbation of the developmental lethality caused by pan-neuronal Gclc knockdown. These copper transporter manipulations both cause copper deficiency (Lye et al. 2011), by ectopic efflux or reduced uptake, respectively (Norgate et al. 2006; Hwang et al. 2014), further supporting the notion that GSH depletion is contributing to copper deficiency. It has been previously shown that Drosophila Ctr1A maternal and zygotic mutants are defective in production of FMRF (Phe-Met-Arg-Phe)-amide-related peptides, as a result of a proposed deficiency in neuroendocrine cell-derived PHM activity (Turski and Thiele 2007).
Visualisation of CCAP neurons revealed that Gclc knockdown caused a decrease in the abundance of axon projections, with no obvious loss of neuronal cell bodies. This finding is consistent with that of El Meskini et al. (2007) who found that axon projections were defective in brindled mice as a direct consequence of reduced ATP7A-mediated copper transport into the brain. Degeneration of axons may be the first stage of progressive neurodegeneration; in the process of post-ecdysis programmed cell death of CCAP neurons, which normally occurs within 24 h post adult emergence (Lee et al. 2013), the axonal projections are the first to degenerate (Lee et al. 2013). We observed Gclc-knockdown-mediated axonal degeneration in third instar larval brains, indicating premature degeneration of these neurons. Interestingly, in combination with ATP7 over-expression, there was further loss of axon projections and shrinkage of CCAP neuron cell bodies, a further indication of the early stages of apoptosis. These Gclc-knockdown/ATP7 over-expression neurons have a similar appearance to wild-type CCAP neurons at 8–9 h post adult emergence, a time point at which they are undergoing programmed cell death (Lee et al. 2013).
It is important to note that while Gclc is the rate-limiting enzyme in GSH biosynthesis, its direct catalytic product is γ-glutamylcysteine (γGC) which is then converted into GSH by Glutathione Synthetase. Knockdown of mammalian GCL has been shown to cause neuronal apoptosis which can be rescued by addition of either γGC or glutathione ethyl ester (Diaz-Hernandez et al. 2005); the ability of glutathione ethyl ester to rescue indicates that this neuronal death is more likely to be caused by lack of GSH than by insufficient γGC. However, generation of γGC targeted to the mitochondria has been shown to detoxify hydrogen peroxide and superoxide anion independently of GSH by acting as a glutathione peroxidase-1 cofactor (Quintana-Cabrera et al. 2012). Therefore, we cannot currently rule out the possibility that some or all of our results are as a result of decreased γGC, not GSH.
Knockdown of Gclc would also be predicted to increase levels of its substrate, the γGC precursor cysteine. Given the known toxicity caused by cysteine and its metabolites to cultured neuronal cells (Parsons et al. 1997, 1998), such an increase could also contribute to the neuronal defects observed here. As mammalian neurons express relatively low GCL levels and rely on astrocytes for adequate supplies of GSH precursors (Dringen et al. 1999), it is reasonable to speculate that cysteine accumulation could have as great, or greater effect than GSH depletion in Gclc-knockdown neurons. Dietary supplementation with γGC and GSH might be able to resolve this issue and also address the question of whether copper deficiency is secondary to glutathione deficiency.
The results of this in vivo study suggest that adequate GSH biosynthesis is required for neuronal function and copper homeostasis. This has implications for age-related neurodegenerative diseases which have been linked to copper dyshomeostasis. An extensive analysis of copper distribution within neurons in response to decreased GSH levels, as well as investigation of Drosophila models of neurodegenerative diseases will provide further insight into the role that GSH levels play in the progression of these diseases.
Acknowledgements and conflict of interest disclosure
This work was supported by Project Grant funding from the National Health and Medical Research Council of Australia (RB, Project Grant #606609). The Australian Drosophila Biomedical Research Support Facility assisted in the import and quarantine of fly strains used in this research. We thank Professor Julian Mercer for critical appraisal of this manuscript. The authors have no conflict of interest to declare.