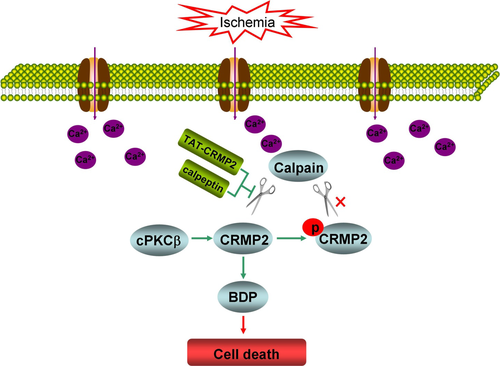
Conventional protein kinase Cβ-mediated phosphorylation inhibits collapsin response-mediated protein 2 proteolysis and alleviates ischemic injury in cultured cortical neurons and ischemic stroke-induced mice
Abstract
We previously reported that conventional protein kinase C (cPKC)β participated in hypoxic preconditioning-induced neuroprotection against cerebral ischemic injury, and collapsin response-mediated protein 2 (CRMP2) was identified as a cPKCβ interacting protein. In this study, we explored the regulation of CRMP2 phosphorylation and proteolysis by cPKCβ, and their role in ischemic injury of oxygen–glucose deprivation (OGD)-treated cortical neurons and brains of mice with middle cerebral artery occlusion-induced ischemic stroke. The results demonstrated that cPKCβ-mediated CRMP2 phosphorylation via the cPKCβ-selective activator 12-deoxyphorbol 13-phenylacetate 20-acetate (DOPPA) and inhibition of calpain-mediated CRMP2 proteolysis by calpeptin and a fusing peptide containing TAT peptide and the calpain cleavage site of CRMP2 (TAT-CRMP2) protected neurons against OGD-induced cell death through inhibiting CRMP2 proteolysis in cultured cortical neurons. The OGD-induced nuclear translocation of the CRMP2 breakdown product was inhibited by DOPPA, calpeptin, and TAT-CRMP2 in cortical neurons. In addition, both cPKCβ activation and CRMP2 proteolysis inhibition by hypoxic preconditioning and intracerebroventricular injections of DOPPA, calpeptin, and TAT-CRMP2 improved the neurological deficit in addition to reducing the infarct volume and proportions of cells with pyknotic nuclei in the peri-infact region of mice with ischemic stroke. These results suggested that cPKCβ modulates CRMP2 phosphorylation and proteolysis, and cPKCβ activation alleviates ischemic injury in the cultured cortical neurons and brains of mice with ischemic stroke through inhibiting CRMP2 proteolysis by phosphorylation.
Abbreviations used
-
- ACSF
-
- artificial cerebral spinal fluid
-
- BDP
-
- CRMP2 breakdown product
-
- CaMKII
-
- calcium/calmodulin-dependent protein kinase II
-
- CBF
-
- cerebral blood flow
-
- CDK5
-
- cyclin-dependent kinase 5
-
- cPKCβ
-
- conventional protein kinase Cβ
-
- CRMP2
-
- collapsin response-mediated protein 2
-
- DAPI
-
- 4′,6-diamidino-2-phenylindole
-
- DOPPA
-
- 12-deoxyphorbol 13-phenylacetate 20-acetate
-
- DTT
-
- dithiothreitol
-
- GAP-43
-
- growth-associated protein-43
-
- GSK3β
-
- glycogen synthase kinase 3β
-
- HPC
-
- hypoxic preconditioning
-
- I/R
-
- ischemia/reperfusion injury
-
- MAP2
-
- microtubule-associated protein 2
-
- MCAO
-
- middle cerebral artery occlusion
-
- mGluR1α
-
- metabotropic glutamate receptor 1α
-
- NMDA
-
- N-methyl-d-aspartic acid
-
- OGD
-
- oxygen–glucose deprivation
-
- PBS
-
- phosphate-buffered saline
-
- PKA
-
- protein kinase A
-
- PSD-95
-
- postsynaptic density protein 95
-
- SDS
-
- sodium dodecyl sulfate
-
- TAT peptide
-
- transduction domain of HIV-1 trans-activator transcription protein
-
- TAT-CRMP2
-
- fusing peptide containing TAT peptide and the calpain cleavage site of CRMP2
-
- TTC
-
- 2,3,5-triphenyltetrazolium chloride
Aside from other cardiovascular diseases, stroke ranks as the fourth fatal disease after heart diseases, cancer, and chronic lower respiratory disease, and on average someone dies of a stroke every 4 min (Mozaffarian et al. 2015). Ischemic/hypoxic preconditioning (I/HPC) is defined as an endogenous phenomenon in which sublethal hypoxia or ischemia exposure makes the tissues/organs resistant to later severe hypoxic/ischemic insults (Murry et al. 1986). The molecular mechanisms underlying I/HPC are of great interest because of their profound therapeutic potential (Gidday 2006; Sanders et al. 2010; Kitagawa 2012). There are multiple reported mechanisms of I/HPC, and protein kinase C (PKC) appears to play an essential role (Zhao et al. 2012).
We previously reported that conventional PKC (cPKC)β participated in HPC-induced neuroprotection against middle cerebral artery occlusion (MCAO)-induced ischemic injury and that 49 cPKCβ-interacting proteins were identified in the cortex of HPC mice (Li et al. 2005; Niu et al. 2005; Bu et al. 2011). Among the 10 cPKCβ-interacting proteins whose detection changed after HPC, collapsin response-mediated protein 2 (CRMP2) was found to be distributed in both cytosolic and particulate fractions, leading to the suggestion that CRMP2 might play an important role in cPKCβ-mediated neuroprotection (Bu et al. 2011).
CRMP2 was identified in 1995 as an intracellular transducer of the extracellular semaphorin 3A signal transduction pathway (Goshima et al. 1995). It was found concentrated in growing axons, dendrites, and cytoplasms of neurons (Inagaki et al. 2001). It is important in the determination of neuronal polarity and axonal elongation during neuron development (Arimura et al. 2004). As the most abundantly expressed member of the CRMP family in the mature brain, CRMP2, is thought to play an important role in pathophysiological processes in adult central nervous system (Wang and Strittmatter 1996). For example, CRMP2 has been reported to be involved in Alzheimer's disease (AD), epilepsy, neuropathic pain, prion disease as well as focal cerebral ischemia, and many other neuropathological processes (Czech et al. 2004; Chung et al. 2005; Shinkai-Ouchi et al. 2010; Wang et al. 2013; Fischer et al. 2014). Several studies have reported the changes of CRMP2 detection during the process of ischemia. Koh (2011) and Chen et al. (2007) reported elevation of CRMP2 protein expression in the ischemic brain, while Sato and Xiong and colleagues reported that hypoxia–ischemia caused dephosphorylation of CRMP2 in neonatal brain (Sato et al. 2011; Xiong et al. 2012). Evidence also showed that the intact CRMP2 decreased, whereas the CRMP2 breakdown product (BDP) appeared in the rat brain during ischemia (Chung et al. 2005; Jiang et al. 2007). Papers also reported that CRMP2 was cleaved by calpain at the C-terminal to form the BDP (Chung et al. 2005; Zhang et al. 2007). Although these studies observed changes of CRMP2 during focal cerebral ischemia, the pathophysiological role of CRMP2 in ischemic injury is still unclear. In this study, the regulation of CRMP2 phosphorylation and proteolysis by cPKCβ and their roles in ischemic injury in cultured cortical neurons and brains of mice with ischemic stroke were explored.
Material and methods
All chemicals were purchased from Sigma-Aldrich (St Louis, MO, USA) except for the individually indicated agents and antibodies. Adult (8–10 weeks old) male BALB/c mice weighing 20–25 g and postnatal 0 day were purchased from the Experimental Animal Center of Chinese Academy of Medical Sciences, PR China. The experimental protocols were approved by the Animal Care and Use Committee of Capital Medical University and are consistent with the National Institutes of Health (NIH, USA) Guide for the Care and Use of Laboratory Animals.
Primary cortical neuron culture and oxygen–glucose deprivation treatment
The postnatal day 0 BABL/c mice were killed within 24 h. The brains were removed and placed in ice-cold 10 mM phosphate-buffered saline (PBS). The cortices were then dissected out, cut into small pieces, and transferred into 0.25% (w/v) Trypsin–EDTA (Gibco Inc., Grand island, NY, USA) for 20 min at 37°C. High glucose Dulbecco's modified Eagle's medium (5 mL; Gibco Inc., Rockville, MD, USA) containing 10% (v/v) fetal bovine serum, 10% (v/v) equine serum, and 2 mM l-glutamine was added to terminate the action of trypsin. Mild trituration using a Pasteur pipette was used to create a cell suspension. Cells were plated onto poly-l-lysine-coated 60-mm culture dishes (2 × 106 cells per dish) for Western blot assay, 24-well plates with cover slips (2 × 105 cells per well) for immunofluorescent staining, or 96-well plates (1.5 × 104 cells per well) for Thiazolyl blue tetrazolium bromide (MTT) assay. The medium was replaced by serum-free neurobasal medium containing 2% B27 supplement (Gibco Inc.) 4 h later.
After culturing in neurobasal medium containing 2% B27 for 7 days, the cortical neurons were subjected to oxygen–glucose deprivation (OGD) treatment. The hypoxia incubator (Thermo Scientific, Marietta, OH, USA) was used in the OGD experiment. The incubator is capable to maintain O2 at a certain level through persistently pumping N2 into the incubator. The neurons were replaced with glucose-free Dulbecco's modified Eagle's medium and placed in the incubator. The time point when the atmosphere in the incubator was stabilized (5% CO2/1% O2/94% N2) was recorded as the starting time. At the end of 1 h OGD exposure, cells were maintained in regular neurobasal medium containing 2% B27 under normoxic conditions for 24 h reoxygenation.
cPKCβ and CRMP2 modification in OGD-injured primary cultured cortical neurons
cPKCβ was activated by the addition of 0.1 μM 12-deoxyphorbol 13-phenylacetate 20-acetate (DOPPA; Enzo Life Sciences Inc., Farmingdale, NY, USA) (Macfarlane and Manzel 1994). Calpeptin (0.2, 0.5, 1.0, and 2.0 μM; Enzo Life Sciences Inc.) was used to inhibit calpain-mediated cleavage of CRMP2. CRMP2 cleavage was also blocked by the addition of a fusing peptide containing TAT peptide and the calpain cleavage site of CRMP2 (TAT-CRMP2) (0.1, 0.25, and 0.5 μM, sequence: YGRKKRRQRRRGVPRGLYDGPVCEV, California Bioscience Inc., Sacramento, CA, USA), a fusion peptide containing TAT peptide (transduction domain of HIV-1 trans-activator transcription protein) and the calpain cleavage site of CRMP2 (Xu et al. 2007). All the chemicals above were added in the medium 1 h before and during the induction of OGD.
Animal models and treatments
The repetitive autohypoxia-induced hypoxic preconditioning (HPC) mouse model was performed as our previous reports (Lu and Liu 2001; Bu et al. 2011; Zhang et al. 2011). Briefly, mice were placed in a 125-mL jar full of normoxic air and then the jar was sealed with a rubber plug so that continued breathing induced progressive hypoxia. The autohypoxia-treated mice were removed from the jar to normoxic conditions when the first signs of gasping appeared and recovered for 30 min. After the recovery period, mice were switched to another hermetically sealed jar. This procedure was repeated four times to obtain the HPC mice; normoxic control mice were placed in open jars for the same amount of times. The oxygen concentration inside the jar at the moment of the first gasping appearance was about 6.6% in the first run and 3.8% in the fourth run, respectively.
Two in vivo models of ischemia were used. The first was induction of 1 h transient MCAO with sacrifice at 24 h. The second was permanent MCAO with sacrifice at 6 h. Briefly, using methods previously described (Bu et al. 2011; Zhang et al. 2011; Feng et al. 2013), mice were anesthetized with sodium pentobarbital (0.06 g/kg i.p.). A ventral midline incision was then made to expose the left common carotid artery, and external and internal carotid arteries. The left common carotid artery and external carotid artery were ligated. For transient MCAO, an arteriotomy was made in the left external carotid artery, and then a blunt-tipped 5-0 surgical nylon filament (0.22 ± 0.01 mm in diameter; Beijing Cinontech, Beijing, China) was inserted through the external carotid artery to the internal carotid to a point approximately 12-mm distal to the carotid bifurcation to occlude the middle cerebral artery. After 1 h occlusion, the filament was carefully withdrawn to restore blood flow and the mice were kept for 24 h. For permanent MCAO, the same filament was inserted via the common carotid artery. Mice were kept for 6 h before being killed. The ischemic penumbra or peri-infarct region exits more apparently, and the effects of cPKCβ on CRMP2 phosphorylation and proteolysis are obvious at this time point. In the sham groups, mice received the same surgical exposure without occlusion. During the surgery, body temperature was maintained at 37°C using a heating lamp and thermal blanket. Mice were placed in a postoperative cage, kept warm, and undisturbed for at least 2 h with regular observation.
cPKCβ and CRMP2 modification in MCAO mice by intracerebroventricularly injection
Dimethylamine analog LY333531 (200 nM, 5 μL, cPKCβ-specific inhibitor) (Jirousek et al. 1996), DOPPA (2 μM, 5 μL, cPKCβ-selective activator), calpeptin (20 μM, 5 μL, calpain inhibitor), and TAT-CRMP2 (10 μM, 5 μL), which can prevent the cleavage of endogenous CRMP2, were intracerebroventricularly injected just before MCAO as in our previous reports (Bu et al. 2011; Zhang et al. 2011). Briefly, mice were anesthetized with pentobarbital sodium (0.06 g/kg i.p.) and fixed in a stereotaxic frame. The cannula (28-gauge, inner diameter 0.18 mm, outer diameter 0.36 mm) tip was inserted into the left cerebral ventricle at a point 0.5 mm posterior to bregma, 1.0 mm lateral to bregma, and 3.5 mm below the skull surface. The dosages of LY333531, DOPPA, calpeptin, and TAT-CRMP2 were determined by our preliminary experiments.
Measurement of cerebral blood flow
Regional cerebral blood flow (CBF) was monitored by laser Doppler flowmetry (Perimed Periflux system 5000, Järfälla-Stockholm, Sweden). The tip of the probe was fixed to the surface of skull over the MCA supplied core area (2 mm posterior to bregma and 6 mm lateral to midline). Cerebral blood flow during MCAO and reperfusion was expressed as a percentage of the baseline value.
Neurological deficit scoring and behavioral test
Neurological deficit scores were evaluated as reported previously (Longa et al. 1989) by an observer blinded to the treatment: 0, no deficit; 1, flexion of contralateral forelimb upon lifting of the whole animal by the tail; 2, circling to the contralateral side; 3, falling to the contralateral side; and 4, no spontaneous motor activity.
Behavioral tests were performed by an observer blinded to the treatment. The wire hanging test and pole test were performed as described previously (Ji et al. 2009). The wire hanging test was used to assess the motor deficits of mice. Mice were trained to cling with their forepaws to a steel wire (1 mm) stretched between two posts 60 cm above a thick protective pad 2 days before the actual test. On the day of testing, latency to fall was tested three times and results were averaged. The pole test was carried out using a vertical steel pole covered with tape to create a rough surface. Mice were placed near the top of the pole with head upward. The time taken to turn completely downward and the total time to reach the floor with all four paws were recorded. Each animal was tested three times and the average time was taken as the results.
Immunofluorescent staining of CRMP2 and cell viability assay in cultured cortical neurons
After the treatment of OGD, cells grown on cover slips were fixed in 4% paraformaldehyde and goat serum in PBST was used for 1 h as a block to prevent non-specific antibody binding. Cells were then incubated with rabbit anti-CRMP2 (1 : 500, #9393; Cell Signaling Technology, Beverly, MA, USA) overnight. After washing, the secondary Alexa Fluor® 488-conjugated goat anti-rabbit IgG (1 : 500, green fluorescent; Invitrogen, Carlsbad, CA, USA) was added and incubated for 2 h. Finally, 4′,6-diamidino-2-phenylindole was used to stain the nuclei. Cover slips were dried at 4°C overnight. Slides were mounted and observed under a fluorescence microscope system (Leica DM4000B, Wetzlar, Germany).
According to the manufacturer's instruction, the cell viability of cultured cortical neurons was assessed by Thiazolyl blue tetrazolium bromide (MTT, 0.5 mg/mL; Applichem Inc., Omaha, NE, USA). The OD490 for the control group was set to 100%, and the other groups were presented as a percentage of control.
Measurements of infarct size by TTC staining
After permanent and transient MCAO, blind measurements of the infarct volume were taken. Mice were killed, and the brains were quickly removed and cut into 1.5-mm thickness coronal sections. Brain sections were incubated in 0.5% 2,3,5-triphenyltetrazolium chloride (TTC) solution at 37°C for 20 min, and then the images of the slices were scanned into a computer. The infarct volume was analyzed according to the evaluation procedure of our previous reports (Bu et al. 2011; Zhang et al. 2011), and expressed as the percentage of total brain through the equation: I = [∑VI × (1 − E)/∑VT × (1 − B)] × 100%. ∑VI is the volume of tissue that is not stained with TTC, ∑VT is the total brain volume, E is edema ratio that was calculated from (∑VL − ∑VR)/(∑VL + ∑VR) × 100%, ∑VL and ∑VR are the volume of left ischemic and right non-ischemic hemisphere, B is background (∑VS/∑VT) × 100%, ∑VS is the volume of the unstained white matter in the sham group, and ∑VT is the total brain volume.
Nissl staining
Nissl staining was used to assess the neural cell damage in brain sections. Mice for this measurement were deeply anesthetized and transcardially perfused with 100 mM PBS containing 4% paraformaldehyde. Brains were removed and postfixed in 4% paraformaldehyde followed by graded sucrose solutions (20% for 24 h and 30% for another 24 h) to dehydrate. Then brains were cut into 20-μm thick slices, washed in fresh PBS, stained with 0.04% cresyl violet (Sigma-Aldrich), and dissolved in acetate buffer for 1 h. Images were photographed under a Nikon 50i light microscopy (Nikon, Tokyo, Japan).
Sample preparation and Western blot analysis
At the end of 1 h OGD followed by 24 h reoxygenation, cells in 60-mm dishes were washed three times with PBS and then stored at −70°C. For the required regions of cortexes, the brains from killed mice were immediately placed into ice-cold artificial cerebral spinal fluid (in mM: NaCl 125.0, KCl 2.5, CaCl2 2.0, NaHCO3 26.0, NaH2PO4 1.25, MgCl2 1.0, glucose 5.0, pH 7.4) bubbled with 95% O2/5% CO2 and dissected as previous reports (Ashwal et al. 1998; Niu et al. 2005). In brief, 2 mm from the anterior tip of the frontal lobe was removed and the remaining brain was sectioned to four 2-mm thickness slices. Each hemisphere was longitudinally cut 1 mm from the midline to remove the tissue supplied by the anterior cerebral artery. A 45-degree angle transverse diagonal cut was then made to separate the ischemic core and peri-infarct region. The corresponding regions from the non-ischemic hemisphere were dissected as contralateral controls. All the tissues were frozen in liquid nitrogen and kept frozen at −70°C for later analysis.
To obtain whole cell lysates, the cultured cortical neurons and tissue samples were rapidly thawed and homogenized at 25°C in buffer C [5 mM Tris-Cl, pH 7.5, containing 2 mM dithiothreitol, 2 mM ethylenediaminetetraacetic acid (EDTA), 1 mM ethyleneglycoltetraacetic acid (EGTA), 5 μg/mL each of leupeptin, aprotinin, pepstatin A, and chymostatin, 50 mM potassium fluoride, 50 μM okadaic acid, 5 mM sodium pyrophosphate, 2% sodium dodecyl sulfate] and sonicated. Protein concentration was determined by bicinchoninic acid kit (Pierce Company, Rockford, IL, USA) with albumin diluted in buffer C as standard.
Samples loaded with the equal amount of proteins (50 μg) were electrophoresed on 10% sodium dodecyl sulfate polyacrylamide gel, and then transferred onto polyvinylidene difluoride (PVDF) membrane (GE Healthcare, Buckinghamshire, UK) at 4°C. After several rinses with Tween/Tris-buffered salt solution (20 mM Tris-Cl, pH 7.5, 0.15 M NaCl, and 0.05% Tween-20), the transferred PVDF membrane was blocked with 10% non-fat milk in Tween/Tris-buffered salt solution for 1 h. The blocked PVDF membrane was then incubated with the primary polyclonal antibodies of rabbit anti-CRMP2 (1 : 1000, #9393; Cell Signaling Technology), rabbit anti-phospho-CRMP2 (T514, 1 : 2000, ab62478; Abcam Inc., Cambridge, MA, USA), or rabbit anti-caspase-3 (1 : 1000, #9662; Cell Signaling Technology) for 3 h at 25°C. To verify equal loading of protein, the blots were reprobed with primary monoclonal antibody of mouse anti-β-actin (1 : 10 000, 60008-1-Ig; Proteintech Group, Chicago, IL, USA) or mouse anti-β-tubulin polyclonal antibody (1 : 10 000, 10094-1-AP; Proteintech Group). The horseradish peroxidase-conjugated goat anti-rabbit or anti-mouse (1 : 5000; Stressgen Biotechnologies Corporation, Victoria, BC, Canada) were incubated as second antibodies for 1 h. After incubation with the secondary antibodies, the enhanced chemiluminescence kit (GE Healthcare) was used to detect the signals.
Statistics and analysis
The Gel Doc-2000 imaging system (Bio-Rad Laboratories, Hercules, CA, USA) was used to perform the quantitative analysis of Western blot results. For CRMP2 protein level, the ratio of intact CRMP2 densities to β-actin band density in the control group was expressed as 1.0; for the changes in CRMP2 phosphorylation, the ratio of p-CRMP2 band density to total CRMP2 band densities was expressed as 1.0 in control group. The ratio of cleaved caspase-3 band density to procaspase-3 and cleaved caspase-3 band densities was normalized to 1.0 in control. The data from other groups were represented as ratio of that from control group. The presented values were expressed as mean ± SE. Statistical analysis was conducted by one-way anova followed by all pairwise multiple comparison procedures using Bonferroni test, with significance cut-off set as p < 0.05.
Results
cPKCβ-mediated phosphorylation inhibits CRMP2 proteolysis and protects cultured cortical neuron against OGD-induced ischemic injury
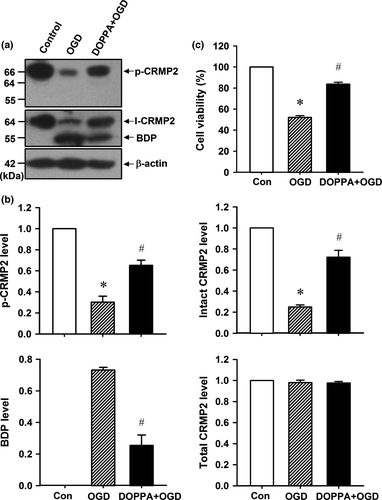
CRMP2 is abundantly expressed in neuron cytosol and has been proved to produce a 55-kD BDP of CRMP2 in cultured neurons under simulated ischemic conditions (Bretin et al. 2006; Zhang et al. 2007). In this study, cortical neurons from postnatal mice were cultured for 7 days, and then subjected to 1 h OGD followed by 24 h reoxygenation in regular medium. We found that the intact CRMP2 (I-CRMP2, Fig. 1a and b, one-way anova followed by Bonferroni test, p < 0.001, n = 6 per group) protein levels reduced significantly, while BDP appeared and the CRMP2 phosphorylation level decreased (p-CRMP2, Fig. 1a and b, p < 0.05, n = 6 per group) in 1 h OGD/24 h reoxygenation-treated cortical neurons. However, the cPKCβ-selective activator DOPPA (0.1 μM) promoted higher p-CRMP2 and intact CRMP2 levels, and inhibited the production of BDP when compared with that of the 1 h OGD/24 h reoxygenation group (Fig. 1a and b, p < 0.05, n = 6 per group). In addition, the MTT assay results in Fig. 1(c) showed that DOPPA could significantly increase the cell survival rate of 1 h OGD/24 h reoxygenation-treated cortical neurons (Fig. 1c, p < 0.05, n = 8 per group). These results suggested that cPKCβ-mediated CRMP2 phosphorylation protects cortical neurons against 1 h OGD/24 h reoxygenation-induced ischemic injuries through inhibiting CRMP2 proteolysis.
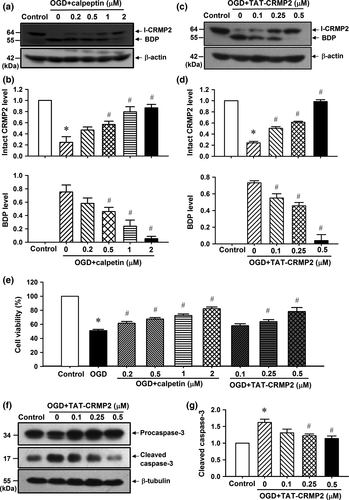
Inhibition of calpain-mediated CRMP2 proteolysis protects cultured cortical neurons against OGD-induced ischemic injury and cell apoptosis
To determine the role of CRMP2 proteolysis in 1 h OGD/24 h reoxygenation-induced ischemic injuries, the calpain inhibitor calpeptin and TAT-CRMP2 were used to block the endogenous CRMP2 proteolysis.
As shown in Fig. 2(a) and (b), calpeptin significantly reduced the endogenous CRMP2 proteolysis at 0.2, 0.5, 1.0, and 2.0 μM (p < 0.05, n = 5 per group) in 1 h OGD/24 h reoxygenation-treated cortical neurons. Similarly, TAT-CRMP2 (0.1, 0.25, and 0.5 μM) also significantly inhibits the production of BDP in 1 h OGD/24 h reoxygenation-treated cortical neurons (Fig. 2c and d, p < 0.05, n = 5 per group). The MTT assay results showed that both calpeptin and TAT-CRMP2 effectively reduced the 1 h OGD/24 h reoxygenation-induced cell death in cultured neurons in a dose-dependent manner (Fig. 2e, p < 0.05, n = 8 per group). In addition, we found that 1 h OGD/24 h reoxygenation induced a significant increase in cleaved caspase-3 (17 kD), while TAT-CRMP2 dose dependently inhibited the cleavage of procaspase-3 (34 kD, Fig. 2f and g, p < 0.05, n = 5 per group). These results suggested that calpeptin and TAT-CRMP2 protect cortical neurons against 1 h OGD/24 h reoxygenation-induced ischemic injury and cell apoptosis through inhibiting CRMP2 proteolysis.
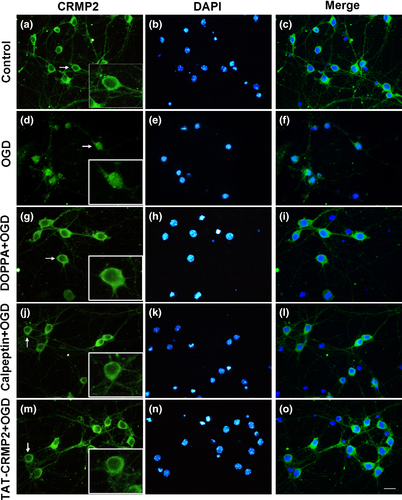
BDP nuclear translocalization is involved in OGD-induced ischemic injury in cultured cortical neuron
To determine whether changes of CRMP2 subcellular distribution occurred after OGD, immunofluorescent staining was applied to observe CRMP2 expression in cortical neurons following 1 h OGD/24 h reoxygenation treatment. As shown in Fig. 3(a–c), CRMP2 was mainly expressed in the cytoplasm of control cortical neurons, while the 1 h OGD/24 h reoxygenation treatment caused an increase of CRMP2 nuclear translocation (Fig. 3d–f). This OGD-induced nuclear localization of CRMP2 could be prevented by pretreatment of cPKCβ-selective activator DOPPA (Fig. 3g–i), calpeptin (Fig. 3j–l), and TAT-CRMP2 (Fig. 3m–o).
cPKCβ-mediated phosphorylation inhibits CRMP2 proteolysis and protects mouse brain after ischemic stroke
cPKCβ-selective activator DOPPA was administered in mouse brain to further determine whether cPKCβ modulates phosphorylation and proteolysis of CRMP2 in vivo. As shown in Fig. 4(a) and (b), there was a significant decrease in CRMP2 phosphorylation and an increase in CRMP2 proteolysis, but DOPPA inhibited the decrease in p-CRMP2 level and the increase in BDP level both in ischemic core and peri-infarct region of mice with 6 h permanent MCAO (p < 0.05, n = 5 per group). Consistent with our previous report (Bu et al. 2011), HPC-induced cPKCβ activation played a similar role to the DOPPA in CRMP2 phosphorylation and proteolysis in ischemic core and peri-infarct region, but intracerebroventricular injection of cPKCβ-specific inhibitor LY333531 could inhibit this effect of HPC on CRMP2 phosphorylation and proteolysis (Fig. 4c and d, p < 0.05, n = 5 per group). In addition, the TTC staining results showed that DOPPA could reduce the neurological deficit scores (Fig. 4e, p < 0.05, n = 8 per group) and the infarct volume (Fig. 4f and g, p < 0.05, n = 5 per group) of mice with 1 h MCAO/24 h reperfusion, which indicates that cPKCβ-mediated CRMP2 phosphorylation can protect the brain against ischemic injury in mice with ischemic stroke through inhibiting CRMP2 proteolysis in vivo.
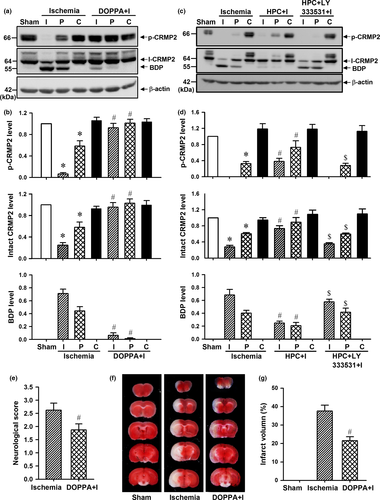
Inhibition of calpain-mediated CRMP2 proteolysis protects brain against ischemic injury of mice with ischemic stroke
To further determine the neuroprotective effect of CRMP2 proteolysis inhibition in vivo, calpeptin and TAT-CRMP2 were intracerebroventricularly injected to inhibit CRMP2 proteolysis in mouse brain with transient MCAO. As shown in Fig. 5(a) and (b), calpeptin effectively suppressed the production of BDP both in ischemic core and in the peri-infarct region (p < 0.05, n = 4 per group) and reduced the neurological deficit scores (Fig. 6a, p < 0.05, n = 9 per group) and infarct volume (Fig. 6b and c, p < 0.05, n = 5 per group) of mice with 1 h MCAO/24 h reperfusion.
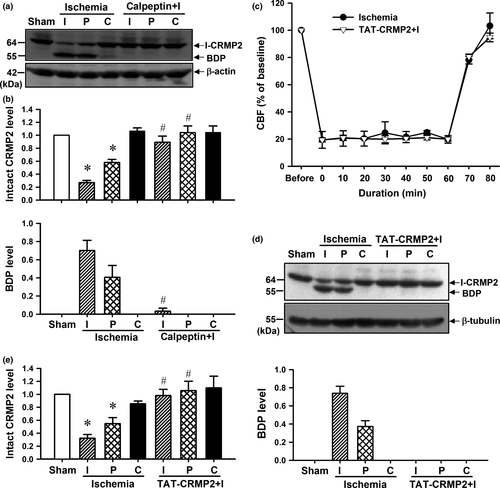
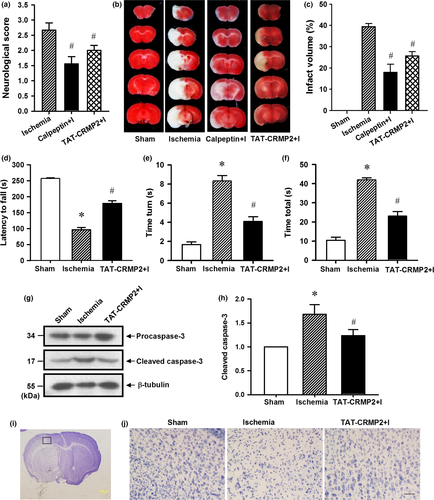
We previously reported that TAT-CRMP2 could inhibit CRMP2 dephosphorylation and proteolysis in ischemic core of mice with 6 h permanent MCAO (Bu et al. 2011). Here, we applied transient MCAO mouse model to determine the neuroprotective effect of TAT-CRMP2 on the brain of mice with ischemic stroke. Cerebral blood flow was monitored during MCAO and 20 min of reperfusion by laser Doppler flowmetry. After the onset of MCAO, CBF sharply reduced to about 20% of baseline value and this was maintained during the 1 h of MCAO. The decrease of CBF in TAT-CRMP2 administrated mice was not significantly different from control ischemic mice (Fig. 5c, n = 4 per group). Intracerebroventricular injection of TAT-CRMP2 could inhibit CRMP2 proteolysis in ischemic core and peri-infact region of mice with 1 h transient MCAO followed by 24 h reperfusion (Fig. 5d and e, p < 0.05, n = 6 per group). Mice treated with TAT-CRMP2 achieved lower scores than the ischemia group (Fig. 6a, p < 0.05, n = 9 per group). The wire hanging test showed that ischemic mice clung to the wire for less time than the sham animals and TAT-CRMP2 treatment made mice cling to the wire for longer (Fig. 6d, p < 0.05, n = 7 per group). The pole test showed that animals from the ischemic group took more time to turn their head downward on the pole (time turn) and reach the ground (time total) compared to the sham-operated groups. TAT-CRMP2 reduced the time for mice to turn their head and reach the ground (Fig. 6e and f, p < 0.05, n = 7 per group). The TTC and Nissl staining results demonstrated that TAT-CRMP2 effectively reduce both the infarct volume (Fig. 6b and c, p < 0.05, n = 5 per group) and proportion of shrunken cells with pyknotic nuclei in peri-infact region (Fig. 6i and j) of brain with 1 h MCAO/24 h reperfusion. In addition, we observed that TAT-CRMP2 reduced the cleavage of caspase-3 in the peri-infarct region of ischemic mice (Fig. 6g and h, p < 0.05, n = 5 per group). These results suggested that calpeptin and TAT-CRMP2 protect brains against ischemic injury of mice with ischemic stroke through inhibiting calpain-mediated CRMP2 proteolysis in vivo.
Discussion
cPKCβ including βI and βII isoforms belongs to the conventional PKC family and its role in ischemia was unclear. A harmful role of cPKCβ has been reported in lung and myocardial ischemia/reperfusion (I/R) injury. Lung I/R-treated cPKCβ knockout and wild-type (WT) mice fed with the cPKCβ inhibitor ruboxistaurin showed increased survival (Fujita et al. 2004). Similarly, cPKCβ knockout and WT mice fed with ruboxistaurin showed significantly decreased infarct size and cellular necrosis in a myocardial I/R model (Kong et al. 2008). Conversely, a protective effect of cPKCβ against ischemic injury has been observed in central nervous system. For example, cPKCβ inhibition aggravated N-methyl-d-aspartic acid (NMDA)-induced excitotoxicity and cell death area in cultured hippocampal slices (Kowalczyk et al. 2012). cPKCβ activation was also reported to be involved in the development of HPC both in vivo and in vitro, and the cPKCβ inhibitors Go6983 and LY333531 could block the neuroprotective effect of HPC, suggesting that cPKCβ mediated HPC-induced endogenous neuroprotection (Niu et al. 2005; Li et al. 2006; Bu et al. 2011). In this study, we further observed that cPKCβ activation by its selective activator DOPPA could alleviate OGD-induced ischemic injury in cultured cortical neurons and reduce the brain infarct volume of mice with ischemic stroke, which confirms the neuroprotective effect of cPKCβ in ischemic-induced injury in vitro and in vivo. In our previous studies to elucidate cPKCβ-mediated signaling networks, we have reported 49 cPKCβII-interacting proteins. Among these proteins, CRMP2 changed significantly in both cytosolic and particulate fraction in HPC mouse brain, suggesting the possibility of CRMP2 as a downstream effector of cPKCβ action (Bu et al. 2011).
During the past decade, studies of the function of CRMP2 have mainly focused on neuronal polarity and neuron development, with little being known about the pathophysiological role of CRMP2. Studies have reported that ischemia could induce CRMP2 dephosphorylation and production of a 55 kD BDP, which was produced by calpain-mediated proteolysis (Chung et al. 2005; Jiang et al. 2007). We also detected the dephosphorylation and proteolysis of CRMP2 both in OGD-treated cortical neurons and ischemic mouse brain. In this study, the permanent 6 h MCAO mouse model was used to determine the effect of cPKCβ activity on CRMP2 phosphorylation and proteolysis. The application of cPKCβ-selective activator DOPPA and HPC-induced cPKCβ activation could inhibit ischemia-induced dephosphorylation and BDP production of CRMP2 in 6 h permanent MCAO mice. These results suggested that cPKCβ could mediate CRMP2 phosphorylation and thereby increase its resistance to calpain-mediated proteolysis of CRMP2.
The C-terminal region of CRMP2 is highly rich in serine and threonine, which can be phosphorylated by a number of kinases, including cyclin-dependent kinase 5, glycogen synthase kinase 3β (GSK3β), Rho kinase, calcium/calmodulin-dependent protein kinase II, and protein kinase A (Arimura et al. 2005; Uchida et al. 2005; Boudreau et al. 2009; Hou et al. 2009; Wilson et al. 2014). Phosphorylation of CRMP2 by cyclin-dependent kinase 5, GSK3β, and Rho kinase lowers its binding affinity to tubulin, thereby mediating the collapse of growth cones (Arimura et al. 2005), and suggests that the biological function of CRMP2 could be regulated by its phosphorylation. Although several studies have reported a decrease of CRMP2 phosphorylation during ischemia, its role in ischemic injury remains unclear. Hou et al. (2009) has described calcium/calmodulin-dependent protein kinase II as a kinase upstream of CRMP2 with phosphorylation preventing CPMP2 from being cleaved by calpain and enhancing the integrity of axons in the face of NMDA-induced excitotoxicity. We found that cPKCβ is also an upstream kinase of CRMP2 increasing its resistance to calpain-mediated cleavage, and the neuroprotective effect of cPKCβ in ischemia is then probably mediated by CRMP2.
Calpain is a calcium-dependent cysteine protease that is ubiquitously expressed in various mammalian tissues and plays a key role in cerebral ischemia. During cerebral ischemia, calpain is activated by increased calcium influx through the hyper activation of glutamate receptors, and inhibition of calpain can protect the brain against ischemic injury (Vosler et al. 2008). In this study, we once again demonstrated the detrimental role of calpain both in vitro and in vivo. Calpain inhibition blocked CRMP2 proteolysis both in the ischemic mouse brain and OGD-treated cortical neurons, further confirmed that CRMP2 was cleaved by calpain. Pathophysiological activation of calpain during ischemia results in the cleavage of a number of neuronal substrates such as microtubule-associated protein 2 (MAP-2), growth-associated protein-43, postsynaptic density protein 95, metabotropic glutamate receptor 1α (mGluR1α), NR2A and NR2B subunits of NMDA receptors that regulate neuronal structure and function, leading to disruption of essential neuronal survival mechanisms (Siman and Noszek 1988; Zakharov and Mosevitsky 2001; Guttmann et al. 2002; Simpkins et al. 2003; Xu et al. 2007; Gascon et al. 2008). As a substrate of calpain, CRMP2 and its BDP may be involved in calpain-modulated neuron death.
To better understand the role of CRMP2 and its BDP in focal cerebral ischemia, we constructed a TAT-CRMP2 fusion peptide which contained the calpain cleavage site of CRMP2. TAT peptide is derived from the transduction domain of HIV-1 TAT protein and contains 11 residues (YGRKKRRQRRR). TAT peptide can effectively transfer the fusion peptide through the membrane to cells without affecting its biological activity (Nagahara et al. 1998; Zhou et al. 2009). The transfer process occurs within 10 min (Pei et al. 2006). When TAT-CRMP2 was applied in cultured cortical neurons and MCAO-treated mice, it effectively inhibited calpain-mediated cleavage of CRMP2 and alleviated ischemic injury.
Calpain-mediated C-terminal cleavage of CRMP2 has been reported to be involved in traumatic brain injury, focal cerebral ischemia, Wallerian degeneration, and prion disease (Jiang et al. 2007; Shinkai-Ouchi et al. 2010; Brittain et al. 2011; Wakatsuki et al. 2011). At present, the role of CRMP2 and its BDP in ischemia process remains unclear. Bretin et al. (2006) observed that over-expression of BDP could reduce the surface N2B subunit of glutamate receptors and improve resistance to NMDA-induced exitotoxicity. Rogemond et al. (2008) found that BDP localized to the nucleus and inhibited neurite outgrowth in PC12 cells after transfection with the short isoform of CRMP2. Tahimic et al. (2006) also reported the nucleus translocation of CRMP2-truncated fragment in fibroblast cells which exhibited apoptosis-like features. Nada and Shah (2012) reported that after exposure of neurons to tert-butyl hydroperoxide (t-BuOOH) for 6 h, the CRMP2 protein was dissociated from the axons and dendrites and was observed concentrated in the nucleus. The structure of BDP gives few clues to its specific function. It is reported to contain a nuclear localization signal (Rogemond et al. 2008) and the primary sequence of CRMP2 (17-471) has some homology with the dihydropyrimidinase enzyme but no enzymatic activity has been detected. In this study, we demonstrated that TAT-CRMP2 inhibited the cleavage of caspase-3 and CRMP2 nuclear localization in OGD-treated cortical neurons; and this OGD-induced CRMP2 nuclear localization could also be blocked by DOPPA-mediated cPKCβ activation and inhibition of calpain-mediated CRMP2 proteolysis. These observations further suggested that CRMP2 is a common target of both cPKCβ and calpain, and BDP as CRMP2 proteolysis product may play an important role in ischemic injuries such as cell apoptosis in the cultured cortical neurons and brains of mice with ischemic stroke. However, further evidence is needed to determine whether BDP works through the nuclear localization.
In conclusion, we demonstrated that cPKCβ mediates the phosphorylation and proteolysis of CRMP2, and cPKCβ activation alleviates ischemic injury in the cultured cortical neurons and brains of mice with ischemic stroke through inhibiting CRMP2 proteolysis by phosphorylation.
Acknowledgments and conflict of interest disclosure
This work was supported by grants from the National Natural Science Foundation of China (Grant Nos. 31171147 and 81301015), the Beijing Natural Science Foundation (Grant Nos. 7132070 and 7141001), and the Seed Grant of International Alliance of Translational Neuroscience (PXM2014-014226-000006). The authors confirm that there are no conflicts.
All experiments were conducted in compliance with the ARRIVE guidelines.