Relationship between maternal egg consumption during lactation and the risk of developing egg allergies in 12-month-old infants: A multicenter cohort study
Abstract
Aim
To determine the effect of maternal egg consumption during lactation on the development of egg allergies in 12-month-old infants. We hypothesized that infants whose mothers consume larger amounts of eggs during the early lactation period acquire oral immune tolerance and are less likely to develop egg allergies at 12 months.
Methods
This study was a part of the Japan Pregnancy Eating and Activity Cohort Study. Mothers answered questionnaires on egg consumption, breastfeeding rates at 1 month, infants' eczema at 6 months, and the development of egg allergies among infants at 12 months. In order to assess the actual impact of maternal egg consumption on infants' egg allergy development, breastfeeding-dependent egg consumption was calculated at 1 month by multiplying maternal egg consumption at 1 month with the breastfeeding rates at the same time. Logistic regression analysis was performed to examine the potential risk and protective factors.
Results
Data from 420 infants were analyzed, of whom 27 had egg allergies at 12 months. No significant impact of breastfeeding-dependent egg consumption was observed on infants' egg allergy development at 12 months. However, infants with eczema at 6 months showed a greater risk of developing an egg allergy at 12 months (adjusted odds ratio, 3.59; 95% confidence interval, 1.59–8.13).
Conclusion
The results suggest that breastfeeding-dependent egg consumption at 1 month did not contribute to sufficient oral immune tolerance in 12-month-old infants. Eczema at 6 months significantly impacted the development of an egg allergy, emphasizing the importance of preventing cutaneous exposure to egg allergens.
1 INTRODUCTION
Food allergies are immune-mediated adverse reactions triggered by otherwise harmless protein antigens. Egg allergy is among the most prevalent food allergies in young children, alongside peanut and cow milk allergies (Loh & Tang, 2018). The clinical symptoms and severity of egg allergy are diverse and can potentially be life-threatening (Caubet & Wang, 2011). In Japan, eggs are the most predominant food allergen among 1-year-old infants; the prevalence of caregiver-reported food allergies and egg allergies was 7.6% and 5.3%, respectively (Yamamoto-Hanada et al., 2020).
A dual allergen exposure hypothesis has been proposed to explain the pathogenesis of food allergies (Lack, 2008). According to this hypothesis, cutaneous exposure to food allergens promotes allergic sensitization, while oral exposure induces immune tolerance. Previous studies support this hypothesis concerning the occurrence and prevention of egg allergies: Infant eczema, which promotes cutaneous exposure to food allergens, was revealed to be an important risk factor for allergies toward eggs and other foods (Hill et al., 2007; Tsakok et al., 2016). Furthermore, the early introduction of eggs as a complementary food from 4 to 6 months of age was demonstrated to be a protective factor (Natsume et al., 2017; Perkin et al., 2016).
Considering the importance of the early introduction of eggs, it is proposed that oral exposure to eggs through breast milk may be more effective than complementary food (Verhasselt, 2015). In a mouse model, egg protein transferred through breast milk induced oral tolerance, which prevented allergic reactions to eggs (Verhasselt et al., 2008). An analysis of breastfed infants with a maternal allergic history showed that egg protein in breast milk at 3 and 6 months could reduce egg allergy incidence (Verhasselt et al., 2020). Consumption of egg protein through breast milk before initiating complementary food could induce immunological tolerance in the gut and prevent egg allergies.
Several studies have been conducted based on the hypothesis that the maternal diet during early lactation promotes immune tolerance. One observational study examined white egg sensitization in 12-month-old infants after adjusting for maternal egg consumption and breast milk volume at 1 month (Shibuya & Saito, 2015). Two randomized controlled trials conducted an intervention of maternal egg consumption; one study period was up to 5 days (Nagakura et al., 2023), and the other was 6 months (Palmer et al., 2022). However, the association between maternal egg consumption and egg allergy development in offspring remain to be elucidated. The reason for this may be that the intervention period was too short (Nagakura et al., 2023), the number of participants and those who developed egg allergies were too small (Palmer et al., 2022), and the analysis did not adjust for eczema (Nagakura et al., 2023; Shibuya & Saito, 2015), which is strongly suggested to be associated with egg allergy development (Hill et al., 2007; Tsakok et al., 2016). Therefore, a large-scale study is necessary to examine the association between maternal egg consumption during lactation and immune tolerance in infants while considering the presence or absence of eczema.
This study aimed to investigate whether maternal egg consumption during the early lactation period is associated with the development of egg allergies while considering the factors of infant feeding methods and eczema. We hypothesized that infants exposed to higher amounts of eggs through breast milk at 1 month would have greater oral tolerance, resulting in a reduced risk of an egg allergy at 12 months.
2 METHODS
2.1 Study design and participants
This study was part of the Japan Pregnancy Eating and Activity Cohort Study (J-PEACH Study), which is a multicenter prospective cohort study. The J-PEACH study aimed to examine the association between mothers' lifestyles and the health outcomes of both mothers and their infants (J-PEACH Study, 2024). The study was conducted across four regions in Japan: Yamagata, Tokyo, Osaka, and Fukuoka. Mothers were recruited during pregnancy, and mother-infant dyads were followed up for 12 months after delivery. The eligibility criteria for J-PEACH study participants were as follows: Women who (1) were aged 18 years and older, (2) were expected to deliver at the study facility, (3) were able to read and write Japanese. Women who were deemed by hospital staff to be difficult cases for psychosocial reasons were excluded from the J-PEACH study.
Researchers who were licensed nurses or midwives recruited mothers at the maternal health checkup for pregnant outpatients. The recruitment period was from March 2020 to October 2022. However, recruitment was halted in Tokyo from April to July 2020 and November 2020 to March 2021 due to the 2019 coronavirus disease pandemic (COVID-19).
Consenting mothers completed a web-based questionnaire detailing their daily lives, health, and daily food intake, and the health status of their infants. In the original cohort study, questionnaires were distributed three times during pregnancy (in the first, second, and third trimesters) and three times after delivery (1, 6, and 12 months postpartum) via email.
In this part of the study, infants whose mothers completed questionnaires at 1, 6, and 12 months (referred to as the 1-month, 6-month, and 12-month questionnaires, respectively) were considered for analysis. The inclusion criteria were as follows: Infants who had answers on (1) both breastfeeding status and maternal egg consumption in the 1-month questionnaire, (2) infant skin conditions in the 6-month questionnaire, and (3) infant food allergies in the 12-month questionnaire. The exclusion criteria were as follows: Infants whose mothers (1) responded to the questionnaires over 2 months later and (2) reported an extremely unrealistic energy intake on the diet history questionnaire at 1 month. Unrealistic energy intake was defined based on a previous study (Takei et al., 2019) as reported energy intake exceeding half of the estimated energy requirement for women with a low-physical activity level in their 30s (Ministry of Health, Labour and Welfare of Japan, 2020): 875 kcal (1750 kcal × 0.5), or exceeding 1.5 times the estimated energy requirement for lactating women with a moderate physical activity level in their 30s (Ministry of Health, Labour and Welfare of Japan, 2020): 3600 kcal ((2050 kcal +350 kcal) × 1.5).
2.2 Data collection
2.2.1 Outcome
The primary outcome measure was the development of an egg allergy in infants at 12 months of age. Egg allergy development was defined as a diagnosis of egg allergy, with data collected through a 12-month questionnaire completed by mothers.
2.2.2 Participants' characteristics
The following sociodemographic data were collected from the medical records and questionnaires: infant sex, gestational age at birth, birth weight, mode of birth, number of siblings, maternal age at delivery, maternal education level, maternal smoking status, and survey regions.
2.2.3 Independent variables
Collected from second trimester of pregnancy questionnaire
For considering potential confounding factors, data on maternal egg consumption during pregnancy were collected (Loewen et al., 2021). Daily egg intake was assessed using a brief self-administered diet history questionnaire (BDHQ), a validated questionnaire for estimating food and nutrition intake in the Japanese population, including pregnant women (Kobayashi et al., 2011; Kobayashi et al., 2012; Shiraishi et al., 2017). In order to reduce the influence of overreporting or underreporting, the energy-adjusted egg consumption by the density method (g/1000 kcal) derived from the questionnaire in pregnancy second trimester, (distributed at 18 weeks of gestation), was used.
Collected from 1-month questionnaire
Maternal egg consumption at 1 month: Energy-adjusted egg consumption data from 1-month BDHQ were used.
Breastfeeding rates at the age of 1 month: Data on the frequency of breastfeeding and formula feeding per day were obtained from the 1-month questionnaire. The breastfeeding rates were calculated by dividing breastfeeding frequency by total feeding frequency.
Breastfeeding-dependent egg consumption at 1 month: To quantify the actual amount of eggs affecting infants and to presume the degree of egg protein transferred to infants, the maternal egg consumption at 1 month was multiplied by breastfeeding rates, based on a method used in a previous study (Shibuya & Saito, 2015). We assumed that the actual amount of eggs that affects children varies depending on the proportion of breastfeeding, even when maternal egg consumption is the same. For example, when infants were fed a combination of 50% breast milk and 50% formula, the amount of eggs that affected the oral immune tolerance of the infant was estimated as 50% of maternal egg consumption.
Collected from 6-month questionnaire
Family history of allergy was defined as any reported maternal or paternal history of atopic dermatitis or food allergies.
Eczema was defined as eczema severity in mother-reported symptoms in adherence to the UK working party's criteria of atopic dermatitis (Williams et al., 1994). Infants who met a mandatory criteria of having an itchy skin condition (or parental report of scratching or rubbing in a child) plus at least 3 major criteria of the following were defined as having eczema: (1) Having history of involvement of the skin creases such as folds of elbows, behind the knees, fronts of ankles or around the neck or cheeks, (2) having a personal history of asthma or history of atopic disease in a parent, (3) having a history of a general dry skin, (4) having a visible flexural eczema or eczema involving the cheeks/forehead and outer limbs.
Collected from 12-month questionnaire
Data on months of complementary food introduction was collected by asking “At what age in months did your infant start eating complementary food?”
Additionally, we also collected data on the introduction of egg as a complementary food by 6 months of age, specifically referring to the feeding of either egg white or egg yolk.
2.3 Statistical analysis
Descriptive statistics included the median with interquartile range (IQR) for continuous variables and frequencies and percentages for categorical variables. Continuous variables were analyzed using the Mann–Whitney U test, and categorical variables using Fisher's exact test or Fisher–Freeman–Halton exact test to compare potential factors between infants with and without egg allergies. Forced-entry binomial logistic regression analysis was performed to identify potential risk and protective factors for egg allergies. The covariates were breastfeeding-dependent egg consumption at 1 month, eczema at 6 months, and family history of allergy. Spearman's correlation was performed to assess multicollinearity between the covariates. All analyses were conducted using SPSS Statistics 29 for Windows (IBM Corp., Armonk, NY, USA). The significance level was set at p < .05.
2.4 Sample size
We calculated the required sample size using the rule of 5–10 events per variable for logistic regression analysis (Vittinghoff & McCulloch, 2007), considering three covariates. This resulted in an estimated need for 15–30 cases of egg allergy.
2.5 Ethical approval
This study was approved by the Ethics Committee of the University of Tokyo (2019318NI-(5)) and the Ethics Review Board for Clinical Research of the Kyushu University Medical District Department (2021–92). Written informed consent was obtained at the time of recruitment from all the mothers who agreed to participate.
3 RESULTS
3.1 Participants
Of the 1374 live infants in the J-PEACH study, 461 mothers answered all the 1-month, 6-month, and 12-month questionnaires. Seven infants were excluded due to missing data on maternal egg consumption (n = 5), breastfeeding method (n = 1), and eczema (n = 1). Additionally, 20 infants were excluded because their mothers responded to the questionnaire over 2 months late. Moreover, 14 infants were excluded because their mothers responded unrealistic BDHQ data. A total of 420 infants were included in the final analysis (Figure 1). Among them, 379 (90.2%) infants were breastfed at 1 month, regardless of the frequency of breastfeeding.
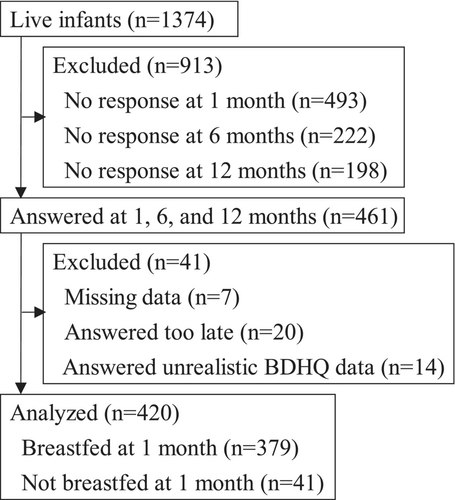
We examined basic infant characteristics between the included and excluded groups. No significant differences were found in sex or birth weight (Fisher's exact test and Mann–Whitney U test, respectively). A significant difference was observed in gestational age, with infants in the excluded group being slightly younger (p = .027, Mann–Whitney U test). The median gestational age was 38 weeks for both groups, but the average gestational age was 38.69 weeks in the included group compared with 38.32 weeks in the excluded group. Overall, the differences in infants' basic characteristics were not considered substantial enough to significantly impact the results.
Regional differences were observed; more than half of the infants represented Tokyo area (n = 237, 56.4%), whereas the smallest cohort (n = 46, 11.0%) was observed in Osaka.
The prevalence of food allergies was 35 (8.3%) among the 12-month-old infants. Eggs were the most common allergen, with 27 (6.4%) cases reported as egg allergies. These 27 infants were classified into the egg allergy group, and the remaining infants were classified into the ’no egg allergy’ group.
Among breastfed infants (n = 379), 32 (8.4%) infants were reported to have food allergies, and 24 (6.3%) had egg allergies. As for no breastfed infants (n = 41), three (7.3%) were reported to have food allergies, all of whom had egg allergies.
3.2 Sociodemographic characteristics
Table 1 shows the sociodemographic characteristics of the infants. In total, 189 males (45.0%) and 231 females (55.0%) were included in this study. The median maternal age at delivery was 35 years. Regarding maternal education level, 289 (70.3%) mothers had completed university or graduate school; none of the mothers smoked during the third trimester of pregnancy.
| All n = 420 | Egg allergy n = 27 (6.4%) | No egg allergy n = 393 (93.6%) | p | |||
|---|---|---|---|---|---|---|
| n or median (IQR) | n (%) or median (IQR) | n (%) or median (IQR) | ||||
| Sex | .235a | |||||
| Male | 189 | 9 (4.8) | 180 (95.2) | |||
| Female | 231 | 18 (7.8) | 213 (92.2) | |||
| Gestational age at birth (week) | 39 (38, 40) | 39 (38, 40) | 39 (38, 40) | .040b | ||
| Birthweight (g) | 3034 (2755, 3292) | 3126 (2860, 3364) | 3030 (2753, 3289) | .368b | ||
| Mode of birth | .834a | |||||
| Vaginal birth | 279 | 19 (6.8) | 260 (93.2) | |||
| Cesarean section | 141 | 8 (5.7) | 133 (94.3) | |||
| Older sibling (n = 403) | .684a | |||||
| At least one | 154 | 9 (5.8) | 145 (94.2) | |||
| No | 249 | 18 (7.2) | 231 (92.8) | |||
| Maternal age at delivery (year) | 35 (31, 38) | 36 (31, 41) | 34 (31, 38) | .304b | ||
| Maternal education level (n = 411) | 1.000c | |||||
| Junior high or high school | 27 | 1 (3.7) | 26 (96.3) | |||
| Junior/technical college | 95 | 6 (6.3) | 89 (93.7) | |||
| University/Graduate school | 289 | 20 (6.9) | 269 (93.1) | |||
| Survey regions | .026c | |||||
| Yamagata | 69 | 3 (4.3) | 66 (95.7) | |||
| Tokyo | 237 | 14 (5.9) | 223 (94.1) | |||
| Osaka | 46 | 8 (17.4) | 38 (82.6) | |||
| Fukuoka | 68 | 2 (2.9) | 66 (97.1) | |||
| Family history of allergy | .120a | |||||
| Yes | 115 | 11 (9.6) | 104 (90.4) | |||
| No | 305 | 16 (5.2) | 289 (94.8) | |||
| Maternal egg consumption during pregnancy (g/1000 kcal) (n = 321) | 16.9 (10.7, 27.1) | 19.0 (13.4, 30.3) | 16.8 (10.5, 27.0) | .344b | ||
| Maternal egg consumption at 1 month (g/1000 kcal) | 18.3 (12.9, 29.3) | 24.4 (12.3, 32.9) | 18.0 (12.9, 29.2) | .292b | ||
| Breastfed (n = 379) | 18.4 (13.1, 29.3) | 25.4 (12.5, 32.0) | 18.3 (13.2, 29.2) | .307b | ||
| Not breastfed (n = 41) | 17.3 (9.8 32.4) | 19.9 (10.5 33.0) | 16.4 (8.6 32.7) | .869b | ||
| Breastfeeding rates at 1 month (%)d | 59.4 (40.3, 90.9) | 76.9 (50.0, 91.7) | 58.3 (39.2, 90.9) | .211b | ||
| Breastfeeding-dependent egg consumption (g/1000 kcal)e | 10.7 (4.7, 17.9) | 15.1 (6.8, 24.7) | 10.5 (4.6, 17.5) | .096b | ||
| Eczema at 6 months | <.001a | |||||
| Yes | 96 | 14 (14.6) | 82 (85.4) | |||
| No | 324 | 13 (4.0) | 311 (96.0) | |||
| Egg introduction by 6 months | .690a | |||||
| Yes | 183 | 13 (7.1) | 170 (92.9) | |||
| No | 237 | 14 (5.9) | 223 (94.1) | |||
| Month of complimentary food introduction (month) (n = 419) | 5 (5, 6) | 5 (5, 6) | 5 (5, 6) | .494b | ||
- Note: Bold values indicate p < .05.
- Abbreviation: IQR, interquartile range.
- a Fisher's exact test.
- b Mann–Whitney U test.
- c Fisher–Freeman–Halton exact test.
- d Calculated by dividing breastfeeding frequency by total feeding frequency.
- e Calculated by multiplying maternal egg consumption at 1 month by breastfeeding rates at 1 month.
Infants from Osaka were more likely to develop egg allergies compared with infants from other regions (p = .026). Fisher's exact test and the Mann–Whitney U test were performed to examine differences in infant characteristics between Osaka and the other three regions. There were no significant differences by region in any of the following variables: family history of allergy, maternal egg consumption at 1 month, breastfeeding-dependent egg consumption at 1 month, eczema at 6 months, egg introduction at 6 months, and the months of complementary food introduction. However, the breastfeeding rates at 1 month were significantly higher in the egg allergy group (p = .012), with a median value of 80.0% in Osaka and 57.1% in the other three regions.
3.3 Risk factors associated with egg allergies
The univariate analysis (Table 1) showed an association between each variable and the development of egg allergies in infants.
Family history of allergy and egg introduction by 6 months was not associated with the development of egg allergies. Furthermore, no significant differences were found in maternal egg consumption during pregnancy (p = .344). However, the development of eczema at 6 months was significantly associated with a higher risk of developing an egg allergy in 12-month-old infants (p < .001).
3.4 Maternal egg consumption and breastfeeding rates
The median maternal egg consumption at 1 month in the egg allergy group was 24.4 g/1000 kcal (for reference, the median frequency was 4–6 whole eggs per week), while it was 18.0 g/1000 kcal (for reference, the median frequency was 2–3 whole eggs per week) in the no egg allergy group; however, the difference was not statistically significant (p = .292). Separate analyses were conducted for breastfed and no breastfed infants to assess the possibility of oral egg protein intake through breast milk. No significant differences in maternal egg intake were observed between the groups.
The median breastfeeding rates at 1 month were 76.9% in the egg allergy group and 58.3% in the no egg allergy group. No statistically significant differences were found between the two groups (p = .211).
Similarly, no significant differences were observed in the breastfeeding-dependent egg consumption at 1 month (p = .096). The median was 15.1 g/1000 kcal in the egg allergy group and 10.5 g/1000 kcal in the no egg allergy group.
The Fisher's exact test was performed to analyze the primary outcome between the two groups based on the presence or absence of maternal egg consumption at 1 month, breastfeeding rates at 1 month, and breastfeeding-dependent egg consumption at 1 month, respectively. Our results revealed that maternal egg consumption at 1 month, breastfeeding rate at 1 month, and breastfeeding-dependent egg consumption at 1 month had no significant effect on the development of egg allergy in 12-month-old infants.
3.5 Effect of breastfeeding-dependent egg consumption adjusted for eczema
Logistic regression analysis was performed to examine the effects of maternal egg consumption on the development of egg allergies (Table 2). The crude model analyzed crude odds ratios with only one independent variable, while the adjusted model calculated adjusted odds ratios with breastfeeding-dependent egg consumption at 1 month, eczema at 6 months, and family history of allergy.
| Crude model | Adjusted model | |||||||
|---|---|---|---|---|---|---|---|---|
| B | cOR | 95%CI | p | B | aOR | 95%CI | p | |
| Breastfeeding-dependent egg consumption at 1 month (g/1000 kcal)a | 0.03 | 1.03 | (1.00–1.05) | .054 | 0.02 | 1.02 | (1.00–1.05) | .104 |
| Eczema at 6 months | ||||||||
| Yes | 1.41 | 4.08 | (1.85–9.03) | <.001 | 1.28 | 3.59 | (1.59–8.13) | .002 |
| No | Reference | Reference | ||||||
| Family history of allergy | ||||||||
| Yes | 0.65 | 1.91 | (0.86–4.25) | .113 | 0.45 | 1.57 | (0.68–3.62) | .286 |
| No | Reference | Reference | ||||||
- Note: Logistic regression analysis (forced entry method) was performed. Bold values indicate p < .05.
- Abbreviations: aOR, adjusted odds ratio (adjusted for 3 variables in this table); B, partial regression coefficient; cOR, crude odds ratio; CI, confidence interval.
- a Multiplied by Maternal egg consumption at 1 month and breastfeeding rates at 1 month, which was a division of breastfeeding frequency by total feeding frequency.
After adjusted for eczema and family history of allergy, there was no significant relationship observed between breastfeeding-dependent egg consumption and the development of infants' egg allergies. However, the significant effect of eczema on the development of egg allergies persisted in the adjusted model (aOR, 3.59; 95% CI, 1.59–8.13). Multicollinearity was not detected between covariates.
4 DISCUSSION
This study examined whether oral egg protein intake through breast milk in early infancy was associated with the risk of developing egg allergy in 12-month-old infants within a Japanese cohort. No significant difference was observed in maternal breastfeeding-dependent egg consumption between the groups of egg allergy infants and no egg allergy infants. Eczema at 6 months of age was significantly associated with the development of egg allergy at 12 months.
4.1 Maternal egg consumption and egg allergies
Contrary to the initial hypothesis, breastfeeding-dependent egg consumption at 1 month was not associated with a reduced risk of egg allergies. This result remained unchanged even after adjusting for the influence of eczema.
One possible reason for this may be the low accuracy of measuring egg consumption. Maternal egg consumption and the level of egg protein in breast milk have dose–response relationships (Metcalfe et al., 2016); however, this study did not directly measure the actual amount of egg protein in breast milk, creating uncertainty regarding its precise impact. Second, the impact of egg protein on the environment has remained unmeasured. In this study, we did not measure the amount of egg protein in the form of house dust (Kitazawa et al., 2019; Trendelenburg et al., 2018), which could increase the risk of cutaneous sensitization. Future studies should measure both egg protein in breast milk and the accompanying environmental egg amounts to understand the precise effect of oral immune tolerance through egg protein in breast milk.
4.2 Eczema and egg allergies
Eczema at 6 months was significantly associated with an increased risk of egg allergies at 12 months. Previous studies have indicated the importance of treating eczema in order to prevent egg allergies (Natsume et al., 2017; Tsakok et al., 2016; Yamamoto-Hanada et al., 2023). Eczema was found to be an indispensable risk factor for egg allergies in other previous studies that examined the associations between infant oral immune tolerance and egg allergies, most of which targeted infants with a family history of allergies (Nagakura et al., 2023; Palmer et al., 2022). The present study included infants at all risk levels, regardless of their family history of allergies. The results highlight that the development of eczema has a strong association with egg allergies, regardless of the risk level. Over time, allergic diseases evolve from eczema to food allergies, asthma, and allergic rhinitis. This phenomenon is known as the atopic march (Hill & Spergel, 2018). Eczema is located at the trigger point of the atopic march. Therefore, the treatment of eczema including atopic dermatitis is thought to be crucial for the prevention of allergic diseases, including egg allergies.
The results of this study indicate that the risk of cutaneous sensitization may be greater than that of oral immunization tolerance. Therefore, it is necessary for healthcare providers to diagnose eczema at an early stage and promote early treatment among parents.
4.3 Regional differences
In this study, the prevalence of egg allergy was significantly higher in Osaka than in the other regions. A comparison of the results of the present study with those of other studies to investigate their validity was precluded, as no studies have reported the prevalence of infant food allergies by region in Japan. However, a global survey done in 2013 reported differences in the prevalence of food allergies across various regions and food cultures (Prescott et al., 2013). Therefore, differences in local dietary habits in Osaka may contribute to the increased incidence of egg allergy within the region. Since the current study could not perform a multilevel analysis due to bias in the number of people in each region, further studies are needed to examine egg allergy risks by region and dietary habits.
4.4 Limitations and strengths
This study has two limitations. First, the generalizability of this study should be interpreted with caution. Infants in this study had mothers with higher education levels. Mothers with advanced academic backgrounds may have higher health literacy levels and a better understanding of infant allergy prevention and lifestyle care. Furthermore, all participants were recruited during the unique period after the outbreak of the COVID-19 began in March 2020, which may have caused some changes in maternal mental health and the division of childcare responsibilities between parents. However, there was no major impact reported on the variables of maternal egg consumption, breastfeeding rates, or infant eczema that were focused on in this study. Second, although a large general population cohort was investigated, there may be limitations in sample size in multiple analyses due to the low prevalence of allergies. Third, since this study was based on self-reported questionnaires, the accurate amount of egg protein in breast milk and in the environment, and the severity and treatment of eczema were unclear. Reporting bias was also presumed to have occurred. However, any effects of reporting bias were minimized by using measurement questionnaires commonly used in previous studies.
Despite the above limitations, the current study had specific strengths. First, to the best of our knowledge, this was the first cohort study examining the effect of maternal egg consumption during lactation and considering both feeding methods and eczema. Second, this was one of the few studies to examine factors related to the development of egg allergies among a general infant population spanning multiple regions in Japan. The results suggest the possibility of regional differences in the prevalence of food allergies among infants in Japan.
4.5 Implications
The current study revealed no significant impact of maternal egg consumption at 1 month on egg allergy development in infants when adjusted for infant eczema at 6 months of age. A systematic review concluded that egg elimination during lactation is not associated with egg sensitization in infants (Kramer & Kakuma, 2012). Therefore, excessive maternal egg consumption or elimination during lactation for egg allergy prevention is not recommended due to lack of scientific evidence.
This study indicates that the risk of cutaneous sensitization had a greater impact than the oral immune tolerance through breast milk. The prevention and treatment of eczema may be important in order to prevent egg allergy caused by percutaneous sensitization.
It is necessary for healthcare providers to emphasize the importance of prevention, early detection, and treatment of eczema, including atopic dermatitis. It is also important for healthcare providers to inform mothers who avoid egg consumption due to concerns about infant egg allergies that there is no evidence indicating that maternal egg consumption affects the development of egg allergies in their infants.
5 CONCLUSIONS
This study suggests that egg consumption through breast milk at 1 month does not provide sufficient oral tolerance to eggs at the age of 12 months. Furthermore, eczema at 6 months was significantly associated with the development of egg allergies, indicating the importance of preventing cutaneous exposure to egg allergens.
AUTHOR CONTRIBUTIONS
Conceptualization: Sakurako Kishino, Kaori Yonezawa, and Megumi Haruna. Data curation: Sakurako Kishino, Megumi Fujita, Masayo Matsuzaki, Yoko Sato, Yoshiko Suetsugu, Riko Ohori, Moeko Tanaka, and Satoko Aoyama. Formal analysis: Sakurako Kishino. Funding acquisition: Megumi Haruna. Investigation: Sakurako Kishino, Megumi Fujita, Masayo Matsuzaki, Yoko Sato, Yoshiko Suetsugu, Riko Ohori, Moeko Tanaka, and Satoko Aoyama. Methodology: Sakurako Kishino, Kaori Yonezawa, Megumi Haruna and Satoshi Sasaki. Project administration: Megumi Haruna. Resources: Satoshi Sasaki. Supervision: Kaori Yonezawa, Megumi Haruna and Satoshi Sasaki. Visualization: Sakurako Kishino. Writing—original draft: Sakurako Kishino. Writing—review and editing: Sakurako Kishino, Kaori Yonezawa, Megumi Haruna, Yuriko Usui, Satoshi Sasaki, Megumi Fujita, Masayo Matsuzaki, Yoko Sato, Yoshiko Suetsugu, Riko Ohori, Moeko Tanaka, and Satoko Aoyama. All authors read and approved the final manuscript.
ACKNOWLEDGMENTS
This study was part of a research project funded by JSPS KAKENHI (Grant Numbers 19H03940, 19K22741, and 22H03399). We are deeply grateful to all participants and hospital staff members for their cooperation. We express our sincere appreciation to the members of the J-PEACH Study, especially the Department of Midwifery and Women's Health at the University of Tokyo, for their invaluable support.
CONFLICT OF INTEREST STATEMENT
The authors declare that there is no conflict of interest.




