Long-term effects of environmental dynamic lighting on sleep–wake rhythm, mood and behaviour in older adults with intellectual disabilities
Abstract
Background
Sleep–wake problems and depressive symptoms are common in people with intellectual disabilities (IDs) and are thought to be related to the unstable sleep–wake rhythm in this population. Previously, we showed that after increasing environmental light exposure, mid-sleep and sleep onset advanced, and mood improved over a period of 14 weeks after installing environmental dynamic light installations in the living room of people with IDs. We invited participants of that short-term study to take part in the current study on sleep–wake rhythm, mood and behaviour in older adults with IDs 1 year after installing environmental dynamic light installations in the common living rooms of six group homes.
Methods
A pre–post study was performed from October 2017 to February 2019. We included 45 participants (63.5 ± 8.5 years, 67% female) from six group home facilities who provided data at baseline (9, 4 and 1 weeks prior to installing light installations), short term (3, 7 and 14 weeks after installing light installations) and 1 year (54 weeks after installing light installations). Wrist activity was measured with actigraphy (GENEActiv) to derive the primary outcome of interdaily stability of sleep–wake rhythms as well as sleep estimates. Mood was measured with the Anxiety, Depression and Mood Scale. Behaviour was measured with the Aberrant Behaviour Checklist.
Results
One year after installing dynamic lighting, we did not find a change in interdaily stability. Total sleep time decreased (β = −25.40 min; confidence interval: −10.99, −39.82), and sleep onset time was delayed (β = 25.63 min; confidence interval: 11.18, 40.08). No effect on mood or behaviour was found.
Conclusions
We did not find a change in sleep–wake rhythm, mood or behaviour in older persons with IDs living in care facilities 1 year after installing the light. We did find evidence for a long-term effect on sleep duration and sleep timing. The results have to be interpreted with care as the current study had a limited number of participants. The need for more research on the long-term effects of enhancing environmental light in ID settings is evident.
Introduction
The sleep–wake rhythm in older adults with intellectual disabilities (IDs) is often unstable and fragmented (Maaskant et al. 2013). The instability and fragmentation are associated with sleep problems (Van De Wouw et al. 2012; Van De Wouw et al. 2013), which are found in up to 72% of this population (Van De Wouw et al. 2012; Van De Wouw et al. 2013) and are more common in people with IDs than in their typical developing peers (Surtees et al. 2018; Böhmer et al. 2020; Browne et al. 2024). Sleep problems are associated with the high prevalence of depressive symptoms in older adults with IDs (Van De Wouw et al. 2012; Hermans et al. 2013; Maaskant et al. 2013; Van De Wouw et al. 2013). Two recent literature reviews showed that non-pharmacological interventions (e.g. activity scheduling, stimulus control, cognitive-behaviour therapy, relaxation and sleep hygiene) focusing on improving the sleep–wake rhythm and/or mood are not yet evidence based for people with IDs (Priday et al. 2017; Shanahan et al. 2019; Korb et al. 2023).
Both the sleep–wake rhythm and mood follow a circadian rhythm that is regulated by the biological clock. As the biological clock is especially sensitive to light exposure (Minors & Waterhouse 1981; Duffy et al. 1996), insufficient light exposure is thought to disrupt the circadian rhythm and thereby affect sleep and mood (Legates et al. 2014). Increasing light exposure using conventional bright light therapy is broadly implemented as an effective treatment of both sleep and mood complaints in various populations (Penders et al. 2016; Van Maanen et al. 2016; Pjrek et al. 2020) and was shown to be a promising treatment for depressive symptoms in adults with IDs as well (Hamers et al. 2020). Administrating bright light by using ceiling-mounted light installations is also shown to be effective in improving sleep, mood and behaviour in older adults with dementia in care facilities (Van Someren et al. 1997; Riemersma-Van Der Lek et al. 2008; Figueiro et al. 2014; Hadi et al. 2019).
Recently, we conducted a study on the short-term effect of increasing environmental light exposure in group homes for older adults with IDs (Böhmer et al. 2022). Installing environmental dynamic light in the common living rooms did not affect stability and fragmentation of the sleep–wake rhythm within 14 weeks after placement, but small advancing effects were seen on sleep onset time and mid-sleep. We did find a clinically relevant decrease in depressive symptoms in older adults with IDs at 14 weeks after exposure to dynamic environmental light. In addition, a decrease was seen in hyperactivity, irritability, social avoidance and lethargic behaviour measured with the Aberrant Behaviour Checklist (ABC) (Aman et al. 1995) without any adverse effects.
Even though environmental dynamic light installations are there for the long run, to date, the long-term effects (>3 months) of increasing light exposure on sleep and mood in healthcare patient populations have not been well studied. A review by Hadi et al. (2019) provided just one study with a study duration of up to 3.5 years: a randomised controlled trial that took place in care facilities for people with dementia (Riemersma-Van Der Lek et al. 2008). Due to the dropout of participants during the study, results are reported over the first 2 years. In this study, dynamic light increased total sleep time (TST) by 10 min/year over the first 2 years. Increasing environmental light ameliorated depressive symptoms over 2 years but had no effect on the circadian sleep–wake rhythm.
The current study presents the long-term measurements of our previous study and investigates the long-term effect of dynamic light 1 year after installation on sleep–wake rhythm, mood, behaviour and adverse effects in older adults with IDs living in group homes. The effect of dynamic light might take more time to fully affect the sleep–wake rhythm and therefore might be more pronounced than after 14 weeks. Second, given the relationship between sleep and mood and behaviour in ID (Van De Wouw et al. 2012), depression in people with IDs often presents itself via worsening of behaviour (Eaton et al. 2021), and improvement in mood can result in an improvement of behaviour as well, we looked into the long-term effect of dynamic light on mood and behaviour. It is hypothesised that, compared with baseline, stability of the sleep–wake rhythm, depressive symptoms and behaviour are improved. When compared with the short-term effects, we expect the stability of the sleep–wake rhythm, depressive symptoms and behaviour to be at the same level or to have improved more.
Methods
Study setting and participants
A complete description of the context, design, recruitment and data collection of the short-term measurement has been presented elsewhere (Böhmer et al. 2022). In short, the study took place in six group homes for older adults (>40 years) with the ID of Middin, a care organisation for people with disabilities in the Netherlands providing care for 4400 people. These group homes are located in a central setting or are community-based settings in which multidisciplinary long-term care is provided for their residents. Residents are assisted by professional caregivers with merely all daily activities like dressing, eating and going to bed. Residents take part in in-home activities that are provided during the day or go to day-care facilities or other residencies. Medical and psychological care is provided by professional caregivers, physicians specialising in ID medicine and behavioural therapists. In October 2017, the dynamic light installations were placed in the six group homes.
The Medical Ethical Committee of Erasmus MC, University Medical Center Rotterdam, the Netherlands, approved both the short-term and long-term measurements (MEC-2017-467).
Inclusion criteria, long-term measurement and consent procedure
Residents were eligible for participation in the short-term study if they (1) were 40 years of age or older; (2) had mild, moderate, severe or profound ID (or IQ below 70); (3) were living in one of the six selected residential homes for people with IDs; and (4) spent at least 1 h daily in the common living room, of which 30 min between 7:00 am and noon, in order to guarantee exposure to the intervention. We chose 40 as the minimum age for our sample because people with IDs aged 50 and over are as frail as older people without IDs aged 70 and above (Schoufour et al. 2013). In addition, we wanted to include as many residents from the selected care facilities as possible to take part in this study. Residents known to be critically ill were excluded from participation. For the present study, we invited all participants in the short-term study to partake in measurements 1 year after installing the light. Residents were eligible for the measurement 1 year after installing the light if they (1) were living in one of the six selected residential homes for people with IDs and (2) had participated in the short-term measurements. Residents known to be critically ill at the time of the long-term measurement were excluded from participation.
Participants in the study or their representatives were asked for informed consent for the study to assess the long-term effects. Participants who could decide on participation themselves received an easy-to-read information letter about the study's long-term effects and provided informed consent. For participants who were not capable of deciding themselves, their legal representative received the information letter and provided informed consent.
Dynamic light installation
The dynamic light installation was placed in the common living room of the group home, where residents can watch TV, have meals and partake in activities like crafts (Fig. 1). The ceiling-mounted dynamic lighting installation (YSELED, Light Technology, the Netherlands) delivered a maximum of 12 000 lumens (120 W) up and 6000 lumens (54 W) down. Per care facility, a professional light plan was designed for the complete living room. As all living rooms differed in size, shape, architecture and access to outdoor light, we aimed to standardise the environmental lighting by programming the installations to deliver an illuminance of 1000 lux and 4500 K at eye level in gaze direction (120 cm from the floor) between 7:00 a.m. and 6:00 p.m. and to dim down to 150 lux and 2700 K at eye level automatically at 6:00 p.m. The average light exposure of participants (n = 52) while in the common living room between 7:00 a.m. and 7:00 p.m. during baseline and intervention was measured twice: once during the baseline period and once in the week after installing the dynamic light. Light exposure was measured in the direction of gaze using a Testo 545 digital lux metre (Testo SE & Co. KGaA, Germany), so the data represent the light levels that enter the eye. Mean light exposure between 7:00 a.m. and 6:00 p.m. was 68 lux (SD = 46 lux) at baseline and 989 lux (SD = 211 lux) during intervention. Light exposure at 7:00 p.m. was 23 lux (SD = 8 lux) during baseline and 85 lux (SD = 34 lux) during intervention. A full description of the dynamic lighting installation and an overview of illuminances during the baseline and intervention periods are presented elsewhere (Böhmer et al. 2022).

Study design
A schematic overview of the study design is presented in Fig. 2. Group homes were non-randomly paired to create three groups with a similar number of participants. Each group followed the same study procedure. Three baseline measurement periods of 1 week took place in weeks 1, 5 and 9. In week 10, the dynamic lights were installed. Measurements to assess the short-term effect of light took place during weeks 3, 7 and 14 after installing light installations. The measurements to assess the effect after 1 year were conducted 54 weeks after installing light installations. The dynamic light installations were operational from installation until long-term measurement and forward. The first baseline measurement took place on 18 October 2017, and the short-term effect was studied up to 24 May 2018. The long-term effect was measured from 9 January to 27 February 2019.

Materials
Sleep–wake rhythm and sleep estimates
The GENEActiv (Activinsights, Kimbolton, UK), a wrist-worn piezo-electric accelerometer, was worn continuously on the non-dominant wrist for seven consecutive days and nights and measured activity at 100 Hz. Sleep–wake rhythm parameters were calculated using non-parametric circadian rhythm analyses (Van Someren et al. 1999). For sleep–wake rhythm analysis, actograms were inspected visually, and all 24-h periods with more than 4 h of missing data were excluded. Measurements were considered valid if they included a total of at least 96 h of valid data. The primary outcome of interest was interdaily stability, the stability of the sleep–wake rhythm. Additionally, the fragmentation of the sleep–wake rhythm intradaily variability and relative amplitude [calculated as the difference of activity during the 10 most active hours (M10) and the 5 least active hours (L5) using the formula relative amplitude = M10 − L5/M10 + L5] were measured. Finally, L5 onset and M10 onset were provided.
For sleep estimates, all 24-h periods with more than 4 h of missing data were excluded, and measurements were considered valid if they included at least one night of valid data. Sleep estimates were calculated using the Actant-Activity Analysis Toolbox (Te Lindert & Van Someren 2013), which has the advantage of estimating bedtimes based on activity levels and thus does not need bedtimes from sleep diaries, as this was proven to be either unreliable or often not reported by the caregiver (Van De Wouw et al. 2013). Sleep estimates of interest were TST (total time asleep between sleep onset and final wake time), waking after sleep onset (WASO; time awake between sleep onset and final wake time), sleep efficiency (percentage of sleep between sleep onset and final wake time), sleep onset, final wake time, number of wake bouts, wake bout duration, number of sleep bouts, sleep bout duration, mid-sleep (the midpoint between sleep onset and final wake time), short sleep duration (TST < 6 h) and night waking (WASO > 90 min).
Mood and behaviour
The Dutch translation of the Anxiety, Depression and Mood Scale (ADAMS) was used to measure mood. The ADAMS is a proxy screening scale for the presence of symptoms of depression and anxiety in adults with IDs. It consists of 28 items scored on a 4-point scale (0–3) covering four sub-scales: depression, anxiety, social avoidance and other symptoms. A cut-off score of ≥14 points (screening depression) was used for the presence of depressive symptoms and a cut-off score of ≥10 (screening anxiety) for anxiety symptoms. The test–retest reliability of the ADAMS for adults with IDs is good to excellent (Hermans et al. 2018).
Behaviour was measured using the ABC (Aman et al. 1985), consisting of 58 items that are scored on a scale ranging from 0 (no problem) to 3 (very problematic). The ABC consists of the sub-scales hyperactivity, irritability, lethargy, stereotypy and inadequate speech (Rojahn et al. 2011). The internal consistency of the ABC in adults with IDs is excellent (Rojahn et al. 2011).
Both questionnaires were filled out by assigned professional caregivers who knew the participant well.
Adverse effects
To account for possible unwanted side effects of the dynamic lighting, adverse effects (e.g. eye complaints, headache and dizziness) in participants were rated on a 4-point scale (0 = absent, 1 = probably absent, 2 = probably present, 3 = present) by the tutor or the professional caregivers once every measurement week (Riemersma-Van Der Lek et al. 2008).
Demographics, medical status and medication use
Information on age and sex was subtracted from the medical records at baseline. The level of ID was obtained from behavioural therapists' records and was classified as mild (IQ 55–70), moderate (IQ 35–55), severe (IQ 25–35) or profound (IQ < 25). Professional caregivers filled out questionnaires on the activities of daily living (ADL) and mobility of the participant.
Basic ADL, instrumental ADL, mobility, comorbidities and medication use were collected at the first baseline and the last short-term and long-term measurements. Basic ADL was assessed with the Barthel index (Mahoney & Barthel 1965), and instrumental ADL was assessed with the Lawton index (Lawton & Brody 1969).
Based on the medical records, a checklist on the medical status was filled out by the general practitioner, ID physician or researcher. The checklist consisted of items on the presence of genetic syndromes, medical conditions and psychiatric disorders. Medication use was retrieved from the medical records. Each medication was scored using the Anatomical Therapeutic Chemical classifications (WHO Collaborating Centre for Drug Statistics Methodology 2020).
Professional caregivers provided information about the number of alcohol units per day, the number of cigarettes smoked per day, whether a participant took part in organised daily activities and the mobility (independent/with support/wheelchair) of participants.
Statistical analysis
Multilevel regression analyses were performed using RStudio with the nlme package and accounted for the one-level nested structure of the data. Missing values were under 5% for all outcomes of demographics, medical status and medication use (instrumental ADL, 2.3%; medical status, 4.4%; medication use, 4.4%).
Only outcome variables with stable baseline measurements would be analysed further. This was tested by selecting only the baseline measurement and building a multilevel regression model that predicts the outcomes at baseline over time. Only when time was not a significant predictor of the outcome was a full analysis performed. All models included the moment of measurement (baseline/short term/1 year after installing light), age and sex. Demographics, medical status or medication use at baseline that correlated significantly (P < 0.05) with the outcome variables during either the baseline or intervention period and that were available to at least seven participants were included as covariates in the analysis of that outcome variable. As changes in covariates over measurements were limited, we included covariates from the first measurements. As TST and WASO are highly correlated, analyses for WASO were corrected for assumed sleep (minutes between sleep onset and final wake time).
The effect of dynamic light was analysed only for outcome variables that met the assumption for multilevel regression analysis and outcome variables that were stable during baseline, as this is the assumption for the analysis of a study following the multiple baseline design. Analysing the effect of dynamic light started with the most elaborate model, including a random intercept for participants. Covariates were kept in the model, irrespective of whether they were significant. We were interested in the development of outcomes from baseline to short term to 1 year after installing light. The final full models are reported in Appendix A.
Because of multiple testing, the P-value for sleep–wake rhythm and sleep estimates was lowered to 0.011 (Sidak's correction) (r = 0.5, alpha = 0.05 with 20 outcome measurements), and the P-value for mood and behaviour was lowered to 0.019 (Sidak's correction) (r = 0.6, alpha = 0.05 with 11 outcome measurements).
It was assessed whether possible adverse effects were due to intervention with the simplest model, with time and intervention as fixed effects and participants as the random effect. No multi-testing correction was applied for side effects.
Finally, possible changes in ADL, instrumental ADL, medical status and medication use over the measurements were checked using a t-test for continuous variables and a χ2 test for categorical variables. As these outcomes were not of primary interest and did not show any change, these data are not presented but are available upon request.
Results
Participants' characteristics
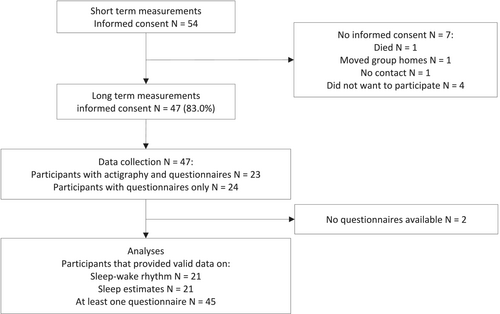
Of the 54 participants in the study on the short-term effect, 47 gave consent for participation in the measurements 1 year after installing the light (Table 1 and Fig. 3). One participant moved, one legal representative could not be contacted in time, one died and four others did not want to participate. Follow-up questionnaires were not available for two participants; therefore, the final dataset consisted of 45 participants.
| Measures* | |||
|---|---|---|---|
| Participants, count | 45 | ||
| Age, years, mean (SD) | 63.5 | (8.5, 46–79) | |
| Female | 30 | 67% | |
| Level of ID | Mild | 27 | 60% |
| Moderate | 12 | 27% | |
| Severe | 1 | 2% | |
| Unknown | 5 | 11% | |
| Genetic syndromes associated with ID | Down syndrome | 9 | 20% |
| No/unknown/not specified | 36 | 80% | |
| Activities of daily living, range 0–20, mean (SD) | 14.95 | (6.1) | |
| Instrumental activities of daily living, range 8–33, mean (SD) | 11.09 | (2.8) | |
| Mobility | Independent | 29 | 64% |
| Support | 9 | 20% | |
| Wheelchair | 7 | 16% | |
| Medication | Antidepressants | 7 | 16% |
| Antipsychotics | 12 | 28% | |
| Sleep medication | 2 | 5% | |
| Benzodiazepines | 15 | 35% | |
| Antiepileptics | 7 | 16% | |
| Beta-blockers | 9 | 21% | |
| Other psychotropics | 5 | 12% | |
| At least one of the above | 28 | 62% | |
| Missing medication use, n | 2 | 4% | |
| Comorbidities | Autism | 1 | 2% |
| Depression | 5 | 11% | |
| Anxiety | 4 | 9% | |
| Sleep problems | 5 | 11% | |
| Cardiovascular risk/disease† | 20 | 44% | |
| Dementia | 5 | 11% | |
| Epilepsy | 10 | 22% | |
| Spasticity | 2 | 4% | |
| Visual impairment | 8 | 18% | |
| Hearing impairment | 14 | 31% | |
| ADHD | 0 | 0% | |
| Missing comorbidities, n | 2 | 4% | |
- * Range is shown from worst to best.
- † The umbrella variable of all reported cardiovascular conditions or risk factors, for example, hypertension, pacemaker or cardiac arrhythmias.
- SD, standard deviation; ID, intellectual disability; ADHD, attention deficit hyperactivity disorder.
Participants who took part in measurements 1 year after installing lights did not differ in demographics, medical status or medication use from the participants in the short-term measurements. Dropout was equal across all group homes.
Sleep–wake rhythm and sleep estimates
Actigraphy data were available for 21 participants, who all provided both valid sleep–wake rhythms and at least one valid night of data for sleep estimates (Table 2). When compared with participants without actigraphy data, participants with actigraphy data more often used beta-blockers (P = 0.007) and antiepileptics (P = 0.033) and had fewer hours of daily activities per week (P = 0.027).
| Assessment scale† | Baseline, mean (SD) | Short term, mean (SD) | Long term, mean (SD) | Baseline to 1 year after installing light | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Valid obs | N | Beta | 5% | 95% | P-value‡ | Effect size | ||||||
| Sleep–wake rhythm | Stability | Range, 0–1 | 0.65 (0.16) | 0.64 (0.17) | 0.57 (0.17) | 123 | 21 | −0.07 | −0.14 | 0.00 | 0.08 | −0.41 |
| Fragmentation | Range, 2–0 | 0.68 (0.31) | 0.73 (0.34) | 0.71 (0.39) | 123 | 21 | 0.03 | −0.04 | 0.10 | 0.40 | 0.19 | |
| L5 | Range, 0–60 | 10.13 (5.74) | 11.1 (6.38) | 11.15 (6.06) | 123 | 21 | 0.57 | −0.65 | 1.79 | 0.38 | 0.19 | |
| L5 onset | hh:mm:ss | 01:00 (1:50) | 00:29 (1:41) | 01:02 (2:55) | 122 | 21 | 3.06 | −45.79 | 51.90 | 0.92 | 0.03 | |
| M10 | Range, 0–60 | 45.43 (10.14) | 45.33 (9.7) | 43.51 (11.2) | 123 | 21 | −2.27 | −4.20 | −0.33 | 0.03 | −0.48 | |
| M10 onset | hh:mm:ss | 10:11 (2:14) | 10:25 (2:14) | 11:14 (2:54) | 113 | 20 | 52.16 | −20.72 | 125.04 | 0.18 | 0.30 | |
| Amplitude | Range, 0–60 | 35.3 (10.55) | 34.22 (10.7) | 32.36 (9.97) | 123 | 21 | −2.80 | −4.89 | −0.72 | 0.01 | −0.55 | |
| Relative amplitude | Range, 0–1 | 0.64 (0.16) | 0.61 (0.16) | 0.6 (0.14) | 123 | 21 | −0.03 | −0.06 | 0.01 | 0.11 | −0.35 | |
| Sleep estimates | Total sleep time | h | 8.05 (2.21) | 7.81 (2.04) | 7.65 (2.22) | 810 | 24 | −24.62 | −42.10 | −7.13 | 0.01* | −0.56 |
| Waking after sleep onset | min | 87.07 (67.04) | 105.6 (75.97) | 86.33 (59.43) | 810 | 24 | 6.6 | −1.73 | 14.96 | 0.12 | 0.31 | |
| Sleep efficiency | % | 84.84 (10.3) | 81.71 (12.14) | 84.21 (9.59) | 810 | 24 | −0.83 | −2.31 | 0.66 | 0.28 | −0.22 | |
| Sleep onset time | hh:mm:ss | 22:36 (01:44) | 22:23 (01:33) | 22:58 (01:43) | 810 | 24 | 25.40 | 10.99 | 39.82 | 0.00* | 0.70 | |
| Final wake time | hh:mm:ss | 08:06 (01:44) | 07:58 (1:21) | 8:14 (01:50) | 749 | 23 | 13.65 | −3.23 | 30.53 | 0.12 | 0.33 | |
| Mid-sleep | hh:mm:ss | 03:21 (01:12) | 03:10 (01:04) | 03:31 (01:04) | 810 | 24 | 13.53 | 3.07 | 23.98 | 0.01** | 0.51 | |
| Number of wake bouts | 55.19 (33.1) | 58.32 (34.44) | 56.36 (35.86) | 810 | 24 | 1.24 | −2.98 | 5.47 | 0.57 | 0.12 | ||
| Wake bout duration | min | 1.75 (1.24) | 2.21 (2.29) | 1.83 (1.44) | 749 | 23 | 0.10 | −0.21 | 0.42 | 0.52 | 0.13 | |
| Number of sleep bouts | 54.94 (33.27) | 58.03 (34.63) | 56.07 (36.1) | 749 | 23 | 3.52 | −0.68 | 7.71 | 0.07 | 0.10 | ||
| Sleep bout duration | min | 12.83 (13.36) | 11.62 (13.67) | 11.03 (5.92) | 749 | 23 | −2.08 | −4.65 | 0.48 | 0.11 | −0.33 | |
| Estimate | SE | Wald | P-value§ | Odds ratio | ||||||||
| Short sleep | % | 15.10 | 17.85 | 21.05 | 749 | 23 | 0.12 | 0.41 | 0.08 | 0.78 | 1.13 | |
| Night waking | % | 35.65 | 47.59 | 37.59 | 810 | 24 | 0.17 | 0.45 | 0.14 | 0.70 | 1.18 | |
- * P < 0.011.
- ** P < 0.05.
- † Range is shown from worst to best.
- ‡ Linear mixed model analysis (lme) is mutually adjusted for time, measurement and covariates (full models in Appendix A), and test results are presented for the measurement ‘1 year’.
- § Linear mixed model analysis (geeglm) is mutually adjusted for time, measurement and covariates (full models in Appendix A), and test results are presented for the measurement ‘1 year’.
- SD, standard deviation; Valid obs, valid observations; N, participants; SE, standard error.
Both interdaily stability (Fig. 4a) and intradaily variability did not change from baseline to short term and 1 year after installing the lights, nor did amplitude, relative amplitude, L5, L5 onset, M10 and M10 onset.

TST shortened significantly from baseline to 1 year after installing light [β = −25.40 min; confidence interval (CI): −39.82, −10.99; Fig. 4b]. In more detail, TST did not change from baseline to short term, but from short term to 1 year after installing light, TST showed a non-significant trend for a decrease of 21.68 min (P = 0.02).
With regard to fragmentation of the nocturnal sleep, we found that the increase of WASO (β = 11.2 min; CI: 5.15, 17.22) at short term was no longer present at long term, as the WASO at baseline did not differ from the WASO 1 year after installing lights. The probability of night waking increased by 8% from baseline to short-term measurement (P < 0.01) but did not differ from baseline to 1 year after installing the light. The decrease of 2.2% in sleep efficiency (P < 0.0001) in the short term was no longer present after 1 year. Mean wake bout time in minutes followed a similar pattern; in the short term, the mean wake bout time increased (β = 0.45 min; CI: 0.21, 0.68), but after 1 year, mean wake bout time did not differ from baseline anymore. Overall, we found no long-term effect of light on the fragmentation of nocturnal sleep.
Regarding sleep timing, the short-term advancing of sleep onset time was found to change into a delaying effect 1 year after installing the light. When compared with baseline, sleep onset time at short term was 16.2 min earlier (CI: −26.72, −5.70; Fig. 4c). However, 1 year after installing the light, sleep onset time was 25.63 min (CI: 11.18, 40.08) later than at baseline and 41.83 min (CI: 27.48, 56.20) later than at short term. Mid-sleep at short-term measurement was 10.3 min (CI: 27.48, 56.20) earlier than at baseline and showed a trend for a delayed mid-sleep of 13.43 min (P = 0.013) from baseline to 1 year after installing light.
We found no short-term and long-term changes in final wake time, number of wake bouts, number of sleep bouts or sleep bout duration. In addition, no changes were found for the presence of short sleep problems.
Mood and behaviour
For mood and behaviour, long-term data from 45 participants were available (Table 3). The short-term improvement of depressive symptoms (β = −2.62; 95% CI: −3.58, −1.65) returned to baseline level at 1 year after installing light. Also, the short-term effect on the prevalence of scoring above the cut-off for depressive symptoms (from 22% to 8%) did not last and returned to 16% at 1 year after installing the lights.
| Assessment scale† | Baseline, mean (SD) | Short term, mean (SD) | Long term, mean (SD) | Baseline to 1 year after installing light | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Valid obs | N | Beta | 5% | 95% | P-value‡ | Effect size | ||||||
| ADAMS | Depressive symptoms | Range, 0–39 | 9.1 (4.95) | 6.31 (4.9) | 7.35 (5.15) | 282 | 43 | −1.45 | −2.78 | −0.13 | 0.03 | −0.62 |
| Anxiety symptoms | Range, 0–21 | 5.3 (3.46) | 4.09 (2.85) | 4.51 (2.86) | 282 | 43 | −0.47 | −1.16 | 0.21 | 0.18 | −0.50 | |
| Social avoidance | Range, 0–21 | 3.79 (2.93) | 2.79 (3.14) | 3.55 (2.55) | 282 | 43 | −0.14 | −0.93 | 0.66 | 0.73 | −0.35 | |
| Other symptoms | Range, 0–33 | 6.78 (4.98) | 4.57 (4.46) | 5.44 (4.56) | 282 | 43 | −1.20 | −2.33 | −0.06 | 0.04 | −0.02 | |
| Estimate | SE | Wald | P-value§ | Odds ratio | ||||||||
| Above cut-off 14: depressive symptoms, % | 21.77 | 7.75 | 15.56 | 282 | 43 | −0.45 | 0.51 | 0.76 | 0.38 | 0.64 | ||
| Above cut-off 10: anxiety symptoms, % | 12.10 | 3.88 | 2.22 | 282 | 43 | −1.88 | 1.04 | 3.28 | 0.07 | 0.15 | ||
| Beta | 5% | 95% | P-value‡ | Effect size | ||||||||
| ABC | Hyperactivity | Range, 0–48 | 4.15 (4.48) | 3.4 (4.57) | 3.62 (4.15) | 280 | 43 | −0.29 | −1.45 | 0.86 | 0.63 | −0.37 |
| Irritability | Range, 0–45 | 4.86 (5.01) | 3.51 (4.22) | 4.04 (4.21) | 280 | 43 | −0.43 | −1.36 | 0.50 | 0.37 | −0.43 | |
| Lethargy | Range, 0–48 | 5.61 (5.17) | 4.06 (4.59) | 5.15 (4.63) | 281 | 43 | −0.28 | −1.65 | 1.08 | 0.69 | 0.00 | |
| Stereotypy | Range, 0–21 | 0.78 (1.66) | 0.52 (1.38) | 0.84 (1.59) | 264 | 42 | 0.11 | −0.35 | 0.57 | 0.65 | 0.00 | |
| Inadequate speech | Range, 0–12 | 1.18 (1.7) | 0.85 (1.47) | 0.86 (1.21) | 281 | 43 | −0.25 | −0.67 | 0.17 | 0.26 | 0.00 | |
- * P < 0.0195.
- † Range is shown from best to worst.
- ‡ Linear mixed model analysis (lme) is mutually adjusted for time, measurement and covariates (full models in Appendix A), and test results are presented for the measurement ‘1 year’.
- § Linear mixed model analysis (geeglm) is mutually adjusted for time, intervention and covariates (full models in Appendix A), and test results are presented for the measurement ‘1 year’.
- Valid obs, valid observations; N, participants; ADAMS, Anxiety, Depression and Mood Scale; ABC, Aberrant Behaviour Scale; SD, standard deviation; SE, standard error.
A pattern similar to that of the depression scores was seen for anxiety (baseline–short β = −1.00; CI: −1.49, −0.51), social avoidance (β = −1.02; CI: −1.59, −0.44), irritability (β = −0.92; CI: −1.59, −0.25) and lethargy (β = −1.44; CI: −2.43, −0.46); all showed a short-term effect returning to baseline during long-term measurements 1 year after installing light. No long-term effect was found on the probability of scoring above the cut-off for anxiety symptoms. In addition, no long-term effect of light was found for hyperactivity, stereotypy or inadequate speech.
Adverse effects and other outcomes
There were no adverse events reported 1 year after installing the light (Appendix B). Installing dynamic lighting did not affect the scores on ADL, instrumental ADL, mobility or medication use (data not shown).
Discussion
We studied the long-term effect of environmental dynamic lighting on the sleep–wake rhythm, mood and behaviour of older persons with IDs living in care facilities. One year after installing dynamic light, we did not find a change in stability or fragmentation of the sleep–wake rhythm or prevalence of sleep problems in the long term, nor did we find a long-term effect on depressive symptoms, mood or behaviour. We did find delayed sleep timing and shortened sleep duration 1 year after installing dynamic light, although the clinical relevance has to be determined. Overall, we could not confirm our hypothesis; we found no evidence for a long-term effect of environmental dynamic lighting on sleep–wake rhythm, mood and behaviour in older persons with IDs living in care facilities.
Although we did not find an effect of light on the circadian sleep–wake rhythm, these results should be interpreted with care due to the small sample size. The initial study on the short-term effects was not designed to also study the long-term effects and therefore did not take into account a possible dropout during follow-up. A possible long-term effect on the sleep–wake rhythm of environmental light might have been too small to be detected by as few as 21 participants.
The shortening of sleep duration and the delaying effect of light on sleep onset time 1 year after installing light might indicate a better consolidation of nocturnal sleep. The delay in sleep onset time by 25 min might be related to the shorter sleep duration of 25 min. We do not have data on the sleep onset latency or get-up latency; therefore, we could not estimate the total time our participants spent in bed. Although previous studies in comparable populations and contexts showed that people with IDs lay in bed for approximately 10 h (Böhmer et al. 2020), this leaves room for improvement with regard to the sleep efficiency of people with IDs.
The effects on sleep duration and sleep onset time should be interpreted with some care. First, the clinical relevance of a shorter sleep duration of 25 min and a delay in sleep onset time of 25 min has to be determined. Second, a possible better consolidation of nocturnal sleep was not represented by a better sleep quality, as we found no change in sleep efficiency, amount or duration of sleep and wake bouts. Therefore, findings on sleep duration and sleep onset time might well be a result of a change in care regime that we did not monitor between the short-term and long-term measurements rather than a long-term effect of the light installations.
The short-term positive effects on mood and behaviour did not last until 1 year after installing the dynamic light. Closer inspection of the short-term results showed that the biggest effect was seen immediately after installing the light in January and February, when the days were shortest (Böhmer et al. 2022). The effect was less pronounced at measurements in the following months, when the days became longer again. Furthermore, long-term measurements were scheduled exactly 1 year and 3 weeks after installation, when days were the shortest again. Therefore, the seasonal effects of increasing light exposure can be excluded as an explanation for the lack of long-term effects of light in this study. We cannot exclude that an initial short-term improvement of depressive symptoms might have been an initial effect of the novelty of the light installation, which returned to baseline 1 year later.
The current study shows the need for more research, with more participants, on the long-term effects of increasing light exposure in the living environment on sleep–wake rhythm, mood and behaviour in care facilities. More studies would increase the possibility of comparing findings. So far, the only other study available on this topic found sleep duration to increase in people with dementia (Riemersma-Van Der Lek et al. 2008), as opposed to the decrease in sleep duration we found. Furthermore, as ceiling-mounted light installations are a long-term investment, it would be especially interesting to study the effects of these installations over the long term.
As we concluded in our study into the short-term effects of dynamic light, the regulation of care in group homes for people with IDs does not always meet the preconditions for healthy sleep–wake rhythm (Böhmer et al. 2021). For instance, clinical experience shows that care schedules often do not consider personal preferences regarding sleep–wake rhythm. Physical activity is needed to build up the sleep pressure needed for consolidated sleep (Borbely 1982), while the physical activity of people with IDs is overall pretty low (Hilgenkamp et al. 2012). With increasing light exposure, one of the preconditions is met, but others need to be improved as well to increase the sleep–wake rhythm in people with IDs living in care facilities.
Strengths, limitations and future research
We conducted one of the first studies into the long-term effect of enhancing environmental light in care facilities on sleep–wake rhythm, mood and behaviour in older adults with IDs. We accounted for the seasonal effect of light by repeating measurements in the darkest period of the year.
This study had a small sample size for the analyses of the sleep–wake rhythm outcomes. Therefore, the results should be interpreted with care. Furthermore, whereas the short-term study followed the multiple baseline design and was therefore designed to account for possible events during the study, the current study followed a conventional repeated measurements design and did not account for this history effect.
Another limitation is the lack of reliable data on personal (day)light exposure prior to and during the intervention. Initially, personal light measurements were taken every measurement week using a Hobo data logger (Onset Computer Corporation 2018). Despite the fact that using the Hobo data logger was shown to be feasible in our population before (Van Duijnhoven et al. 2017), the amount of missing data on light exposure in our study was high (up to 51%) due to wearing these light sensors incorrectly, for example, underneath sweaters and jackets (Böhmer et al. 2021). This made it impossible to report reliable data on personal daily light exposure during the study period. In order to measure whether the light levels in the common living room increased after the placement of the light installations, light exposure in the living room was measured 1 day during the last week of baseline and 1 day during the first week after the placement of the light installation, and we presented these data in the paper of the initial study (Böhmer et al. 2022). Over the study period, the light levels in the common rooms increased from 68 lux (SD = 46 lux) at baseline to 989 lux (SD = 211 lux) during intervention. In future research, the reliability of (day)light exposure measurements could be increased by using two light sensors, one on a broche above the clothing worn inside and one on the jacket worn outside (Te Lindert et al. 2018; Itzhacki et al. 2019).
There was insufficient monitoring of what happened between the short-term and long-term measurements and whether the program of the light installation was working correctly. It might be that the light installations did not run the proper program throughout the period between short-term and long-term measurements. Also, we did not monitor any changes in the care regime that might have affected the sleep rituals and bedtimes of our participants.
The current study shows the need for research on the long-term effects of enhancing environmental light on sleep–wake rhythm, mood and behaviour. Future research should focus on well-powered, randomised, controlled trials into the long-term effect of dynamic light on sleep–wake rhythm and mood in people with IDs throughout the year. Special attention should be given to populations with known mood and/or sleep problems. The preventative properties of light and the effectiveness of increasing light exposure should be studied as well. In addition, the interaction of the effects of light on both the regulation of the circadian rhythm and visual functioning could be studied in both the residents and the caregivers.
Conclusions
We conducted the first study into the long-term effect of enhancing environmental light in care facilities for people with IDs. We found no evidence for a long-term effect of environmental dynamic lighting on sleep–wake rhythm, mood and behaviour in older persons with IDs living in care facilities. However, our results should be interpreted with care due to the small sample size. Overall, the current study shows the need for research on the long-term effects of enhancing environmental light.
Acknowledgements
The authors want to thank the participants, their families, professional caretakers and care provider Middin for their collaboration in this study. The authors would like to thank Light Technology Netherlands for the supply of the light installations used in this study. Advise on and assistance with the analysis of the data was provided by Grigorios Papageorgiou of the Department of Biostatistics at Erasmus MC, University Medical Centre Rotterdam, and Bart te Lindert from the Netherlands Institute for Neuroscience, Amsterdam. All authors made substantial contributions to the design of this study and/or were involved in the interpretation of the data. All authors drafted the work and reviewed and revised the manuscript critically, and all approved the final version to be published.
Source of Funding
The current project is funded by subsidy provider Zorgondersteuningsfonds. Additional financial and organisational support was provided by Middin, a care organisation for people with intellectual disabilities in Rijswijk, the Netherlands. Light Technology Netherlands did not sponsor the current project. Zorgondersteuningsfonds, Middin and Light Technology had no influence on the study design, data collection, data analysis and interpretation or the writing of the report.
Conflict of Interest
The authors declare that there is no conflict of interest.
Ethics Statement
The Medical Ethical Committee of Erasmus MC, University Medical Centre Rotterdam, the Netherlands, approved the study presented here (MEC-2017-467).
Appendix A: Full models
| Sleep–wake rhythm | |
|---|---|
| Stability | IS ~ measurement + age + sex + mobility + antiepileptics + ADL, random = ~1 | participant |
| Fragmentation | IV ~ measurement + age + sex + beta-blockers + IADL + antiepileptics + mobility + ADL, random = ~1 | participant |
| L5 | L5 ~ measurement + age + sex + vision impairment + antidepressants + ADL, random = ~1 | participant |
| L5 onset | L5onsetphase ~ measurement + ns (age, df = 2) + sex + CVDrisk + daily activities (days) + benzodiazepines, random = ~1 | participant |
| M10 | M10 ~ measurement + age + sex + CVDrisk + antiepileptics + mobility + antidepressants + ADL + beta-blockers + hearing impairment, random = ~1 | participant |
| M10 onset | M10onsetphase ~ measurement + age + sex + antiepileptics + CVDrisk + vision impairment + mobility + daily activities (hours per week), random = ~1 | participant |
| Amplitude | AMP ~ measurement + age + sex + ADL + mobility + antidepressants + antiepileptics + antipsychotics + IADL, random = ~1 | participant |
| Relative amplitude | RA ~ measurement + age + sex + ADL + IADL + mobility + antiepileptics, random = ~1 | participant, |
| Sleep estimates | |
|---|---|
| Total sleep time | TST ~ measurement + age + sex + ADL + mobility + daily activities (days) + antipsychotics + benzodiazepines + antidepressants + hearing impairment, random = ~1 | participant |
| Waking after sleep onset | WASO ~ measurement + age + sex + ADL + IADL + mobility + daily activities (days) + antidepressants + antipsychotics + benzodiazepines + beta-blockers + MDepilepsie + Assumed sleep, random = ~1 | participant |
| Sleep efficiency | SE ~ measurement + age + sex + ADL + IADL + mobility + daily activities (days) + antidepressants + antipsychotics + benzodiazepines + beta-blockers + vision impairment, random = ~1 | participant |
| Sleep onset | SOT ~ measurement + age + sex + ADL + IADL + mobility + daily activities (days) + antidepressants + benzodiazepines + antipsychotics + CVDrisk + epilepsy + vision impairment + hearing impairment, random = ~1 | participant |
| Final wake time | FWT ~ measurement + age + sex + ADL + IADL + daily activities (hours per week) + mobility + vision impairment + antidepressants + antiepileptics + beta-blockers, random = ~1 | participant |
| Mid-sleep | Mid-sleep ~ measurement + age + sex + ADL + IADL + mobility + CVDrisk + daily activities (days) + benzodiazepines + antipsychotics + epilepsy + vision impairment + hearing impairment, random = ~1 | participant |
| Number of wake bouts | Numberofwakebouts ~ measurement + age + sex + ADL + IADL + mobility + CVDrisk + daily activities (days) + antipsychotics + beta-blockers + hearing impairment + vision impairment + MDepilepsie, random = ~1 | participant |
| Wake bout duration | Meanwakebouttimemin ~ measurement + age + sex + ADL + IADL + CVDrisk + daily activities (hours per week) + antidepressants + benzodiazepines + antiepileptics + beta-blockers + hearing impairment, random = ~1 | participant |
| Number of sleep bouts | Numberofsleepbouts ~ measurement + age + sex + ADL + IADL + mobility + CVDrisk + daily activities (hours per week) + antipsychotics + beta-blockers + vision impairment + MDepilepsie, random = ~1 | participant |
| Sleep bout duration | Meansleepbouttimemin ~ measurement + ns (age, df = 2) + sex + ADL + mobility + daily activities (hours per week) + antipsychotics + vision impairment, random = ~1 | participant |
| Short sleep | shortsleep ~ measurement + age + sex + ADL + daily activities (hours per week) + antidepressants + antipsychotics + epilepsy + vision impairment, random = ~1 | participant |
| Night waking | nightwaking ~ measurement + ns (age, df = 2) + sex + ADL + IADL + mobility + daily activities (days) + antipsychotics + benzodiazepines + hearing impairment, random = ~1 | participant |
| Mood and behaviour | |
|---|---|
| Depressive symptoms | ADAMS_Depressive symptoms ~ measurement + age + sex + antipsychotics + IADL, random = ~1 | participant |
| Anxiety symptoms | ADAMS_Anxiety ~ measurement + age + sex + antipsychotics + epilepsy + daily activities (days), random = ~1 | participant |
| Social avoidance | ADAMS_Social ~ measurement + age + sex + mobility + ADL + antipsychotics + vision impairment, random = ~1 | participant |
| Other symptoms | |
| Above cut-off depression | ScreeningDepr ~ measurement + age + sex + antipsychotics, random = ~1 | participant |
| Above cut-off anxiety | ScreeningAnx ~ measurement + age + sex + daily activities (days) + antipsychotics, random = ~1 | participant |
| Hyperactivity | ABC_hyperactiviteit ~ measurement + age + sex + vision impairment + hearing impairment + antipsychotics + benzodiazepines + level of ID + daily activities (days), random = ~1 | participant |
| Irritability | ABC_prikkelbaarheid ~ measurement + age + sex + antipsychotics + benzodiazepines + daily activities (days) + MDepilepsie, random = ~1 | participant |
| Lethargy | ABC_lethargie ~ measurement + age + sex + ADL + mobility + antipsychotics + level of ID + IADL, random = ~1 | participant |
| Stereotypy | ABC_stereotypie ~ measurement + age + sex + mobility + antidepressants + antiepileptics + antipsychotics + beta-blockers + level of ID + daily activities (hours per week), random = ~1 | participant |
| Inadequate speech | ABC_inadequate_spraak ~ measurement + age + sex + mobility + antidepressants + antipsychotics, random = ~1 | participant |
- IS, interdaily stability; ADL, activities of daily living; IV, intradaily variability; IADL, instrumental activities of daily living; CVD, cardiovascular disease; ADAMS, Anxiety, Depression and Mood Scale; ABC, Aberrant Behaviour Checklist.
Appendix B: Test results of dynamic light on adverse effects
| Assessment scale† | Baseline, mean (SD) | Short term, mean (SD) | Long term, mean (SD) | Baseline to 1 year after installing light | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Valid obs | N | Beta | 5% | 95% | P-value‡ | Effect size | |||||
| Dizziness | Range, 0–3 | 0.55 (0.88) | 0.28 (0.65) | 0.6 (0.91) | 292 | 45 | 0.08 | −0.09 | 0.24 | 0.36 | 0.14 |
| Drowsiness | Range, 0–3 | 1.19 (1) | 0.95 (0.95) | 1.11 (1.09) | 292 | 45 | −0.04 | −0.30 | 0.22 | 0.76 | −0.05 |
| Eye complaints | Range, 0–3 | 0.82 (1.04) | 0.6 (0.95) | 0.88 (1.04) | 292 | 45 | 0.06 | −0.18 | 0.31 | 0.61 | 0.08 |
| Feebleness | Range, 0–3 | 0.89 (0.98) | 0.71 (0.95) | 0.8 (0.96) | 292 | 45 | −0.06 | −0.31 | 0.19 | 0.62 | −0.07 |
| Headache | Range, 0–3 | 0.8 (0.93) | 0.78 (0.96) | 0.75 (0.93) | 292 | 45 | −0.03 | −0.26 | 0.20 | 0.79 | −0.04 |
| Hunger | Range, 0–3 | 0.78 (0.89) | 0.64 (0.83) | 0.57 (0.78) | 292 | 45 | −0.20 | −0.44 | 0.03* | 0.09 | −0.25 |
| Hyperactivity | Range, 0–3 | 0.35 (0.73) | 0.24 (0.61) | 0.28 (0.66) | 292 | 45 | −0.06 | −0.24 | 0.12 | 0.53 | −0.09 |
| Inability to sleep | Range, 0–3 | 0.67 (0.77) | 0.62 (0.83) | 0.68 (0.82) | 292 | 45 | 0.04 | −0.19 | 0.27 | 0.73 | 0.05 |
| Irritability | Range, 0–3 | 1.22 (1.07) | 1.09 (1.14) | 1.17 (1.19) | 292 | 45 | 0.02 | −0.23 | 0.27 | 0.87 | 0.03 |
| Nausea | Range, 0–3 | 0.42 (0.7) | 0.25 (0.53) | 0.33 (0.56) | 292 | 45 | −0.08 | −0.27 | 0.11 | 0.40 | −0.13 |
| Constipation | Range, 0–3 | 0.77 (0.96) | 0.63 (0.81) | 0.77 (0.9) | 292 | 45 | 0.09 | −0.14 | 0.32 | 0.44 | 0.12 |
| Nervous | Range, 0–3 | 1.14 (1.01) | 1.09 (1) | 0.91 (1.04) | 292 | 45 | −0.18 | −0.40 | 0.04* | 0.11 | −0.24 |
| Anxious | Range, 0–3 | 0.9 (0.94) | 0.92 (0.97) | 0.93 (1) | 292 | 45 | 0.09 | −0.14 | 0.32 | 0.44 | 0.12 |
| Stomach ache | Range, 0–3 | 0.73 (0.97) | 0.64 (0.93) | 0.8 (1.01) | 292 | 45 | 0.04 | −0.18 | 0.26 | 0.73 | 0.05 |
| Sweating | Range, 0–3 | 0.47 (0.74) | 0.28 (0.62) | 0.35 (0.67) | 292 | 45 | −0.10 | −0.28 | 0.07 | 0.25 | −0.17 |
| Trembling hands | Range, 0–3 | 0.54 (0.9) | 0.38 (0.83) | 0.46 (0.84) | 292 | 45 | −0.05 | −0.25 | 0.15 | 0.65 | −0.07 |
| Other complaints | Range, 0–3 | 0.58 (1.02) | 0.37 (0.83) | 0.53 (0.89) | 292 | 45 | −0.02 | −0.27 | 0.22 | 0.84 | −0.03 |
- *P < 0.05.
- †Range is shown from best to worst.
- ‡Linear mixed model analysis is mutually adjusted for time, and test results are presented for the measurement ‘1 year’.
- SD, standard deviation; Valid obs, valid observations; N, participants.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, M. N. B., upon reasonable request.




