Paternally imprinted LATE-FLOWERING2 transcription factor contributes to paternal-excess interploidy hybridization barriers in wheat
Edited by: Bao Liu, Northeast Normal University in Changchun, China
Abstract
Interploidy hybridization between hexaploid and tetraploid genotypes occurred repeatedly during genomic introgression events throughout wheat evolution, and is commonly employed in wheat breeding programs. Hexaploid wheat usually serves as maternal parent because the reciprocal cross generates progeny with severe defects and poor seed germination, but the underlying mechanism is poorly understood. Here, we performed detailed analysis of phenotypic variation in endosperm between two interploidy reciprocal crosses arising from tetraploid (Triticum durum, AABB) and hexaploid wheat (Triticum aestivum, AABBDD). In the paternal- versus the maternal-excess cross, the timing of endosperm cellularization was delayed and starch granule accumulation in the endosperm was repressed, causing reduced germination percentage. The expression profiles of genes involved in nutrient metabolism differed strongly between these endosperm types. Furthermore, expression patterns of parental alleles were dramatically disturbed in interploidy versus intraploidy crosses, leading to increased number of imprinted genes. The endosperm-specific TaLFL2 showed a paternally imprinted expression pattern in interploidy crosses partially due to allele-specific DNA methylation. Paternal TaLFL2 binds to and represses a nutrient accumulation regulator TaNAC019, leading to reduced storage protein and starch accumulation during endosperm development in paternal-excess cross, as confirmed by interploidy crosses between tetraploid wild-type and clustered regularly interspaced palindromic repeats (CRISPR) – CRISPR-associated protein 9 generated hexaploid mutants. These findings reveal a contribution of genomic imprinting to paternal-excess interploidy hybridization barriers during wheat evolution history and explains why experienced breeders preferentially exploit maternal-excess interploidy crosses in wheat breeding programs.
INTRODUCTION
Tetraploid introgression into hexaploid wheat has occurred repeatedly during wheat evolution, strongly increasing the genetic diversity of hexaploid populations and contributing to their divergent adaptations to changing environments (He et al., 2019; Zhou et al., 2020). Hexaploid wheat is thought to have served as the maternal parent during interploidy introgression because the average diversity index value of the cytoplasmic genome in hexaploid wheat is ~14-fold smaller than that of tetraploid wheat (Ishii et al., 2001), whereas paternal-excess crosses (in which the male parent is of higher ploidy) with multiple groups of tetraploid wheat species would have led to progeny with similar levels of cytoplasmic diversity.
Interploidy hybridization is often exploited in hexaploid wheat breeding programs to introduce genomic fragments from tetraploid into hexaploid genotypes to improve the grain quality potential and biotic/abiotic stress resistance of the progeny (Martin et al., 2011; Martin et al., 2013; Han et al., 2014; Kalous et al., 2015; Han et al., 2016). To efficiently obtain fertile F1 progeny from interploidy crosses, breeders have widely employed maternal-excess hybridization (in which the female parent is of higher ploidy) because paternal-excess crosses often result in poor seed set, seed germination, and seedling establishment (Kihara, 1982; Sharma and Gill, 1983). However, the inheritance efficiency of the tetraploid genome is lower in the progenies of maternal- versus paternal-excess crosses. Maternal-excess crosses also show a lower capacity to retain D chromosomes in the F2 populations compared with paternal-excess crosses (~1.8 vs. ~2.83 per line, Hirosawa et al., 2004). These factors limit the utilization efficiency of maternal-excess crosses in wheat improvement programs. Despite their relevance to these issues, the molecular mechanisms underlying the biased use of hexaploid wheat as the maternal parent in introgression events and breeding programs are largely unknown.
The contribution of the parental genome plays a crucial role in regulating endosperm development in interploidy crosses, resulting in altered timing of endosperm cellularization in the progenies (Scott et al., 1998; Leblanc et al., 2002; Li and Dickinson, 2010; Zhang et al., 2016). A paternal-excess cross tends to prolong the syncytial stage, delay the initiation of starch accumulation, and lead to the production of large but shriveled seeds. A maternal-excess cross acts in the opposite manner, leading to precocious endosperm cellularization and generating small but plump seeds. Emerging evidence suggests that genomic imprinting, an epigenetic phenomenon that causes differences in the expression patterns of parental alleles depending on the parent-of-origin, is involved in regulating endosperm development following interploidy crosses (Erilova et al., 2009; Jullien and Berger, 2010; Li and Dickinson, 2010; Wolff et al., 2011; Wang et al., 2018).
In Arabidopsis thaliana, similar endosperm phenotypes resulting from paternal-excess crosses are observed in loss-of-function mutants of the Polycomb Repressive Complex 2 (PRC2)-associated imprinted genes MEDEA (MEA) and FERTILIZATION INDEPENDENT SEED 2 (FIS2) (Chaudhury et al., 1997; Scott et al., 1998; Kohler et al., 2003; Dilkes et al., 2008; Erilova et al., 2009). Genomic imprinting is thought to establish a barrier for reproductive isolation during interploidy hybridization (Haig and Westoby, 1991; Kradolfer et al., 2013; Wolff et al., 2015; Huang et al., 2017). The paternally imprinted gene PHERES1 (PHE1) is a central player in establishing a reproductive barrier of interploidy crosses, because upregulation of PHE1 causes deregulation of genes that are required for endosperm proliferation and cellularization (Batista et al., 2019). In addition, knockout of the paternally imprinted genes PATERNALLY EXPRESSED GENE 2, PATERNALLY EXPRESSED GENE 9, ADMETOS, SU(VAR)3-9 HOMOLOG 7 partially restores seed fertility and suppresses seed abortion in Arabidopsis interploidy crosses (Kradolfer et al., 2013; Wolff et al., 2015; Huang et al., 2017). Consistent with this finding, parental genome imbalance extensively alters the expression profiles of imprinted genes and deregulates parent-of-origin-dependent allele-specific expression patterns (Erilova et al., 2009; Jullien and Berger, 2010; Li and Dickinson, 2010; Tiwari et al., 2010; Zhang et al., 2016). For example, in Arabidopsis, an increased dosage of the paternal genome causes a loss of maternal imprinting of FIS2, leading to a predominantly paternal expression pattern of MEA (Jullien and Berger, 2010). Similarly, intraploidy crosses affect the expression of maize (Zea mays) maternally expressed gene1 in endosperm (Li and Dickinson, 2010); and in rice (Oryza sativa), 10 out of 18 imprinted genes examined were found to exhibit altered parent-of-origin-dependent allele-specific expression (Zhang et al., 2016).
Endosperm overgrowth or undergrowth due to a variation in the timing of developmental transitions has also been observed in interspecific hybrids, depending on the cross combination and direction; these changes in the endosperm always cause hybrid incompatibility between plant species (Cooper and Brink, 1942; Ishikawa et al., 2011; Rebernig et al., 2015; Lafon-Placette et al., 2017; Roth et al., 2018; Tonosaki et al., 2018). Reciprocal crosses of A. thaliana and Arabidopsis arenosa show loss of imprinting at the maternal PHERES1 (PHE1) locus, and the upregulation of the maternal PHE1 allele contributes to postzygotic incompatibility, whereas its deletion improves the fertility of interspecific crosses (Josefsson et al., 2006).
Genomic imprinting deregulation has also been documented in interspecific crosses within other genera, including Solanum, Oryza, and Capsella (Josefsson et al., 2006; Walia et al., 2009; Burkart-Waco et al., 2015; Kirkbride et al., 2015; Rebernig et al., 2015; Florez-Rueda et al., 2016; Tonosaki et al., 2018). Moreover, manipulating the parental ploidy level influences the compatibility of interspecific crosses in Arabidopsis and rice (Josefsson et al., 2006; Tonosaki et al., 2018). These findings suggest that parent-of-origin-dependent effects play a crucial role in establishing the reproductive barrier for hybrid incompatibility (Kinoshita et al., 1999; Dilkes and Comai, 2004; Lafon-Placette and Köhler, 2016; Gehring and Satyaki, 2017). In addition, these results indicate that the molecular mechanisms underlying the endosperm barrier in interploidy and interspecific crosses overlap and could at least be partially attributed to the misexpression of imprinted genes (Ishikawa et al., 2011; Sekine et al., 2013).
In this study, we performed the first detailed analysis of phenotypic variation and the molecular mechanisms associated with interploidy crosses between tetraploid (Triticum durum, AABB) and hexaploid (Triticum aestivum, AABBDD) wheat. Although the general effects of interploidy crosses in wheat were conserved with respect to other plant species—that is, endosperm cellularization and starch accumulation were dramatically impaired in the paternal-excess versus the maternal-excess cross—we also observed several wheat-specific features, especially regarding effects on seed size and germination percentage. In addition, the expression patterns of the parental alleles for genes in subgenomes A and B were fundamentally disturbed in the interploidy crosses, resulting in significant deviation from a 2:1 maternal: paternal ratio (hereafter referred to as 2m:1p). Loss of function of the paternal allele of LATE-FLOWERING 2 (TaLFL2) improved nutrient accumulation and seed germination percentage in the paternal-excess cross. Further investigation revealed that TaLFL2 binds to and represses the activity of TaNAC19, encoding an endosperm-specific NAC transcription factor that activates glutenin and starch accumulation (Gao et al., 2021). Overall, these findings provide insights into the molecular mechanisms underlying the interploidy block of paternal-excess crosses during the history of wheat evolution. In addition, they offer candidate genes for improving the utilization efficiency of paternal-excess crosses in wheat breeding programs.
RESULTS
Genomic imbalance affects endosperm development and starch accumulation in wheat interploidy crosses
To investigate the effects of parental genome dosage on wheat seed development, we performed self-pollination and interploidy crosses using tetraploid (T. durum, AABB (4n), JIN8) and hexaploid (T. aestivum, AABBDD (6n), CB037) wheat, including 4n × 4n (the maternal parent is listed before the paternal parent in each cross), 6n × 6n, 4n × 6n, and 6n × 4n. We examined ploidy number and chromosome structure in the parental lines by fluorescence in situ hybridization (FISH). CB037 (6n) contained 21 chromosomes pairs, whereas JIN8 (4n) contained 14 chromosomes pairs (Figure S1). Seeds from self-pollinated plants (4n × 4n and 6n × 6n) developed normally, while seeds from interploidy crosses exhibited distinct morphological abnormalities (Figure 1A). Specifically, seeds from interploidy crosses with paternal genomic excess (4n × 6n) were large and shriveled, while the reciprocal cross (6n × 4n) generated small, plump seeds. Histological assays revealed that at 3 d after pollination (DAP), endosperms derived from 4n and 6n self-pollinated plants were undergoing cellularization and formed several layers of cells around the peripheral embryo sac (Figure 1B). At the same developmental stage, endosperms from the 6n × 4n cross showed complete cellularization, while those from the 4n × 6n cross were still in the syncytial stage, with a large cavity at the center. At 10 and 15 DAP, plump kernels with solidified endosperm were produced in the 6n × 4n cross, while swollen kernels filled with viscous liquid were produced in the reciprocal cross (Figure 1B).
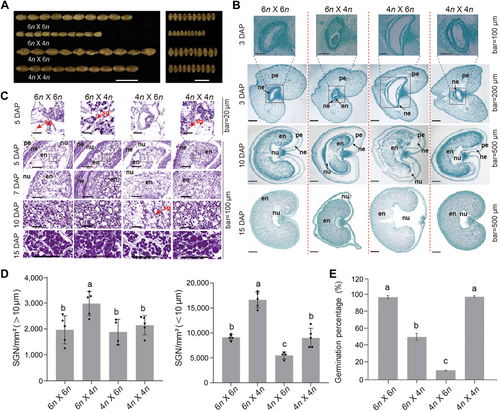
Asymmetric genome dosage results in developmental abnormality of wheat endosperm in reciprocal interploidy crosses
(A) Phenotypes of seeds from the reciprocal crosses and their parents. 6n: CB037; 4n: JIN8. Scale bar: 1 cm. (B) Cross-section micrographs of wheat kernels collected at different developmental stages; 6n: CB037; 4n: JIN8. Scale bar: 500 μm. en: endosperm; ne: nucellar epidermis; nu: nucellus; pe: pericarp. The figure is a representative example of three replicates. (C) Periodic acid-Schiff staining of endosperms from different crosses. Starch granules are indicated by red arrows. 6n: CB037; 4n: JIN8. Scale bar: 100 μm. en: endosperm; ne: nucellar epidermis; nu: nucellus; pe: pericarp; sg: starch granule. The figure is a representative example of three replicates. (D) Amounts of type A (>10 μm) and type B (<10 μm) starch granules. Lowercase letters indicate statistical significances (P < 0.05) according to one-way analysis of variance (ANOVA). SNG: starch granule number. 4n: JIN8; 6n: CB037. (E) Germination percentages of interploidy reciprocal crosses together with self-pollinated crosses. Three biological replicates were performed for each cross. Lowercase letters indicate statistical significance (P < 0.05) according to one-way ANOVA.
The abnormal seed development in wheat interploidy crosses could be associated with a defect in nutrient uptake and allocation. Therefore, we examined the timing and localization of starch accumulation during endosperm development. Periodic acid-Schiff staining showed that starch accumulation occurred earlier (at 5 DAP) in the maternal-excess than in the paternal-excess cross, where it was evident only at 10 DAP (Figure 1C). Consistently, the starch biosynthetic related genes of ADP-glucose pyrophosphorylase small subunit 1 (AGPS1), starch branching enzyme II b (BEIIb) and granule-bound starch synthase I (GBSSI) exhibited elevated expression abundance in maternal-excess cross compared with paternal-excess cross (Figure S2). Moreover, the amounts of starch granules per square millimeter in endosperm, including both A type (>10 μm) and B type (<10 μm) granules, were higher in the maternal-excess cross and lower (for B type only) in the paternal-excess cross than in intraploidy crosses (Figure 1D). Both A and B type starch granules accumulated to lower levels in the paternal- versus maternal-excess cross, which was probably associated with the significantly decreased germination percentage in the paternal-excess cross (11.08%) compared with the maternal-excess cross (50.62%) (Figure 1E).
Together, these results indicate that in wheat interploidy crosses, the timing of endosperm cellularization and starch accumulation is strongly affected by the origin of parental genome dosage.
Parental genomes contribute asymmetrically to transcriptomic changes in the endosperm
To investigate whether the differential endosperm development between interploidy crosses was associated with changes in gene expression, we performed transcriptome sequencing (RNA-seq) analysis using 15-DAP hybrid endosperms derived from reciprocal crosses between tetraploid and hexaploid lines, as well as from self-pollination of each line. The presence of homeologs in polyploid wheat makes it challenging to identify genotype-dependent single nucleotide polymorphisms (SNPs) and could lead to bias in allele-specific expression analysis. Hence, to minimize artifacts from ambiguous mapping, we considered only those RNA-seq reads uniquely mapped to the subgenome A, B, or D and used them to detect informative SNPs (see Methods, Table S1). In total, we identified 4,760 upregulated and 6,695 downregulated genes in the endosperm of the 4n × 6n cross compared with the 6n × 4n cross (fold change >2, false discovery rate (FDR)-adjusted P-value < 0.05, Dataset S1). Specifically, the downregulated genes were 1,912, 1,823, and 956 in subgenomes A, B, and D, respectively, while 69 were in unknown chromosomes due to gaps in the wheat reference genome; and the upregulated genes were 1,289, 1,347, and 3,937 in subgenomes A, B, and D, respectively, and 122 were in unknown chromosomes. In agreement with the observed phenotypes, Gene Ontology (GO) enrichment analysis showed that the genes upregulated in the 6n × 4n cross versus the 4n × 6n cross were enriched in categories such as “nutrient reservoir activity” (GO:0045735), “starch biosynthetic process” (GO:0019252), and “glycogen (starch) synthase activity” (GO:0004373) (Figure 2A; Dataset S2). GO enriched categories for genes upregulated in the 4n × 6n cross included terms related to different enzyme activities and cell wall organization. These findings are thus consistent with the phenotypes observed in crosses with maternal or paternal-excess.
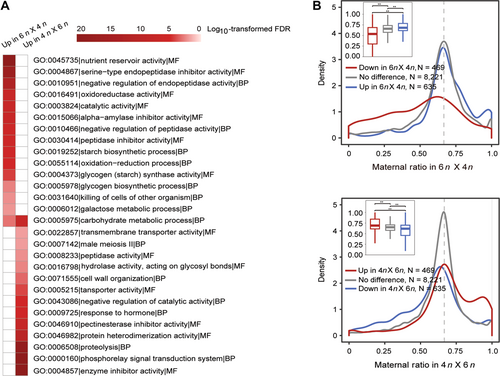
Imbalanced genomic contribution leads to different transcriptome profiles between endosperms of wheat interspecific crosses
(A) Gene Ontology enrichment analysis of genes that are differentially expressed between 4n × 6n and 6n × 4n crosses. (B) Maternal ratio distribution of expressed genes in 15-d after pollination (DAP) wheat endosperm of interploidy reciprocal crosses. Maternal ratio was calculated for transcripts that had ≥10 single nucleotide polymorphism-associated reads from both reciprocal crosses. Double asterisks indicate statistically significant differences estimated by Student's t-test at P < 0.01.
Next, we only investigated the parental allele expression ratio of endosperm genes on subgenome A and B, because the parental genome contribution follows the 2m:1p ratio for subgenome A and B in 6n × 4n and 4n × 6n crosses, whereas those reads on subgenome D would only come from the hexaploidy parent in the wheat endosperm. A comparison of paternal versus maternal expression of SNP-associated genes (read counts >10 per cross; Datasets S3, S4) demonstrated that the majority of non-differentially expressed genes exhibited the expected 2m:1p ratio in the reciprocal crosses (Figure 2B). However, downregulated genes exhibited an overall shift toward paternal-biased expression patterns, whereas upregulated genes showed maternal-biased expression patterns. Interestingly, of the 469 downregulated genes in 6n × 4n compared with 4n × 6n cross, 70.1% (329/469) showed a lower maternal expression ratio in 6n × 4n than in 4n × 6n cross, with an average of 0.5036 versus 0.6931; of the 635 downregulated genes in 4n × 6n compared with 6n × 4n cross, 65.0% (413/635) exhibited a lower maternal expression ratio in 4n × 6n than in 6n × 4n, with an average of 0.5807 versus 0.6828. Taken together, we proposed that maternal and paternal alleles do not contribute equally to the parental-biased patterns of differentially expressed genes in wheat interploidy reciprocal crosses, which is mainly attributed to the expression changes of maternal originated transcripts.
Imbalanced parental genome dosage deregulates genomic imprinting in interploidy crosses
Interploidy hybrids exhibit deregulation of genomic imprinting in various plant species (Josefsson et al., 2006; Walia et al., 2009; Jullien and Berger, 2010; Burkart-Waco et al., 2015; Kirkbride et al., 2015; Rebernig et al., 2015; Florez-Rueda et al., 2016; Zhang et al., 2016; Tonosaki et al., 2018). Therefore, we investigated genomic imprinting patterns in wheat interploidy crosses, and considered only genes located in the A and B subgenome because only one parent provides the D subgenome. We found that 30.4% (2,832/9,325) and 42.5% (3,693/9,325) of genes deviated from the 2m:1p ratio in the 6n × 4n and 4n × 6n cross, respectively (χ2 test, FDR-adjusted P-value < 0.05; Figure 3A). Genes with at least 90% maternal reads or 70% paternal reads in both reciprocal crosses (SNP-associated reads >10 per cross and FDR-adjusted P-value < 0.05 in both biological replicates) were defined as maternally (MEGs) or paternally (PEGs) expressed genes, respectively. According to this criterion, 183 imprinted genes in subgenomes A and B of the interploidy crosses were identified, including 110 MEGs and 73 PEGs (Figure 3B; Dataset S5). We performed reverse transcription – polymerase chain reaction (RT-PCR) followed by Sanger sequencing to validate the imprinted status of 10 selected genes; all genes showed allele-specific expression pattern dependent on the parent-of-origin (Figure S3).
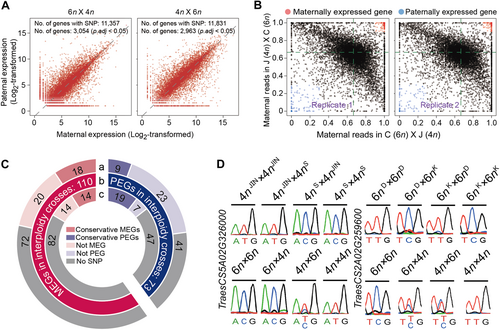
Genomic imprinting is disturbed in interploidy wheat endosperm
(A) Parental allele expression ratio of genes in 15-d after pollination (DAP) endosperms of reciprocal interploidy crosses. Paternal (y-axis) and maternal (x-axis) expression levels are represented as log2-transformed single nucleotide polymorphism (SNP)-associated read counts (≥10). The deviation from 2m:1p was calculated by χ2 goodness-of-fit test (false discovery rate-adjusted P < 0.05). Dashed diagonal line represents the expected 2m:1p ratio. 6n: CB037; 4n: JIN8. (B) Ratio-based cutoff to identify maternally expressed genes (MEGs) and paternally expressed genes (PEGs). Red dots in the upper-right corners have >90% maternal reads (MEGs), whereas blue dots in the lower-left corners have <30% maternal reads (PEGs). (C) Comparison of imprinted genes between intraploidy and interploidy crosses. Lane a: parent-of-origin expression patterns of the 183 genes in the intraploidy cross of hexaploid wheat; Lane b: 110 MEGs and 73 PEGs in interploidy crosses; Lane c: parent-of-origin expression patterns of the 183 genes in the intraploidy cross of tetraploid wheat. In Lanes a and c, red and light red boxes indicate the number of MEGs that are conserved or not conserved between interploidy and intraploidy crosses, respectively; dark purple and light purple boxes indicate the number of PEGs that are conserved or not conserved between interploidy and intraploidy crosses, respectively; gray boxes indicate the number of genes without SNPs in the intraploidy cross parents. (D) Sanger sequencing validation of imprinted genes between interploidy crosses and intraploidy crosses. JIN: JIN8 (4n); SC: SCAUP (4n); K: Keyi5214 (6n); D: Doumai (6n).
Compared with the imprintome of intraploidy crosses (4n × 4n and 6n × 6n) identified in our previous study (Yang et al., 2018), we identified more imprinted genes in the interploidy crosses (110 MEGs in subgenomes A and B of interploidy crosses vs. 90 and 86 MEGs in tetraploid (4n × 4n) and hexaploid (6n × 6n) crosses, respectively, and 73 PEGs in subgenomes A and B of interploidy crosses vs. 45 and 43 PEGs in tetraploid (4n × 4n) and hexaploid (6n × 6n) crosses, respectively). This observation suggests that genomic imprinting could be deregulated by subgenome dosage imbalance in interploidy cross, and interestingly, the imprinting changes of PEG were obviously higher than that of MEG (~39.9% vs. ~20.0%). We assessed the conservation of parental allele expression patterns between interploidy and intraploidy crosses. Among the 183 imprinted genes in the interploidy crosses, 54 and 70 possessed informative SNPs between the two parents in tetraploid (4n × 4n) and hexaploid (6n × 6n) intraploidy crosses, respectively. Among these, 33 imprinted genes (14 MEGs and 19 PEGs) were conserved between interploidy crosses and tetraploid intraploidy crosses, and 27 (18 MEGs and nine PEGs) were conserved between interploidy crosses and hexaploid intraploidy crosses. In contrast, the other 21 (14 MEGs and seven PEGs) and 43 (20 MEGs and 23 PEGs) of imprinted genes showed altered parental allele expression status between interploidy crosses and tetraploid or hexaploid intraploidy crosses, respectively (Figure 3C; Dataset S5). Of the 21 and 43 genes with varied imprinting patterns between interploidy and intraploidy crosses, 10 and 14 genes did not fulfill the cutoffs of 90% maternal reads or 70% paternal reads, respectively, in the reciprocal crosses, although all of these genes contained >10 SNP-associated reads per cross; seven and 20 genes met the screening criteria but contained <10 SNP-containing reads in at least one cross; three and nine genes did not satisfy the cutoff criteria and the number of SNP-associated reads; and only one gene (TraesCS7A02G036400) showed the paternally imprinted pattern in the interploidy cross but not in 4n × 4n reciprocal crosses, because its FDR value was >0.05 (Figure S4; Dataset S5). To confirm imprinting differences between intraploidy and interploidy crosses, we performed PCR followed by sequencing analysis of two genes. The first gene, TraesCS5A02G326000 (encoding an F-box-containing protein), was classified as a MEG in intraploidy reciprocal crosses but exhibited a bi-allelic expression pattern in one reciprocal cross of the interploidy crosses. The second gene, TraesCS2A02G259600 (encoding an U3 small nucleolar RNA-associated protein 6) was predominantly paternal-biased in the hexaploid reciprocal crosses, but not in the interploidy reciprocal crosses (Figure 3D).
Collectively, the results reveal that the parental allele expression patterns are dramatically disturbed due to imbalanced parental genome dosage, resulting in increased occurrence of genomic imprinting.
Paternally expressed TaLFL2 negatively regulates starch accumulation and seed germination in interploidy crosses
To investigate whether genomic imprinting is functionally associated with endosperm development and seed germination in interploidy crosses, we focused on the paternally expressed homeolog pair TraesCS2A02G554300 and TraesCS2B02G589800, encoding a not-yet-characterized transcription factor, because their expression levels were notably higher in the paternal-excess cross (4n × 6n) than in the maternal-excess cross (6n × 4n, Dataset S1). RT-qPCR analysis revealed that the target genes were predominantly expressed in wheat endosperm, with peak expression at 7 DAP, followed by a progressive reduction during subsequent stages of development (Figure 4A). These two homeologs encode members of the B3 domain transcription factor family according to ENSEMBL Plant (https://plants.ensembl.org/index.html) are potential orthologs of the rice genes Os04g0676600 and Os04g0676650, both named LATE-FLOWERING2 (OsLFL2). Accordingly, we named TraesCS2A02G554300 and TraesCS2B02G589800 as TaLFL2-A and TaLFL2-B, respectively. We confirmed the imprinting patterns of TaLFL2-A and TaLFL2-B by PCR followed by sequencing (Figure 4B).
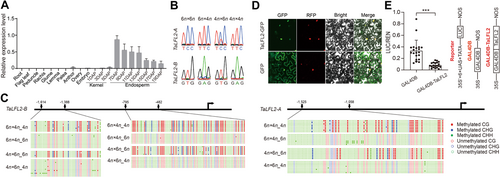
The paternally imprinted pattern of TaFLF2 is attributed to allele-specific DNA methylation
(A) Reverse transcription – quantitative polymerase chain reaction (RT-qPCR) analysis of TaFLF2 expression in different tissues. Data are reported as the average from three biological replicates and three technical replicates for each biological replication. (B) Validation of the imprinting patterns of TaFLF2-A and TaFLF2-B using PCR followed by sequencing. (C) Results from bisulfite sequencing analysis of TaFLF2-A and TaFLF2-B parental alleles. Red dots: CG methylation; blue dots: CHG methylation; green dots: CHH methylation; red circle: unmethylated CG; blue circle: unmethylated CHG; green circle: unmethylated CHH. (D) Subcellular localization analysis of TaLFL2 protein. Squamosa promoter binding protein-like 14 – red fluorescent protein (SPL14-RFP) was used as nucleus marker. The figure is a representative example of three replicates. (E) Transcriptional repression assay of TaLFL2. 35S: 35S promoter without the TATA box; 6×UAS: six copies of the GAL4 binding site (UAS); NOS: terminator of the nopaline synthase gene; GAL4DB: GAL4 DNA-binding domain; GAL4DB-TaLFL2: GAL4DB fused with TaLFL2. The triple asterisks indicate significant difference (***P<0.001).
Imprinting is often associated with cytosine methylation (Batista and Köhler, 2020). Hence, we performed bisulfite sequencing PCR analysis to analyze the cytosine methylation status of TaLFL2. CG and CHG methylation occurred extensively in the promoter regions of the paternal but not the maternal alleles of both homeologs (Figure 4C). When we transiently expressed TaLFL2 fused with green fluorescent protein (GFP) in Nicotiana benthamiana, the fusion protein localized in the nucleus (Figure 4D). Using a transient reporter/effector system, we found that, like OsLFL1, TaLFL2 has the ability to repress transcription (Figure 4E). Finally, we generated Talfl2 knockout wheat mutants in the hexaploid CB037 background (the same genotype used for interploidy hybridization) via CRISPR (clustered regularly interspaced short palindromic repeats) (CRISPR)/CRISPR-associated protein 9 (Cas9) gene editing. To simultaneously knock out all three TaLFL2 homeologs, we designed two reversely connected single guide RNAs (sgRNAs) targeting a conserved region within the first exon of this gene (Figure 5A). We obtained two independent mutant lines with frame-shift mutations leading to premature termination of all three homeologs, which we named 6n7-2 and 6n5-3 (Figure 5A). We found that mutation of TaLFL2 did not cause obvious defects in early endosperm development, like at syncytial and cellularization stages, with seeds of WT and two knockout lines all at syncytial stage at 2 DAP, and accomplished cellularization by 4 DAP (Figure S5A). In addition, knockout of TaLFL2 does not lead to significant variation of germination percentage between each other, although it results in reduced grain length and 1,000 kernel weight compared with WT (Figure S5B, C).
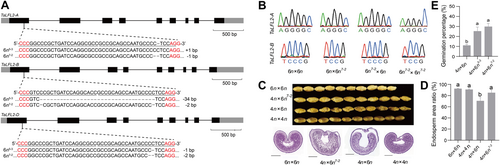
Knock out of paternal allele of TaLFL2 contributes to nutrient accumulation in interploidy crosses
(A) Schematic diagram of the strategy used to generate Talfl2 knockout mutants. The sequences of the two single guide RNAs (sgRNAs) targeting a region in the first exon conserved in the three TaLFL2 homeologs is shown. The protospacer-adjacent motif (PAM) sequence is highlighted in red. Sequencing results from the two mutant lines 6n5-3 and 6n7-2 are illustrated to indicate base insertion (+) and deletion (–) with respect to the wild-type sequences of the three TaLFL2 homeologs. (B) Validation of the imprinted patterns of TaLFL2-A and TaLFL2-B in the endosperm of the reciprocal cross between 6n and 6n7-2. The experiments were performed using reverse transcription – polymerase chain reaction followed by sequencing. Polymorphisms between 6n and 6n7-2 are shown in panel (A). (C) Phenotypic analysis of mature seeds and 15-d after pollination (DAP) endosperms from the crosses 6n × 6n, 4n × 6n, 4n × 6n7-2, and 6n × 6n. Periodic acid-Schiff staining was performed on cross-sections of 15-DAP endosperm. 6n: CB037; 4n: JIN8; scale bar: 1 mm. The figure is a representative example of three replicates. (D) Analysis of endosperm area ratios of 6n × 6n, 4n × 6n, 4n × 6n7-2, and 4n × 4n. Lowercase letters indicates statistical significance (P < 0.05) according to one-way analysis of variance (ANOVA). (E) Analysis of seed germination percentages of 4n × 6n, 4n × 6n5-3, and 4n × 6n7-2. Lowercase letters indicate statistical significance (P < 0.05) according to one-way ANOVA.
We used the two knockout mutants for reciprocal interploidy crosses with tetraploid wheat JIN8. The edited transcripts still exhibited paternally imprinted expression patterns in the reciprocal interploidy crosses (Figure 5B). Seeds from the 4n × 6n7-2 and 4n × 6n5-3 crosses appeared less shriveled than seeds from the 4n × 6n cross (Figures 5C, S6). Histological analysis of developing seeds revealed increased starch levels in the endosperm of the 4n × 6n7-2 compared with the 4n × 6n cross. Furthermore, TaLFL2 knockout resulted in plumper endosperm (Figure 5D, 85.19% vs. 70.66%) and an increase in germination percentage (from 11.08% in the 4n × 6n cross to 28.61% in 4n × 6n7-2 and 25.34% in the 4n × 6n5-3 cross; Figure 5E).
Taken together, these results indicate that the paternally imprinted gene TaLFL2 plays an important role in repressing starch accumulation and seed germination following interploidy crosses, suggesting that deregulated genomic imprinting of TaLFL2 contributes to the deleterious seed phenotypes observed in interploidy crosses with paternal-excess.
TaLFL2 negatively regulates TaNAC019 expression in the paternal-excess cross
To explore the molecular mechanisms underlying the role of TaLFL2 in interploidy crosses, we performed RNA-seq using total RNA extracted from 10-DAP endosperm of plants derived from the 4n × 6n7-2 and 4n × 6n crosses. After read mapping and data processing, we calculated the relative expression abundance of uniquely aligned reads. We identified 1,744 differentially expressed genes, including 792 upregulated genes and 952 downregulated genes, in the 4n × 6n7-2 cross compared with the 4n × 6n cross (fold change >2, FDR-adjusted P-value < 0.05; Dataset S6). GO enrichment analysis showed that the upregulated genes were significantly enriched for the term “nutrient reservoir activity” (GO:0045735, FDR = 4.4 × 10−69, Dataset S2). This finding is consistent with the enhanced nutrient accumulation in 4n × 6n7-2 compared with 4n × 6n endosperm.
Since TaLFL2 displayed transcriptional repression activity (Figure 4E), we reasoned that its direct targets (i.e., genes that are bound by TaLFL2, leading to transcriptional repression) were likely to be among the 792 genes that were upregulated in 4n × 6n7-2. The rice TaLFL2 homolog OsLFL1 binds to the RY cis-element (CATGCATG) in the promoters of its target genes (Peng et al., 2007). Among the 792 4n × 6n7-2 upregulated genes, 113 genes contained the RY cis-element in the region 2 kb upstream of the ATG starting codon. GO analysis indicated that these genes were also enriched for the term “nutrient reservoir activity” (GO:0045735, FDR = 6.59 × 10−15, Dataset S2). RT-qPCR analysis showed that the TaNAC019 homeologs were upregulated in the 4n × 6n7-2 compared with the 4n × 6n cross (Figure 6A), whereas in intraploidy crosses, the A homeolog of TaNAC019 showed similar expression levels among 6n × 6n, 6n × 6n7-2, and 6n7-2 × 6n7-2, and the expression level of B homeolog was also comparable between 6n × 6n and 6n7-2 × 6n7-2 (Figure S7). These results indicated that TaLFL2 might play different roles in the regulation of seed development between interploidy and intraploidy crosses. TaNAC019 concomitantly activates starch and seed storage protein accumulation during endosperm development (Gao et al., 2021), thus representing a key regulator of nutrient accumulation in endosperm. We confirmed the in vitro binding of TaLFL2 to the RY motif in the TaNAC019 promoter by electrophoretic mobility shift assays (EMSAs) (Figure 6B). In addition, yeast one-hybrid (Y1H) assay further confirmed the observation (Figure 6C). Finally, transient assays showed that TaLFL2 represses the transcription of TaNAC019 (Figure 6D).
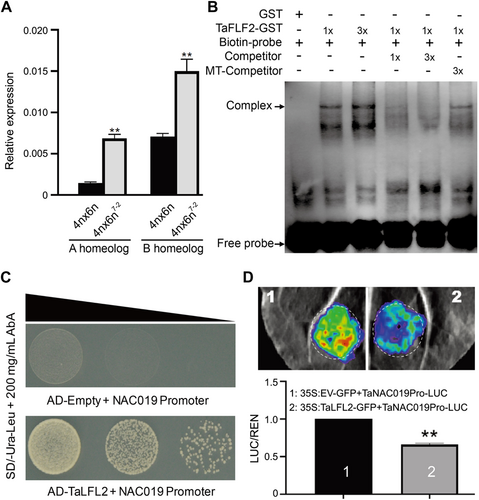
TaLFL2 binds to the TaNAC019 promoter and represses its transcription
(A) Reverse transcription – quantitative polymerase chain reaction analysis of TaNAC019 expression in wild-type (4n × 6n) and Talfl2 (4n × 6n7-2). Data are reported as the average from three biological replicates and three technical replicates for each biological replication. Double asterisks indicate statistically significant differences estimated by Student's t-test at P < 0.01. (B) Results of electrophoretic mobility shift assays. TaLFL2 binds to the CATGCA motif within the TaNAC019 promoter. The symbols “+” and “−” indicate the presence and absence, respectively, of the corresponding probes, proteins, or competitors. “1×” and “3×” TaFLF2-GST (glutathione S-transferase) indicate the protein levels used in the reaction. “1×” and “3×” competitor indicate the molar ratios of wild-type and mutated (MT) competitor sequences with respect to biotin-labeled probe. (C) Yeast one-hybrid (Y1H) analysis indicates that TaLFL2 binds to the promoter of TaNAC019. Co-transformed cells were grown on synthetic defined medium containing 200 mg/mL Aureobasidin A but lacking two amino acids (−Ura/−Leu). (D) Transcriptional repression assay indicates that TaLFL2 represses gene transcription driven by the TaNAC019 promoter. On the y-axis, LUC/REN indicates the ratio of the signal detected for firefly luciferase (LUC) vs. Renilla reniformis luciferase (REN) activity. **P < 0.01.
These results indicate that TaLFL2 binds to the 5ʹ-CATGCATG-3ʹ motif to repress TaNAC019 expression, thus impairing the TaNAC019-mediated accumulation of starch and storage proteins in developing endosperm.
DISCUSSION
Pentaploid wheat hybrids are useful resources for wheat breeding programs
Polyploidy is common in plants and is a driving force which can integrate beneficial traits to enable plant adaptation and colonization (Comai, 2005). Common wheat, a typical hexaploid species, arose from a hybridization event between a maternal tetraploid (Triticum turgidum) and a paternal diploid species (Aegilops tauschii). The polyploidization event that generated hexaploid wheat led to a genetic bottleneck. However, comprehensive genomic introgression occurred from tetraploid into hexaploid wheat during their history of co-evolution, which diversified the genomic composition, compensated for the genetic bottleneck, and promoted the global expansion of hexaploid wheat (Zhou et al., 2020). Interestingly, hexaploid wheat is often recognized as the maternal parent during the process of interploidy introgression, whereas the reciprocal cross evidently occurs at low frequency, as the genetic diversity of the cytoplasmic genome is significantly lower in hexaploid than in tetraploid wheat (average diversity index value: 0.04 vs. 0.57) (Ishii et al., 2001). Theoretically, interploidy reciprocal hybridizations are expected to occur at equal frequencies in natural environments, resulting in similar levels of genetic diversity of the cytoplasmic genome between tetraploid and hexaploid wheat. Thus, the observed bias indicates that primarily maternal-excess interploidy crosses occurred during the introgression process because, if the opposite were true, multiple groups of tetraploid wheat would have contributed to the increased genetic diversity of hexaploid wheat through introgression events. Therefore, maternal-excess crosses were the primary successful form of hybridization during wheat evolutionary history.
Pentaploid hybrid wheat, derived from interploidy crosses between hexaploids and tetraploids, has great potential for improving agronomic traits in wheat breeding programs, such as grain quality and abiotic and biotic stress tolerance (Martin et al., 2011; Martin et al., 2013; Han et al., 2014; Kalous et al., 2015; Han et al., 2016). Experienced breeders often perform interploidy crosses using hexaploid wheat as the maternal parent to obtain fertile F1 progeny, because the reciprocal cross usually leads to poor seed set and low rates of seed germination (Kihara, 1982; Sharma and Gill, 1983). Although maternal-excess interploidy crosses between hexaploid and tetraploid wheat frequently occurred during wheat evolutionary history and are commonly employed in wheat breeding programs, the underlying molecular mechanisms have not yet been elucidated.
Endosperm development and genomic imprinting are deregulated in wheat interploidy crosses
Dysfunctional endosperm has been recognized as a major cause of seed lethality in plant interploidy and/or interspecific crosses (Gehring and Satyaki, 2017). Our current results indicate that wheat interploidy crosses lead to abnormal endosperm development with either delayed or precocious cellularization, depending on whether excess genomes are inherited from the male or female parent. The timing and localization of starch accumulation, as well as the composition of starch granules and the misregulation of genes involved in starch and nutrient metabolism, also depend on the direction of the interploidy crosses. In our wheat reciprocal interploidy crosses, maternal-excess cross and paternal-excess cross show different starch granule numbers, especially for the B type starch granule (Figure 1). While the basis of this phenomenon has been a matter of debate, the kinship theory proposes a conflicting relationship between the two parental genomes: maternal alleles tend to restrict the growth of offspring by optimizing nutrient allocation to all progeny, whereas the paternal alleles tend to increase the size of the offspring (Haig and Westoby, 1989; Wilkins and Haig, 2003). In agreement with findings from interploidy and interspecific crosses in other plant species (Erilova et al., 2009; Jullien and Berger, 2010; Li and Dickinson, 2010; Tiwari et al., 2010; Zhang et al., 2016; Kirkbride et al., 2015; Erdmann et al., 2017; Huang et al., 2017; Tonosaki et al., 2018), we observed that interploidy crosses deregulated genomic imprinting in 15-DAP wheat endosperm. It is possible that a greater number of expressed genes with disturbed imprinting patterns will be found if we perform the examination with different developmental stages. This deregulation of genomic imprinting in the paternal-excess crosses may disrupt endosperm development and storage product accumulation either directly or indirectly. By contrast, nutrient uptake and allocation in the endosperms of the maternal-excess cross were not seriously affected, allowing more fertile seeds to be produced.
Although the developmental effects and underlying mechanisms of wheat interploidy crosses resemble those reported for other plant species, the current study uncovered some wheat-specific features. These are mainly associated with the effects of interploidy crosses on seed size and more importantly, seed germination percentage. In wheat, the paternal-excess cross (4n × 6n) generated large but shriveled seeds, while the maternal-excess cross (6n × 4n) produced small but plump seeds. Correspondingly, the seed germination percentage depended on the direction of the interploidy cross (50.62% in 6n × 4n vs. 11.08% in 4n × 6n).
By contrast, in maize, more than 99% of kernels were highly defective in a maternal-excess cross (4n × 2n), and ~9.7% plump kernels with normal phenotypes were observed in a paternal-excess cross (2n × 4n; Leblanc et al., 2002). In addition, both reciprocal interploidy crosses exhibited low germination percentages, that is, 1.7% in 2n × 4n compared with 0.83% in 4n × 2n crosses (Leblanc et al., 2002). In rice, similar to what we observed in wheat, an interploidy cross with paternal-excess strongly affects seed starch accumulation and gives rise to progeny with a zero germination percentage; however, unlike in wheat, both paternal- and maternal-excess interploidy crosses generate smaller kernels (Sekine, 2013; Zhang et al., 2016). Finally, in Arabidopsis, a paternal-excess cross generates heavier seeds compared with a maternal-excess cross and a balanced cross, and the seed germination percentages (>90%) for both reciprocal crosses are high (Scott et al., 1998).
Some of the differences observed in wheat compared with maize and rice interploidy crosses may be related to wheat's polyploidy status. During its evolution, wheat experienced two rounds of hybridization and polyploidization, which may have partially improved its capability to endure genomic imbalance, thus resulting in a less dramatic effect on the germination percentage compared with these other plants. Unlike wheat, Arabidopsis is a dicot, with endosperm that disappears during seed development and an embryo constituting a larger portion of the mature seed. Therefore, the effects of interploidy crosses on endosperm development may have a minimal effect on seed germination in this plant. Another possible reason for the specific effects of interploidy crosses on wheat is that both the hexaploid and tetraploid wheat cultivars used in this study are allopolyploids, whereas autopolyploids were used in similar studies performed with other plant species. In any case, it is evident that, even if general mechanisms such as changes in genomic imprinting and misregulated gene expression are conserved, interploidy crosses have specific effects on different plant species that are likely to reflect the presence of distinctive underlying mechanisms. Therefore, to improve the usage of interploidy crosses in breeding programs, it is essential to study the detailed mechanisms in each species, especially crops.
Paternally expressed imprinted gene TaLFL2 is involved in the regulation of endosperm development in paternal-excess interploidy crosses
In this context, we revealed that parental allele expression patterns were dramatically disturbed in interploidy crosses, leading to the increased occurrence of gene imprinting compared with intraploidy crosses. Of these genes, we found that allele-specific DNA methylation contributes to the paternally biased expression of the endosperm-specific gene TaLFL2. Like many imprinted genes identified in other plant species (Satyaki and Gehring, 2017), TaLFL2 exhibits a different cytosine methylation status between the paternal and maternal alleles, with a higher methylation level in the paternal allele. However, hypermethylated paternal alleles of TaLFL2 are expressed for both A and B homeologs, whereas hypomethylated maternal alleles are silenced. Hence, the cytosine methylation status shows the opposite correlation with gene expression compared with that usually reported for imprinted genes, whereby higher methylation is associated with lower expression (Niederhuth et al., 2016; Hornslien et al., 2019). It is possible that other epigenomic marks involved in modulating chromatin structure are involved in the uniparental allele expression of TaLFL2 (Batista and Köhler, 2020). A similar scenario has been proposed to explain the finding, strongly resembling that reported in the current study, that cytosine methylation is necessary for the paternal-allele-specific expression of PHE1 in Arabidopsis (Makarevich et al., 2008).
TaLFL2 had a partial but significant effect on establishing the defective seed phenotypes observed in paternal-excess interploidy crosses in the current study. Although knockout of Talfl2 results in decreased 1,000 kernel weight in hexaploid wheat (Figure S5), interploidy crosses using hexaploid Talfl2 mutants as the paternal parent resulted in improved nutrient accumulation and seed germination percentage compared with crosses using WT hexaploid plants as the paternal parent. Loss of function of the paternal allele of TaLFL2 de-repressed endosperm genes enriched in “nutrient reservoir activity” in the paternal-excess cross, pointing to the involvement of TaLFL2 in the repression of starch and nutrient metabolism in interploidy crosses. Specifically, TaLFL2 mutation de-represses the expression of TaNAC019 in the paternal-excess cross, but not in hexaploid intraploidy crosses especially for the A homeolog of TaNAC019 (Figure S7), which encodes a NAC family transcription factor that was recently shown to concomitantly activate starch and storage protein accumulation during endosperm development (Gao et al., 2021). Collectively, we propose that paternally imprinted TaLFL2 contributes to paternal-excess interploidy hybridization barriers in wheat.
Although additional investigation is still required to shed light on the molecular mechanisms involved in the predominant occurrence and high utilization efficiency of maternal-excess crosses during the history of hexaploid and tetraploid wheat introgression and in breeding programs, respectively, we now provide the first experimental evidence that endosperm cellularization is delayed and starch accumulation repressed, resulting in a decreased seed germination percentage, in paternal-excess versus maternal-excess crosses. Importantly, we also identified a previously uncharacterized paternally expressed transcription factor gene, TaLFL2, and uncovered the role of the TaLFL2 protein in negatively regulating starch accumulation in an interploidy cross with paternal-excess. Collectively, these results reveal an important molecular mechanism that contributes to the predominant occurrence of paternal-excess crosses during introgression events between hexaploid and tetraploid wheat. In addition, they provide genomic targets that could be used to improve the efficiency of paternal-excess crosses in wheat breeding programs by screening for natural genetic variations in germplasm collections and/or using biotechnological approaches.
MATERIALS AND METHODS
Plant materials
Seeds of hexaploid wheat (T. aestivum, AABBDD, 6n) cultivar CB037, tetraploid wheat (T. durum, AABB, 4n) cultivar JIN8, and CB037 knockout mutants were sown in the Shangzhuang experimental field or greenhouse at China Agricultural University, Beijing, China. The reciprocal interploidy crosses were made by pollinating the tetraploid pistil (JIN8) with hexaploid pollen (CB037, 4n × 6n) or the hexaploid pistil (CB037) with tetraploid pollen (JIN8, 6n × 4n). As a control, tetraploid (4n × 4n) and hexaploid (6n × 6n) plants were also manually pollinated. Similarly, the crosses of Talfl2 knockout mutant (CB037 background, designed as 6n7-2 and 6n5-3) and JIN8, including 4n × 6n7-2 and 4n × 6n5-3, were conducted. We conducted the crosses as follows: the base and top spikelets of the spike, as well as central florets were removed before anthesis, and the tops of the florets were cut off and bagged. Pollination was then performed 1–2 d later using the corresponding pollen. Endosperm tissues of 4n × 4n, 4n × 6n, 6n × 4n and 6n × 6n at different developmental stages (3 DAP, 5 DAP, 7 DAP, 10 DAP and 15 DAP) as well as endosperms of 4n × 6n7-2 at 15 DAP were collected from at least nine different ears to achieve a total of three biological replicates. The endosperm tissues were manually isolated and immediately frozen in liquid nitrogen.
Fluorescence in situ hybridization
The chromosomes were analyzed by FISH as previously described (Tang et al., 2014). Oligonucleotide probes Oligo-pTa535 and Oligo-pSc119.2 were used for FISH analysis and were synthesized by Tsingke Biological Technology Co. Ltd. (Beijing, China). Oligo-pTa535 and Oligo-pSc119.2 probes were 5ʹ-end-labeled with 6-carboxytetramethylrhodamine (TAMRA) and 6-carboxyfluorescein (6-FAM), respectively. Root tips from primary roots were placed in N2O for 1.5–2 h and fixed in fixative fluid (90% acetic acid) on ice for 5–10 min. The root tips were washed three times in deionized water. The meristematic portions of the root tips were cut on a glass slide, placed into a 1.5-mL microcentrifuge tube containing enzyme solution (2% cellulase and 1% pectolyase), and incubated at 37°C for ~1 h. The root tips were washed twice with 70% ethanol, the ethanol was discarded, and the root tips were tapped with a dissecting needle until the tissue was almost invisible in the spreads. The root tissue was centrifuged at 3,000 × g for 2 min and resuspended in 30 μL 90% acetic acid. A 7–9 μL aliquot of the slurry was dropped onto a glass slide and allowed to air dry. The spreads were examined under a phase contrast microscope, and the best ones were selected for ultraviolet crosslinking followed by FISH.
Synthesized oligonucleotide probes were diluted with 1× TE (Tris-ethylenediaminetetraacetic acid (EDTA)) solution (pH 7.0). A probe mixture containing appropriate probe, 2× SSC (saline-sodium citrate), and 1× TE buffer (pH 7.0, total volume = 10 µL) was dropped onto the center of the chromosome spreads and covered with a glass coverslip. The slides were heated at 80°C for 3 min and immediately stored in a moist box at 37°C for 1 h. The slides were washed for 15 s in 2× SSC at 37°C, and the samples were incubated with 20 μL of 4,6-diamidino-2-phenylindole (DAPI: 1 μg/mL) for 10 min at room temperature in the dark. Images were acquired using an epifluorescence microscope (BX51, Olympus) equipped with a cooled charge-coupled device camera operated with HCIMAGE Live software (version 2.0.1.5, Hamamatsu Corporation, Sewickley, NJ, USA). A minimum of three replicates per sample were analyzed.
Histological analysis of seed development and starch accumulation
Seeds from reciprocal crosses and self-pollinated plants of tetraploid (JIN8) and hexaploid (CB037), including 4n × 4n, 6n × 6n, 4n × 6n, and 6n × 4n, were harvested at 3, 10, and 15 DAP and fixed in FAA solution (5% v/v formaldehyde, 5% v/v acetic acid, 50% v/v ethanol). After fixation, the samples were dehydrated through an ethanol series and embedded in liquid paraffin. Sections were cut using an RM2016 microtome (Leica, http://www.leica-microsystems.com). Safranin O-fast green staining was used to visualize seed development status. Briefly, the sections were deparaffinized and rehydrated in distilled water, immersed in Safranin O solution for 1–2 h, rinsed in water to remove excess dye, and dehydrated successively through an ethanol series. The slides were then immersed in fast green solution for 30–60 s, cleared in xylene for 5 min, and mounted with resin mounting medium.
The seeds from 4n × 4n, 6n × 6n, 4n × 6n, and 6n × 4n, harvested at 5, 7, 10 and 15 DAP as well as seeds from 6n × 6n, 6n5-3 × 6n5-3, and 6n7-2 × 6n7-2, harvested at 2 DAP and 4 DAP, were collected for periodic acid-Schiff staining to investigate starch granule accumulation and early seed development, respectively. The slides were deparaffinized, rehydrated, and immersed in periodic acid solution for 15 min and washed in distilled water. The slides were stained in Schiff reagent for 30 min and kept in the dark, followed by washing in distilled water for 5 min. The slides were rinsed in hematoxylin solution for 1–3 min, washed in distilled water, dipped in 1% hydrochloric acid for a few seconds, and washed in distilled water. The sections were blued in 0.2% ammonia water and washed in tap water. Finally, the samples were dehydrated by immersion in absolute alcohol three times for 5 min each, cleared in xylene, and mounted with resin mounting medium. A minimum of three replicates were analyzed for each sample.
RNA extraction and RT-qPCR
Endosperm tissue (three biological replicates at 15 DAP) was ground to a fine power in liquid nitrogen and total RNA was isolated using a TransZol Plant Kit (TransGen Biotech) according to the manufacturer's instructions. The gene ATG8d (TraesCS2A02G224000/TraesCS2B02G274200/TraesCS2D02G229900) was used as internal reference gene chosen for gene expression analysis (Mu et al., 2019). Primers are listed in Table S2.
RNA-seq analysis
The 15-DAP endosperm from 4n × 4n, 6n × 6n, 4n × 6n, and 6n × 4n as well as the 10-DAP endosperm from 4n × 6n7-2 and 4n × 6n crosses were collected for transcriptome analysis. The quality of the extracted RNA samples was detected using an Agilent 2100 Bioanalyzer. RNA-seq libraries were prepared and sequenced by Berry Genomics Co., Ltd. on the Illumina HiSeq. 2500 or NovaSeq platform. High-quality total RNA was sequenced with two or three biological replicates per sample. In total, ~288 Gb sequencing data with ~156 million reads per sample were generated (Table S1). The sequencing quality was examined by FastQC software (v0.11.5). Adaptor sequences and low-quality ends were trimmed using Trimmomatic (v0.36) (Bolger et al., 2014). Transcripts per million was used to normalize read counts and to calculate the relative gene expression levels. DESeq. 2 was employed for differential gene expression analysis (Love et al., 2014). Genes with log2 (fold change) >1 and adjusted P-value < 0.05 were considered to be differentially expressed genes.
SNP calling and imprinted gene identification
We mapped the high-quality reads to the reference sequence IWGSC RefSeq v1.1 (Appels et al., 2018) using Tophat2 (“--b2-D 20 --b2-R 3 -N 3 --read-edit-dist 3”), and only retained uniquely mapped reads with no more than three mismatches. SNP calling was conducted using the mpileup function of Samtools (v1.4) (Li et al., 2009) and the view function of BCFtools (v1.4) as previous described (Li, 2011).
RNA-seq reads from each biological replicate of reciprocal crosses were mapped to the reference genome separately using Tophat2 (v2.1.1) (Trapnell et al., 2009), and only uniquely mapped reads (<three mismatches) were considered. SNP-containing reads were distinguished according to the maternal and paternal SNPs identified in the previous step and counted using custom Perl scripts. A χ2 test was performed to determine whether the parental ratio deviated from the normal ratio of 2m:1p in each cross. Genes with a ratio deviating from 2m:1p (χ2 goodness-of-fit test, FDR-adjusted P-value < 0.05) and ≥90% maternally or ≥70% paternally derived SNP-containing reads in both biological replicates of the two reciprocal crosses (≥10 SNP-associated reads per cross) were considered as imprinted genes.
Identification of differentially expressed genes and GO enrichment analysis
The number of reads aligning to each gene was calculated using featureCounts (Liao et al., 2014) version 1.5.2 (“--readExtension5 70 --readExtension3 70 –p –C –s 0”). Differential expression analysis was performed using edgeR (Robinson et al., 2010) with an FDR-adjusted P-value < 0.05. GO enrichment analysis was performed using the GOEnrichment program in the Triticeae-GeneTribe software interface (http://wheat.cau.edu.cn/TGT/; Chen et al., 2020).
Validation of imprinted genes by Sanger sequencing
Total RNA from reciprocally crossed and self-pollinated endosperms was used for complementary DNA (cDNA) synthesis using HiScript II Q RT SuperMix for qPCR (+gDNA wiper) (Vazyme Biotech Co., Ltd) according to the manufacturer's instructions. The PCR products were visualized on agarose gel and then submitted to direct sequencing. Primers are listed in Table S2.
Subcellular localization analysis
The full-length open reading frame (ORF) of TaLFL2 was cloned into the pCAMBIA1300-GFP vector to generate the fusion protein driven by MAS gene promoter. The fusion construct pMAS::TaLFL2-GFP and the pMAS::GFP were separately introduced into appropriate leaves of N. benthamiana by Agrobacterium tumefaciens -mediated transient transformation (Liu et al., 2010). pActin::OsSPL14-RFP was co-transformed as a nuclear marker. Transformed plants were grown at 22°C for 48 h, and GFP signal was detected under a confocal microscope (Eclipse TE2000; Nikon, https://www.nikon. com).
Transcriptional repression assay
For transcriptional repression analysis, full-length TaLFL2 cDNA was cloned in frame with the GAL4 DNA-binding domain into the pHB vector (35S::GAL4DB-TaLFL2) (Gao et al., 2021), which expresses the fusion protein driven by the 35S promoter (35S::GAL4DB-TaLFL2 effector construct). The 35S promoter plus a six-fold repeat of an upstream activating sequence (6×UAS) was cloned into the pGreenII 0800-LUC vector to produce the reporter construct (35S-6×UAS::LUC). The 35S::GAL4DB-TaLFL2 and the 35S-6×UAS::LUC constructs were co-transformed into young leaves of N. benthamiana plants via Agrobacterium (strain GV3101)-mediated transient transformation. Co-transformation with the empty 35S::GAL4DB vector and 35S-6×UAS::LUC was used as a control. The transformed plants were grown at room temperature for 48 h, and signal quantification was performed using the Dual-Luciferase Reporter Assay system (Promega). Data were reported as the average of three independent replications.
To examine TaLFL2-mediated repression of the TaNAC019 promoter, the 2-kb sequence upstream of the TaNAC019 start codon was cloned into the pGWB35 LUC vector to generated the reporter plasmid (Nakagawa et al., 2007). To generate the effector construct, the ORF of TaLFL2 was cloned into the pCAMBIA1300 vector driven by pMAS. The two constructs were co-transformed into N. benthamiana leaf epidermal cells via Agrobacterium strain GV3101-mediated transient transformation (Liu et al., 2010). Firefly luciferase activity driven by the TaNAC019 promoter and 35S::REN were quantified using the Dual-Luciferase Reporter Assay system (Promega) with a Synergy 2 Multi-Detection Microplate Reader (BioTek Instruments). Normalized data are presented as the ratio of luminescent signal intensity for the reporter versus internal control reporter (35S:REN) from three independent biological samples.
DNA extraction and bisulfite sequencing
Genomic DNA was extracted from 15-DAP endosperm using a Qiagen DNeasy Plant Mini Kit (Qiagen) according to the manufacturer's instructions. Approximately 500 ng genomic DNA was used for bisulfite conversion using an EZ DNA Methylation-Lightning Kit (Zymo Research,) according to the manufacturer's instructions. Primers flanking the SNP sites were designed using the MethPrimer 2.0 (Li and Dahiya, 2002) and are listed in Table S2. Bisulfite-treated DNA was used to amplify the targeted sequences by PCR in a 10 μL reaction using TaKaRa EpiTaq™ HS (Takara, https://www.takarabio.com/). The PCR products were cloned into the pTOPO-T Simple vector (Aidlab Biotech, http://www.aidlab.cn/) and sequenced. DNA methylation was analyzed using the Kismeth program (Gruntman et al., 2008).
Generation of TaLFL2 knockout mutants
To obtain Talfl2 knockout mutants, CRISPR/Cas9-mediated genome editing was performed in the hexaploid wheat cultivar “CB037” background. Two reversely connected sgRNAs targeting the first exon of the three TaLFL2 homeologs were designed using the E-CRISP Design website as previously described (Xing et al., 2014). The plasmids were transformed into wheat cultivar CB037 via Agrobacterium-mediated transformation (Ishida et al., 2015). DNA samples were isolated from individual plants of the T2 generation, and the site-specific mutations were detected by PCR followed by sequencing. At least three clones were sequenced for each sample. The primers used for sequencing are listed in Table S2.
EMSA
The full-length protein coding sequence of TaLFL2 was amplified and cloned into the pGEX-6P-1 vector (ClonExpress Ⅱ One Step Cloning Kit, Vazyme, China). Glutathione S-transferase (GST)-tagged TaLFL2 was expressed in Escherichia coli BL21 (DE3) cells and purified using ProteinIso® GST Resin (TransGen, Beijing). The cells were incubated at 37°C and shaken at 200 rpm until the optical density at 600 nm (OD600) reached 0.5. Following the addition of 0.3 mmol/L isopropyl-β-D-thiogalactopyranoside (IPTG), the cells were shaken for 12 h at 16°C and harvested by centrifugation at 4°C. The cells were lysed by sonication on ice in lysis buffer (137 mmol/L NaCl, 2.7 mmol/L KCl, 10 mmol/L Na2HPO4, 2 mmol/L KH2PO4, 1 mmol/L phenylmethylsufonyl fluoride (PMSF), and 1/2 tablet of protease inhibitor cocktail, Roche, Germany). Following centrifugation at 13,000 g for 30 min at 4°C, the supernatant was incubated with glutathione agarose resin for 1 h. The fusion proteins were eluted from the GST resin by incubation at 4°C for 1 h with 50 mmol/L Tris-HCl (pH 8.0) supplemented with 10 mmol/L reduced glutathione. EMSAs were performed as previously described using a Light Shift Chemiluminescent EMSA Kit (Thermo Fisher Scientific, Guo et al., 2018).
The primers used for vector construction are listed in Table S2.
Yeast-one-hybrid assay
The 150-bp TaNAC019 promoter sequence with CATGCA motif (from 1,678 to 1,529 upstream of the start codon) was inserted into the pAbAi vector at the KpnI and XhoI sites. The full-length cDNA of TaLFL2 was cloned into pGADT7-AD at the BamHI and EcoRI sites. The vectors were transformed into yeast strain Y1HGold (Clontech, USA), and grown on synthetic defined medium lacking uracil and leucine containing 200 mg/mL Aureobasidin A (AbA) for 3 d at 30°C. All primers used for vector construction are listed in Table S2.
Accession numbers
Sequence data from this article can be found in the GenBank data libraries under accession number PRJNA758178.
ACKNOWLEDGEMENTS
We thank Zongxiang Tang (Sichuan Agricultural University, China) for helping with the FISH analysis. This work was supported by the National Natural Science of China (31471479), and the Chinese Universities Scientific Fund (2017TC035).
CONFLICTS OF INTEREST
The authors declare they have no conflict of interests.
AUTHOR CONTRIBUTIONS
M.X., Z.N., and Q.S. conceived the project; G.Y., M.F., K.Y., L.G., G.C. L.S., and Y.Z. collected plant materials; G.Y., and M.F. performed the research; G.Y., K.Y., Y.Y., Z.H., and H.P. analyzed the data; M.X., Q.S., Z.N., I.D.S., and V.R. wrote the manuscript. All authors read and approved the contents of this paper.






