The plant cell wall: Biosynthesis, construction, and functions
Edited by: Zhizhong Gong, China Agricultural University, China
Abstract
The plant cell wall is composed of multiple biopolymers, representing one of the most complex structural networks in nature. Hundreds of genes are involved in building such a natural masterpiece. However, the plant cell wall is the least understood cellular structure in plants. Due to great progress in plant functional genomics, many achievements have been made in uncovering cell wall biosynthesis, assembly, and architecture, as well as cell wall regulation and signaling. Such information has significantly advanced our understanding of the roles of the cell wall in many biological and physiological processes and has enhanced our utilization of cell wall materials. The use of cutting-edge technologies such as single-molecule imaging, nuclear magnetic resonance spectroscopy, and atomic force microscopy has provided much insight into the plant cell wall as an intricate nanoscale network, opening up unprecedented possibilities for cell wall research. In this review, we summarize the major advances made in understanding the cell wall in this era of functional genomics, including the latest findings on the biosynthesis, construction, and functions of the cell wall.
INTRODUCTION
The plant cell wall is a natural nanoscale network structure that primarily consists of polysaccharide polymers such as cellulose, hemicellulose, and pectin, but often includes glycoproteins and lignin as well. This intricate structure encases plant cells and provides them with diverse shapes, sizes, and distinct physicochemical properties that allow them to fulfill their roles in different organs and at various developmental stages (Figure 1). The plant cell wall plays many fundamental roles, including determining plant morphogenesis and architecture, providing mechanical support for the plant body, conducting water and nutrients, defending against biotic and abiotic stresses, and so on (Bacic et al., 1988; Carpita and Gibeaut, 1993; Somerville et al., 2004). In addition, the plant cell wall is the most abundant renewable resource on Earth, which has great practical value for humans, including use for animal nutrition and heat production, as a building material, and as a source of natural fibers for textile and paper production (Pauly and Keegstra, 2010). The potential of this material for conversion into biofuels to replace fossil fuels has increased its significance (Himmel et al., 2007; Ragauskas et al., 2014). Despite its importance for plant growth and its economic uses, the plant cell wall is the least understood cellular structure in plants.
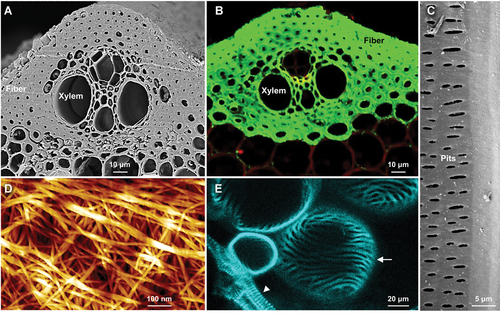
Diversity and complexity of plant cell walls at the micro and nano levels
(A) Scanning electron microscopy (SEM) image of a rice internode section showing the epidermal, xylem, and fiber cells with thickened secondary cell walls in contrast to the cortex and phloem cells. (B) Rice internode section immunolabeled with xylan-targeted LM11 antibodies, showing the uneven distribution of xylan. The signals from xylan antibodies are shown in green, and the cells were stained with propidium iodide (shown in red). (C) SEM image of a rice internode section, showing pits in the xylem cell wall (inner surface). (D) Atomic force microscopy (AFM) image showing cellulose nanofibers with multilevel assemblies at the surface of the xylem cell wall. (E) Induction of ectopic xylem cells with spiral secondary cell wall bands in Arabidopsis cotyledon epidermal cells observed using the VISUAL approach. The arrowhead and arrow indicate native and ectopic xylem cells, respectively. Bars = 10 μm in (A) and (B), 5 μm in (C), 100 nm in (D), 20 μm in (E).
The major barriers to the study of the plant cell wall come from its heterogeneous chemistry and structure. Plants harbor more than 40 types of cells (Farrokhi et al., 2006); each cell type possesses distinct and dynamic cell wall compositions and organization, resulting in cell type-specific cell wall construction (Burton et al., 2010; Loqué et al., 2015). This structural diversity is due to the diverse, multi-step biosynthetic pathways involved, which begin in an intracellular compartment and end in the wall itself. Although it has been estimated that among the ~27, 000 Arabidopsis genes, approximately 15% are dedicated to cell wall biogenesis and modification (Carpita et al., 2001), few such genes were identified before the era of functional genomics. Molecular and genetic studies have identified many genes related to cellulose synthesis and assembly (Arioli et al., 1998; Taylor et al., 2003; Liu et al., 2013; Lei et al., 2015), matrix polysaccharide production (Madson et al., 2003; Burton et al., 2006; Brown et al., 2007; Persson et al., 2007), lignin metabolism and polymerization (Jones et al., 2001; Lu et al., 2013), and wall polymer modification and deposition (Jiang et al., 2005; Gille and Pauly, 2012; Grantham et al., 2017; Zhang et al., 2018c; Kang et al., 2019), offering an excellent opportunity to reveal how the cell wall is organized. Furthermore, the use of cutting-edge technologies, such as nuclear magnetic resonance (NMR), single-molecule plus super-resolution imaging techniques, and atomic force microscopy (AFM) has advanced our understanding of the cell wall as a sophisticated nanoscale network (Figure 1D), launching a new era for the study of cell wall construction and function. In this review, we summarize recent advances in elucidating cell wall biosynthesis and organization, providing an updated systematic understanding of this fundamental plant structure.
CELL WALL COMPOSITION AND BIOSYNTHESIS
Cell wall composition is one of the most important issues affecting cell wall structure and function. As plants adapted to terrestrial habitats, cell wall composition evolved and diversified, ultimately resulting in diverse chemical constituents in different plant species and generating intrinsic heterogeneity in terms of chemistry and structure (Somerville et al., 2004; Burton et al., 2010). Identifying the proteins that are required for biosynthesis of major cell wall components, including cellulose, hemicellulose, pectin, and lignin, is an essential step in uncovering plant cell wall structure and function. The enzymes required for nucleotide sugar interconversions and Golgi transporters represent other aspects of cell wall biosynthesis. As several reviews have detailed the generation and supply of nucleotide sugar substrates (Bar-Peled and O'Neill, 2011; Temple et al., 2016), this topic will not be discussed here.
Cellulose: multiscale linear fibrils assembled from glucan
The possession of cellulose-rich cell walls is an important characteristic of terrestrial vascular plants. Cellulose, consisting of unbranched β-(1,4)-linked glucan chains, is the main polymer in most plant cell walls. Although the chemical nature of cellulose is very simple, its assembly appears complex due to the presence of multiscale cellulosic fibrils. Crystalline cellulose elemental fibers are formed from glucan chains, which are then bundled into nanofibers and aggregate into cord-like structures of various diameters, as revealed by high-resolution electron microscopy and AFM (Ding et al., 2012; Zhang et al., 2019). These multiscale cellulosic fibrils constitute a fibrillar network (Figure 1D), resulting in wall nanostructure. Therefore, cellulose is a load-bearing polymer of the plant cell wall. Investigating how multiscale cellulosic fibrils are organized is an important topic, but it is also quite challenging.
The structural inhomogeneity of cellulose reflects the complexity of its biosynthesis, which requires multiple proteins. One of these proteins is cellulose synthase, which is designated as CESA (Delmer 1999): CES (for cellulose synthase) followed by A (presumed catalytic subunit to indicate a homolog of bacterial catalytic subunits). CESA directly catalyzes glucan chain elongation at the plasma membrane (PM). Genetic studies revealed that Arabidopsis cellulose synthase complex (CSC) contains three CESA proteins (CESA1, CESA3, and either CESA2, CESA5, CESA6, or CESA9). The complex forms via protein-protein interactions to produce cellulose in the primary cell wall (PCW) (Arioli et al., 1998; Scheible et al., 2001). CSC consisting of CESA4, CESA7, and CESA8 is indispensable for cellulose synthesis in the secondary cell wall (SCW) (Taylor et al., 2003). This synthetic machinery is highly conserved in plant species, even in the monocot plant rice. Defects in any of these CESAs results in brittle culms, dwarfism, and collapsed xylem tissue, accompanied by growth abnormalities (Arioli et al., 1998; Tanaka et al., 2003; Somerville et al., 2004; Zhang et al., 2009). CESA proteins in different plant species share high sequence similarity. Therefore, the identification of CESA proteins represents a breakthrough in addressing how plants make cellulose. CESAs are crucial proteins that directly catalyze cellulose elongation and have become the major focus in understanding cellulose production. Heterologously expressed poplar CesA8 catalyzes β-(1,4)-linked glucan biosynthesis in reconstituted proteoliposomes, validating the biochemical activity of CESA in vitro (Purushotham et al., 2016, 2020).
The discovery of the three-dimensional structure of plant CSCs represents another breakthrough. Freeze etching electron microscopy performed in 1980 revealed that plant CSCs exhibit a hexagonal “rosette” structure (Mueller and Brown, 1980), making CSCs one of the most complex membrane protein complexes in plants. The structure of CSCs could not be elucidated by traditional structural analysis techniques prior to the development of cryo-electron microscopy (cryo-EM). This technique was first used to characterize the structure of the bacterial cellulose synthase BcsA. The observation of native processes of bacterial cellulose biosynthesis and the synthesis of nascent glucan polymers by each subunit provided new and exciting insights (Morgan et al., 2013, 2016). These findings highlight the importance of uncovering the atomic structures of CSCs for understanding the cellulose synthesis mechanism. The structure of a homotrimeric poplar cellulose synthase (PttCESA8) complex was recently determined at angstrom (10−1 nm) resolution using cryo-EM (Purushotham et al., 2020, 2016, 2020), providing the first view of how plant CSCs are assembled and operate at this level. The newly determined cryo-EM structure reveals that PttCESA8 possesses seven transmembrane helices (Figure 2), which does not conform to the eight helices predicted previously in a hypothetical structural model. This discrepancy likely results from misinterpretation of the interfacing helix IF3 as a transmembrane helix. Moreover, the cryo-EM structure of PttCESA8 shows that the conserved cytosolic regions of this protein are pivotal for stabilizing the trimeric complex (Purushotham et al., 2020, 2016, 2020). Independent channels occupied by nascent cellulose polymers have been observed in each subunit, which is consistent with findings for bacterial cellulose synthase (Morgan et al., 2013, 2016). The nascent glucan polymers are extruded toward a common exit point, likely appearing as protofibrils. Although homotrimeric CSC is only present sparsely in plants, we expect that a cryo-EM structure of native plant CSC will be released, which should provide us with a crystallographic view of authentic plant CSCs and uncover the native processes of plant cellulose synthesis.
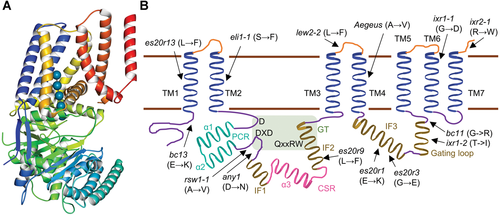
Updated structural model of plant cellulose synthases
(A) 3D structural diagram of PttCESA8. The cryo-electron microscopy structure of PttCESA8 was visualized using the online tool Molmil (PDB ID: 6wlb). (B) Updated model of the structural organization of CESA, showing the seven transmembrane domains (TM, in blue), class-specific region (CSR, in pink), plant-conserved region (PCR, in cyan), and the catalyzing domain between TM2 and TM3 (the light green rectangle) according to Purushotham et al. (2020, 2016, 2020). The arrows indicate the point mutations identified in each CESA protein domain.
Cellulose synthase complexes are thought to be assembled in the Golgi apparatus and translocated to the PM via vesicle trafficking. The identification of Stello, a glycosyltransferase-like protein localized in the Golgi apparatus, has provided proof for this hypothesis (Zhang et al., 2016). As only PM-localized CSCs can synthesize cellulose, the direction and velocity of CSC movement on the PM are crucial factors affecting the physicochemical properties of cellulose. CSC movement along cortical microtubules (CMTs) was first visualized by spinning disk confocal microscopy (Paredez et al., 2006). Since then, several proteins that facilitate CSC trafficking and movement have been characterized. Using this live cell imaging system, cellulose synthase interactors (CSI1–3) were found to mediate the tethering of CSCs to CMTs (Gu et al., 2010; Li et al., 2012b; Lei et al., 2013, 2015). The CSC-CMT association could be uncoupled by cellulose synthase microtubule uncoupling (CMU) proteins (Liu et al., 2016b), the actions of which are modulated by the kinesin protein Fragile fiber1 (FRA1) (Zhong et al., 2002; Ganguly et al., 2020). Cellulose synthase companions are proteins that connect CSCs with CMTs, especially under stress conditions (Endler et al., 2015). Brassinosteroid insensitive2 phosphorylates CESA1 and thereby regulates the bidirectional mobility of CSCs during anisotropic cell expansion (Sanchez-Rodriguez et al., 2017). With the aid of these proteins, CSCs are nicely monodispersed on the PM during PCW formation (Paredez et al., 2006) and condense and align into specific patterns with increasing velocity during SCW formation (Watanabe et al., 2015). Moreover, SCW deposition patterns during xylem vessel differentiation are dependent on a pair of microtubule depletion domain1 (MIDD1)-Kinesin-13A proteins, the interaction of which defines CMT-devoid domains (Oda and Fukuda, 2012a).
Cellulose synthesis is responsive to changes in the plant that occur under stress conditions (Lei et al., 2013; Endler et al., 2015; Watanabe et al., 2015). Under stress conditions, this process is modified by the residence/recycling of CSCs from the PM via the actions of certain small CESA-containing compartments (SmaCCs) (Crowell et al., 2009; Gutierrez et al., 2009). S-acylation, which provides the cysteine residues of Arabidopsis CESAs with acyl-epitope, is also essential for CSC trafficking, but the enzymes that catalyze this process remain unknown (Kumar et al., 2016). Moreover, although proteins that participate in different trafficking pathways for cellulose biosynthesis have been identified, such as the dynamin protein BC3 (Xiong et al., 2010), TPLATE members (Sanchez-Rodriguez et al., 2018), and the AP2 complex (Bashline et al., 2013, 2015), the secretion routes of CSCs are still not fully understood.
Other accessory proteins are also required for cellulose production. The genetic deficiency of Arabidopsis COBRA, rice Brittle Culm1, or maize Brittle Stalk2 severely reduces cellulose content (Schindelman et al., 2001; Li et al., 2003; Sindhu et al., 2007). These COB and COB-like proteins likely participate in cellulose assembly by binding to cellulose and affecting the crystallization of cellulosic microfibrils (Liu et al., 2013). Similarly, Arabidopsis chitinase-like proteins AtCTL1 and AtCTL2 (Sanchez-Rodriguez et al., 2012), rice BC15/OsCTL1 (Wu et al., 2012), and maize BK4/ZmCTL1 (Jiao et al., 2019), as well as the CSC-associated protein Korrigan, a glycosyl hydrolyase 9 (GH9) protein (Vain et al., 2014), are also involved in cellulose production, but the underlying mechanisms remain elusive. Our knowledge of cellulose biosynthesis is still rudimentary. Due to its importance, cellulose biosynthesis has been described in several reviews (McFarlane et al., 2014; McNamara et al., 2015; Polko and Kieber, 2019).
Hemicellulose: β-glycan backbones with diverse substitutions
Most cell wall polymers obtained by alkaline extraction are hemicellulosic polysaccharides, whereas pectic polymers are easily extracted using hot acid or chelators (Scheller and Ulvskov, 2010). Hemicellulosic polysaccharides, including xylans, xyloglucans, mannans, glucomannans, and β-(1,3;1,4)-glucan, all harbor β-(1,4)-glycosyl linked backbones with similar equatorial configurations (Scheller and Ulvskov, 2010). Most hemicellulosic backbones are synthesized by cellulose synthase-like proteins (CSLs) from the GT2 family (Richmond and Somerville, 2000; Scheller and Ulvskov, 2010), although the glycosyl residues vary. The exception is xylans, the backbones of which are produced by Type II membrane proteins from the GT47 and GT43 families (Brown et al., 2007, 2009; Wu et al., 2010). Hemicellulosic backbones are often substituted by diverse glycosyl residues with certain patterns, which determine the variations in their physicochemical properties and structures.
Xylans are the most abundant hemicellulosic polymers in vascular plants. The same β-(1,4)-linked xylosyl backbone is present in different plant species, pointing to a conserved biosynthetic machinery in plants. Because the pentose-containing backbone is different from the hexose-containing backbone, xylan is produced in a distinct manner (Smith et al., 2017). IRX10 and its homolog IRX10L (members of the GT47 family) have been identified as putative β-(1,4) xylosyl transferases based on genetic evidence (Brown et al., 2009; Wu et al., 2009); biochemical analysis confirmed the elongation activities of these enzymes on the xylan backbone (Urbanowicz et al., 2014). IRX10 and its homologs from rice and other plant species successfully complemented Arabidopsis irx10 mutants, validating the cross-species conservation of their role in xylan backbone biosynthesis (Chen et al., 2013). IRX9, IRX9L, IRX14, and IRX14L (of the GT43 family) are another group of components required for xylan backbone synthesis, as lesions in these proteins led to reduced xylan contents (Brown et al., 2007; Wu et al., 2010). However, to date, no GT43 family member has been enzymatically confirmed. The presence of these multiple components suggests they may form protein complexes, but how xylan synthase complexes are organized remains unclear (Zeng et al., 2016). Moreover, the reducing end of xylan in dicots contains the pentasaccharide structure β-D-Xyl-(1,4)-β-D-Xyl-(1,3)-α-L-Rha-(1,2)-α-D-GalA-(1,4)-D-Xyl, which is thought to be a primer to initiate xylan chain synthesis (Smith et al., 2017). This structure is absent in monocot xylans, but putative genes for its biosynthesis were discovered in monocots, leaving this topic an open question.
Most xylosyl residues of the xylan backbone are substituted with (4-O-methyl) glucuronic acids at the O-2 sites in dicot plants and with L-arabinose at the O-3 sites in grasses (Poaceae). The L-arabinosyl side chain may be further substituted with D-xylosyl residues at the O-2 site or modified at the O-5 site with ferulate esters that can be oxidatively cross-linked in a variety of ways (Figure 3; Scheller and Ulvskov, 2010; Chiniquy et al., 2012). In grass endosperm, the double arabinosyl substitution on the xylosyl residues in the backbone (at the O-3 and O-2 sites) is abundantly present, with nearly equal amounts of double and single arabinosyl substitutions (Pellny et al., 2012). The xylosyl substituent is also present in some secretory xylan backbones, such as mucilage (Zhong et al., 2018b). GTs are required for the formation of these side chains. In Arabidopsis, GLUCURONIC ACID SUBSTITUTION OF XYLAN1−3 (GUX1−3) from the GT8 family are capable of adding glucuronic acid substitutions onto xylan, which can be further methylated by glucuronoxylan methyltransferases (GXMT1−2; from domain of unknown function family 579) to produce (methyl)glucuronic xylan (GX) (Mortimer et al., 2010; Urbanowicz et al., 2012). Xylan arabinosyl transferases (XATs) from the GT61 family transfer arabinosyl residues onto the xylan backbone to form arabinoxylan (Anders et al., 2012). XAX1 (another GT61 family member) mediates the substitution of xylosyl residue onto the arabinosyl side chain (Chiniquy et al., 2012), while XYXT1 catalyzes the substitution of xylosyl residue on the xylan backbone (Zhong et al., 2018b). The diversified enzymatic activities of GT61 family members are in agreement with the expansion of this family in grasses (Mitchell et al., 2007).
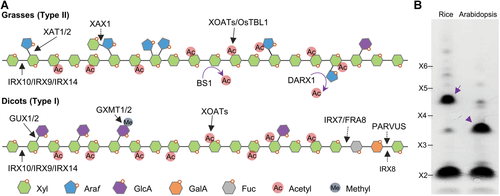
The diversity of the biosynthesis of the prominent hemicellulose xylan in dicots and grasses
(A) Diagram showing the structural models of xylans in dicots and grasses labeled with synthetic glycosyltransferases as well as modifying enzymes, including acetyltransferases (XOATs, OsTBL1), acetyl esterases (BS1 and DARX1), and methyltransferases (GXMT1/2). (B) Electrophoretogram showing the distinct substitutions on xylans from a dicot (Arabidopsis) and a grass (rice). The arrow indicates xylotetraose substituted with arabinosyl residue, and the arrowhead indicates xylotetraose substituted with (methyl) glucuronic acid residue. X2–X6 indicate the xylan oligosaccharide standards in DP 2 to 6.
Xylan is a highly acetylated polysaccharide, which incorporates a majority of cell wall acetyl esters (Gille and Pauly, 2012; Xiong et al., 2013). Monoacetylation at the O-3 or O-2 sites is a dominant acetyl profile on the xylan backbone (Figure 3); diacetylation at both sites O-3 and O-2 is also present in plant xylans (Zhang et al., 2017a). Recent solid-state NMR and molecule simulation studies have demonstrated the role of acetylation in controlling the folding of xylan and its interactions with cellulose and other cell wall polymers (Simmons et al., 2016; Kang et al., 2019). Moreover, acetylation on the arabinosyl side chains of xylan affects its folding and alters the orientation and bundling of cellulose nanofibrils (Zhang et al., 2019). All of these findings suggest that xylan functions as a cross-linking polysaccharide in the construction of cell wall architecture. For a summary of the enzymes that control xylan acetylation, please see the section “Polysaccharide Folding is Required for Cell Wall Construction”.
Xyloglucans contain a β-(1,4)-linked glucosyl backbone that is consecutively substituted with two or three α-D-xyloses at the O-6 sites to form block-wise side chains (Figure 4). Xyloglucan is the most abundant primary-wall hemicellulose in all spermatophytes except grasses. Due to its highly diverse side chain profiles, a special one-letter code system was designed to denote the repetitive units. In brief, G represents an unsubstituted glucosyl residue; X represents the xylosylated glucosyl residue α-D-Xyl-(1,6)-Glc; L represents the further galactosylated X side chain β-D-Gal-(1,2)-α-D-Xyl-(1,6)-Glc; F represents the additionally fucosylated L side chain α-L-Fuc-(1,2)-β-D-Gal-(1,2)-α-D-Xyl-(1,6)-Glc; S represents α-L-Araf-(1,2)-α-D-Xyl-(1,6)-Glc; T represents β-L-Araf-(1,2)-α-D-Xyl-(1,6)-Glc; and U represents β-D-Xylp-(1,2)-α-D-Xyl-(1,6)-Glc (Pena et al., 2008). Galactosyl residues in L and F can be replaced by β-D-galacturonic acid, resulting in the structures denoted as Y and Z found in root hairs (Pena et al., 2012). Additional diversified xyloglucan side chains have been found in non-seed plants (Pena et al., 2008). In addition, acetylation often occurs on the galactosyl residues of side chains in dicots and on the backbone in grasses (Gille et al., 2011; Liu et al., 2016a).
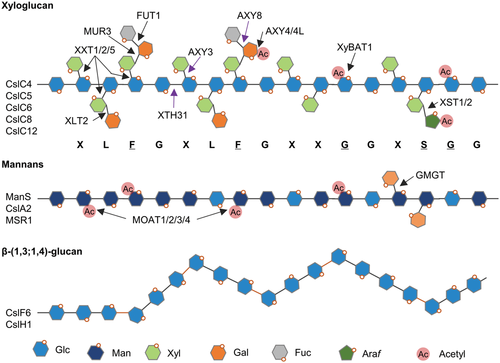
The biosynthesis of hemicelluloses harboring a hexosyl backbone
Diagram showing structural models of the hemicelluloses, xyloglucan, mannans, and β-(1,3;1,4)-glucan labeled with the synthetic glycosyltransferases and modifying enzymes described in the “hemicellulose” section.
The xyloglucan backbone is synthesized by cellulose synthase-like C (CslC) family members; the Arabidopsis cslc4 mutant and the quintuple mutant cslc4 cslc5 cslc6 cslc8 cslc12 have undetectable xyloglucan levels (Cocuron et al., 2007; Kim et al., 2020). The enzymatic activity of AtCslC4 has been examined (Cocuron et al., 2007). Xyloglucan xylosyl transferase1, 2, and 5 (XXT1, 2, 5) are GT-A fold xylosyl transferases that catalyze the first step in side chain formation (Cavalier et al., 2008; Culbertson et al., 2018); the xxt1 xxt2 xxt5 mutant has undetectable levels of xyloglucan (Cavalier et al., 2008). Unexpectedly, Arabidopsis plants appear to grow normally in the absence of xyloglucan (Cavalier et al., 2008; Kim et al., 2020). This finding disagrees with the hypothesis that xyloglucan constitutes a load-bearing tether between cellulose fibrils and is thus important for plant growth (Somerville et al., 2004; Kim et al., 2020). Therefore, the role of xyloglucan remains to be clarified. Furthermore, Xyloglucan L side chain galactosyl transferase position2 (XLT2) and Murus3 (MUR3) from the GT47 family add galactose specifically to the second and third xylosyl residues of the XXXG side chains, respectively, in Arabidopsis (Madson et al., 2003; Jensen et al., 2012). XST1 (Xyloglucan “S”-side chain transferase1) and XST2 are responsible for adding arabinofuranosyl residues to xyloglucans in tomato; expressing these proteins in Arabidopsis mutants partially rescued the growth defects arising from disordered xyloglucan (Schultink et al., 2013). Xyloglucan-specific galacturonosyltransferase1 (XUT1) mediates the addition of galacturonoyl residues to the Y and Z side chains (Pena et al., 2012). Protein structure analysis indicated that fucosyltransferase1 (FUT1, also called Murus2) is a GT-B fold xyloglucan fucosyltransferase that adds terminal fucose to the F side chain (Vanzin et al., 2002; Rocha et al., 2016). In addition, α-fucosidase Altered xylogucan8 (AXY8; from the GH95 family) and AXY3 (α-xylosidase; from the GH31 family) trim fucosyl and xylosyl residues from xyloglucan side chains (Gunl and Pauly, 2011; Gunl et al., 2011). AXY4 and AXY4L specifically mediate the addition of acetyl residues onto the galactosyl side chains of xyloglucans (Gille et al., 2011). Brachypodium XyBAT1 adds acetyl residues onto the xyloglucan backbone, thereby modulating the xylosylation patterns on xyloglucans (Liu et al., 2016a; Zhong et al., 2020). Indeed, much more is known about the biosynthesis of xyloglucans compared to other hemicellulosic polymers (Figure 4).
Mannans are linear glycan chains of β-(1,4)-linked mannose residues, while glucomannan refers to mannan containing intercalary β-(1,4)-linked glucose in its backbone (Figure 4). Galactomannan and galactoglucomannan possess partially galactosylated mannosyl residues at the O-6 site. Mannan levels are usually low in flowering plants, but galactoglucomannan is the major component in the SCW in gymnosperms. Mannans are also storage forms of polysaccharides in some plant species. By tracing the storage of polysaccharides in the endosperm of guar (Cyamopsis tetragonoloba) seeds, the mannan synthase ManS (a CslA protein) was identified as a galactomannan synthase (Dhugga et al., 2004). ManS was also the first hemicellulose backbone synthase to be characterized. More and more CSL proteins have since been characterized, verifying the hypothesis that Csl genes are required for β-glycan formation (Richmond and Somerville, 2000; Dhugga et al., 2004; Burton et al., 2006; Kim et al., 2020). Meanwhile, galactomannan galactosyl transferase (GMGT) was isolated using cDNA from Lotus japonicus endosperm (Edwards et al., 2004). Mannan O-acetyltransferases (MOATs) modify the mannosyl residues of mannan backbone with acetyl epitopes (Zhong et al., 2018c). Mannan and glucomannan were successfully produced in the yeast Pichia pastoris by expressing different plant-derived enzymes to reconstitute mannan synthesis (Voiniciuc et al., 2019). Coexpressing the Arabidopsis cofactor Mannan-synthesis related1 (AtMSR1) enhanced the catalytic activities of AkCslA3 and AtCslA2, allowing AtCSLA2 to produce glucomannan instead of mannan (Goubet et al., 2009; Voiniciuc et al., 2019). However, CcMANS1 from coffee cannot synthesize mannan in the absence of CcMSR1 (Voiniciuc et al., 2019), highlighting the flexibility of (gluco)mannan biosynthesis in plants.
β-(1,3;1,4)-glucans are unbranched β-(1,4)-linked glucans interspersed with β-(1,3)-glucosyl linkages (Figure 4). In general, cellotriosyl (DP3) and cellotetrasyl (DP4) units are connected by β-(1,3) linkages, which can be specifically cleaved by lichenases. The ratio of these two repetitive units (DP3:DP4) is correlated with the physicochemical properties of β-(1,3;1,4)-glucans. Glucans with DP ratios ranging from 1.8 to 2.3 are soluble. Unlike rice glucan, in which the DP ratio is ~1.8, the DP ratios of glucans in oat, rye, and barley range from 1.8 to 3.4. In wheat, the DP ratio ranges 3.0-4.5, and it can reach 5.8 in Brachpodium distachyon grains (Burton and Fincher, 2014; Marcotuli et al., 2016). Expressing rice CslF6 in Arabidopsis (which lacks CslF and its homologs) led to the successful production of β-(1,3;1,4)-glucan in Arabidopsis tissues (Burton et al., 2006; Vega-Sanchez et al., 2012). CslH family members are another group of proteins that synthesize this polymer (Doblin et al., 2009). An investigation of the membrane pore architecture of CslF6 provided a mechanistic view of the formation of β-(1,3;1,4)-glucan structure (Jobling, 2015). Moreover, the observation that CslF6 can target the PM suggests that β-(1,3;1,4)-glucan production likely occurs at various locations more than the Golgi apparatus (Wilson et al., 2015). Indeed, many issues related to β-(1,3;1,4)-glucan synthesis remain to be clarified.
Pectins: structural polymers enriched in galacturonic acids
Pectins are a group of cell wall polysaccharides that possess α-(1,4)-linked galacturonic acids (GalA) in their backbone and are classified into three types or domains: unbranched homogalacturonan (HG), rhamnose-alternated rhamnogalacturonan I (RG-I), and complex branched rhamnogalacturonan II (RG-II) (Mohnen, 2008; Atmodjo et al., 2013). Homogalacturonan harbors an unsubstituted linear chain of α-(1,4)-linked GalA. In addition to occasional acetylation at O-2 and O-3 sites, the α-(1,4)-linked GalA can be methylesterified and demethylesterified at the O-6 sites of the backbone, which affects calcium-bridge formation between HGs (Miao et al., 2011). Xylosylation at O-3 in xylogalacturonan and apiosylation at O-2 and/or O-3 in apiogalacturonan results in the production of HG variants in some plant species or tissues (Jensen et al., 2008; Atmodjo et al., 2013). RG-II is the most complex pectic polymer, as it possesses more than 20 linkages on 12 different sugars to form the HG-like backbone and four oligosaccharide side chains (A–D). Whereas side chains C and D are disaccharides, side chain A is an invariant octasaccharide with varied methylation modifications that has interchangeable L-fucose or L-galactose substitutions. Side chain B possesses six to nine sugars with varied terminal residues and acetylation modifications (Pabst et al., 2013). RG-I has a repetitive disaccharide unit backbone [α-D-GalA-(1,2)-α-L-Rha-(1, 4)-], which is highly acetylated at the GalA residues. The branches of RG-I, such as the α-1,5-linked L-arabinan and β-1,4-linked D-galactan, are often attached onto rhamnosyl residues at O-4, increasing the size of RG-I and exhibiting structural flexibility in different cell types and developmental stages. It is likely that HG, RG-I, and RG-II covalently interconnect via their backbones (Atmodjo et al., 2013). Calcium-bridged HG and dimerized RG-II (via borate diester) further strengthen the pectin network, contributing to robust PCW construction in land plants (Kobayashi et al., 1996; O'Neill et al., 2001; Liu et al., 2011). Crosslinks with the hydroxyproline-rich glycoproteins extensins and the arabinoxylan pectin arabinogalactan protein1 (APAP1) have also been reported (Qi et al., 1995; Tan et al., 2013), highlighting the unusual structural complexity of pectins.
Homogalacturonan is synthesized by galacturonosyl transferases (GAUTs) belonging to the GT8 family (Sterling et al., 2006; Atmodjo et al., 2013; Amos et al., 2018). In contrast to processive glycosyltransferases such as CESAs and CSLs, GAUT1 and GAUT7 form a heteromeric complex to initiate HG formation de novo during the slow phase and switch to a rapid phase when the intermediates reach DP11 in chain length (Atmodjo et al., 2011; Amos et al., 2018), representing an unusual way to synthesize polysaccharide polymers. RG-I:rhamnosyl transferases (RRTs), which transfer rhamnose residues onto RG-I oligosaccharides, belong to the new GT family GT106 (Takenaka et al., 2018), whereas RG-I:galacturonosyl transferase has not yet been identified. Moreover, many glycosyl transferases have been shown to function in side chain formation. Xylogalacturonan deficient1 (XGD1) is required for the xylosylation of xylogalacturonan (Jensen et al., 2008). Four rhamnogalacturonan xylosyl transferases (RGXTs) transfer the xylosyl residues onto L-fucose of RG-II side chain A (Egelund et al., 2006; Liu et al., 2011). Two sialyl transferase-like proteins are thought to add the rare sugars 2-keto-3-deoxy-D-lyxo-heptulosaric acid (Dha) and/or 2-keto-3-deoxy-D-manno-octulosonic acid (Kdo) to side chains C and D (Dumont et al., 2014). Galacturonosyltransferase-like5 (GATL5) is required for mucilage RG-I synthesis in Arabidopsis (Kong et al., 2013). Galactan synthase1-3 (GALS1-3; from the GT92 family) functions as a galactosyl transferase of pectic galactan (Liwanag et al., 2012), while Arabinan deficient1 (ARAD1) and ARAD2 are responsible for pectic arabinan synthesis (Harholt et al., 2012). Before nascent HG is secreted into the apoplast, it is methylated by methyltransferases such as Quasimodo2/Tumorous Shoot Development2 in the Golgi apparatus (Du et al., 2020). Pectin methyl-esterases (PMEs) remove the methyl-ester groups from HG; this action is antagonistically inhibited by PME inhibitors (Peaucelle et al., 2011). Polygalacturonases (PGs) and pectate lyases (PLs) cleave HG (Ogawa et al., 2009; Xiao et al., 2014). Hence, these proteins modify the physiochemical properties of pectin, thereby affecting cell wall structure and function for plant growth and development.
Lignin: polymerized aromatic substances from the phenylpropanoid pathway
Lignin is unordered polymer composed of phenylalanine-derived (and tyrosine-derived in grasses) aromatic monomer substances, representing a characteristic cell wall component in vascular plants (Barros et al., 2016; Vanholme et al., 2019). Although lignin is amorphous, it reinforces cell wall structure and often functions as a physical barrier against pathogen attack. Lignin is usually composed of guaiacyl (G), syringyl (S), and sometimes p-hydroxyphenyl (H) monomers derived from complex phenylpropanoid metabolic pathways (Vanholme et al., 2019). To date, at least 35 natural lignin monomers have been discovered, highlighting the metabolic complexity of lignin. In principal, Phenylalanine ammonia lyases (PALs) convert phenylalanine to cinnamic acids. The resulting products are successively reduced by 4-Coumarate:CoA ligase (4CL), Cinnamoyl-CoA reductase (CCR), and Cinnamyl alcohol dehydrogenase (CAD) to generate CoA-thioester, aldehyde, and alcohol, respectively (Mottiar et al., 2016). The aromatic rings of lignin intermediates may be hydroxylated by cytochrome P450s, including Cinnamate 4-hydroxylase (C4H), p-Coumarate 3-hydroxylase (C3H), and Ferulate 5-hydroxylase (F5H). The products can be further methylated at 3-O and 5-O via O-methyltransferases, such as Caffeoyl-CoA O-methyltransferase (CCoAOMT) and Caffeic acid O-methyltransferase (COMT), to produce various lignin monomers (Vanholme et al., 2019). In addition, Caffeoyl shikimate esterase (CSE) hydrolyzes the intermediate ester caffeoyl shikimate to produce caffeic acid, representing an unusual but dispensable reverse reaction in the monolignol pathway (Vanholme et al., 2013). The multiple substrates and grid-like metabolic flux have led to the diversity in lignin monomer composition among different plant species and tissues. A recent interesting finding in lignin monomer metabolism is the characterization of scaffold proteins that may facilitate substrate channeling to enhance the catalytic activities of enzymes (Gou et al., 2018), representing a potential way to manipulate lignin content and properties. Lignin monomers are passively exported to the apoplast via some undetermined transporters (Perkins et al., 2019; Vermaas et al., 2019). These monolignols are linked by ester/ether bonds to form hydrophobic polymers, while the local oxidation systems promoted by laccases/oxygen and peroxidases/hydrogen peroxide are activated in lignifying cells (Lee et al., 2013, 2018; Lu et al., 2013; Tobimatsu and Schuetz, 2019). For a comprehensive summary of lignin metabolism, please see these reviews (Dixon and Jaime, 2019; Perkins et al., 2019; Tobimatsu and Schuetz, 2019; Vanholme et al., 2019).
ASSEMBLY OF CELL WALL STRUCTURE
Compared to determining the biosynthesis of cell wall components, clarifying how cell wall polymers are organized and assembled into a functional structure appears to be more difficult. The first proposed model was based on characterization of enzyme degradation products, in which matrix polysaccharides were thought to be covalently cross-linked (Keegstra et al., 1973). Approximately 20 years later, updated models were proposed that incorporated advances in polymer structure and insights from electron microscopy observations (Fry, 1989; Hayashi, 1989; Carpita and Gibeaut, 1993; Cosgrove, 2001). These models suggest that there are at least two cell wall types in flowering plants (Carpita and Gibeaut, 1993). The type I cell wall differs from the type II cell wall found in Poaceae in terms of composition and organization. Recent solid-state NMR spectroscopy and genetic analyses have suggested that the cellulose-xylan connection is the major structural framework in the SCW (Simmons et al., 2016; Kang et al., 2019; Zhang et al., 2019). This model appears to be applicable to the type II PCW of grasses, in which glucuronoarabinoxylan is the major hemicellulose. However, the previously proposed load-bearing tether generated by cellulose-xyloglucan links in the type I PCW has been questioned, as Arabidopsis mutants with undetectable xyloglucan levels grow almost normally (Fry, 1989; Hayashi, 1989; Cosgrove, 2001, 2018; Kim et al., 2020). The cellulose-pectin network in the PCW and the cellulose-xylan network in the SCW are two widely accepted models that have been validated by solid-state NMR spectroscopy, AFM, and genetic analysis (Peaucelle et al., 2011, 2014; Wang et al., 2015; Grantham et al., 2017; Kang et al., 2019; Phyo and Hong, 2019; Zhang et al., 2019). On the other hand, increasing evidence indicates that the plant cell wall is a plastic structure that is associated with unresolved mechanical processes, including extending, loosening, softening, strengthening, flexing, and so on (Cosgrove, 2018; Kang et al., 2019). Therefore, clarifying the mechanism underlying cell wall construction is becoming an important topic of study. The current model of cell wall structure is far from the last word on this topic.
Bundling and orienting cellulose microfibrils
Under AFM, cellulose appears as a fibrillar network with multiscale fibrils. The nascent glucan chains are bundled and oriented into a functional architecture, a process involving many factors. While newly synthesized cellulosic chains are extruded outward from the PM, they aggregate laterally and crystallize via Van der Waals forces and hydrogen bonding. Therefore, the status of CSCs and activity of CESAs appear the key factors affecting cellulose crystallization and bundling. The Arabidopsis mutants cesa1aegeus and cesa3ixr1-2 produce aberrant cellulose microfibrils with severely reduced crystallinity (Harris et al., 2012), genetically verifying this hypothesis. Moreover, the COBRA-like protein BC1 regulates cellulose crystallite size in rice (Liu et al., 2013), suggesting that auxiliary proteins are required for stacking nascent glucan chains into a crystalline structure. Cellulosic elementary fibrils (CEFs) likely form from 18 glucan chains that are simultaneously synthesized by a CSC rosette with six lobes (Purushotham et al., 2016, 2020). The single CEFs could further aggregate into nanofibrils/macrofibrils with multiple diameters, and even into cord-like structures (Ding et al., 2012; Zhang et al., 2019). However, the mechanisms underlying these processes are currently unclear. Other models for cellulose crystallization and bundling cannot be excluded (Fernandes et al., 2011).
As cellulosic microfibrils usually attach to matrix polysaccharides, the orientation of these microfibrils is dependent on the cell wall matrix. Disruptions in arabinoxylan, pectins and xyloglucan alter the orientation of ordered microfibrils, thereby modifying cell wall mechanics (Xiao et al., 2016; Zhang et al., 2019; Du et al., 2020). Xylan binds directly to crystalline cellulose via a two-fold screw conformation. This conformation is essential for the normal orientation of multiscale cellulose microfibrils to form the SCW network at the nanoscale level (Simmons et al., 2016; Zhang et al., 2019). The regulation of the interaction between cellulose and xylan is discussed in the following section.
By applying mechanical stress and enzymatic treatments to onion epidermis, diverse microfibril movements, including kinking, axial shearing, separating, and sliding were observed in the onion PCW (Zhang et al., 2017b). Unexpectedly, slippage between microfibril lamella is limited and is only present at the nanoscale level (Zhang et al., 2017b), corroborating the existence of various molecular connections within cellulosic nanofibrils. Unfortunately, in planta analysis of microfibril movement is still impractical, but cellulose nanofibrils can be isolated from cell walls and extensively characterized. For example, right-handed chirality has been observed in both bundles and single fibrils (Ding et al., 2012; Usov et al., 2015). Kinking of cellulosic nanofibrils can be induced by mechanical deformation, which is usually accompanied by the breakage of chemical bonds in glucan chains (Ciesielski et al., 2019), suggesting that matrix polysaccharides play important roles in wall mechanics. Studies on cellulosic nanofibril performance during growth-triggered wall construction and deformation may provide crucial clues for understanding the nature of plant life.
Polysaccharide folding required for cell wall construction
Matrix polysaccharides were long thought to be amorphous and diffuse in cell walls (Somerville et al., 2004). These polysaccharides are usually modified with chemical groups, such as acetyl, methyl, and phenyl epitopes, which alter their physicochemical properties. Among these, acetylation and methylesterification are the major modifications. Hemicellulosic and pectic polymers usually possess specific acetylation and methylesterification profiles in different tissues and across various developmental stages, pointing to the roles of these modifications in cell wall organization. Recent high-resolution spectroscopy and microscopy studies showed that these modifications can mediate previously uncharacterized types of folding, ultimately determining cell wall nanostructure (Grantham et al., 2017; Zhang et al., 2019; Haas et al., 2020). These findings open a new direction in the study of cell wall construction.
O-acetylation is a widespread modification found in most cell wall polymers (Kiefer et al., 1989; Ishii, 1991; Gille and Pauly, 2012). The naturally acetylated forms of at least eight of 14 monosaccharides have been detected in the cell wall, including galactosyl residue on xyloglucan branches, xylosyl residue on the xylan backbone, and galacturonosyl residue on pectic polymers (Gille and Pauly, 2012). Some glycosyl residues can even be doubly substituted. Interestingly, the wall polymer xylan incorporates the majority of acetyl esters based on whole cell wall analysis (Xiong et al., 2013; Gao et al., 2017). However, until recently, the underlying mechanism for how plants establish acetylation profiles in their cell walls remained a mystery. Trichrome birefringence-like (TBL) proteins function as glycosyl acetyltransferases (Gille et al., 2011; Gille and Pauly, 2012), while reduced wall acetylation (RWA) proteins, the sequences of which are similar to that of the fungal glucuronoxylomannan acetyltransferase Cas1p, might function as a channel of acetyl donors (Janbon et al., 2001). Since the genetic identification of AXY4 and AXY4L as putative galactosyl acetyltransferases of xyloglucan (Gille et al., 2011), a group of TBL proteins was shown to be required for xylan acetylation (Xiong et al., 2013; Urbanowicz et al., 2014; Gao et al., 2017; Zhong et al., 2018a). Deacetylases might be involved in maintaining specific acetylation profiles on cell wall polysaccharides, but the characterization of these proteins has lagged behind that of glycosyl acetyltransferases. A carbohydrate esterase (CE) family member removes acetate from pectins (Gou et al., 2012). Two GDSL esterase/lipase proteins (GELP) from the GELP supergene family, Brittle leaf sheath1 (BS1) and Deacetylase on arabinosyl side chain of arabinoxylan1 (DARX1), trim acetate from the xylan backbone and side chains in the Golgi apparatus (Figure 3; Zhang et al., 2017a, 2019). The presence of multiple GDSL family members suggests that there may be equivalent deacetylases and acetyltransferases that help maintain the acetylation profiles on cell wall polymers (Zhang et al., 2019).
Genetic evidence suggests that the acetylation profiles of polysaccharides are important for the construction of the functional cell wall structure (Zhong et al., 2017; Zhang et al., 2017a). Although β-(1,4)-xylan is favored to form a three-fold screw symmetry based on modeling simulations (Almond and Sheehan, 2003), the two-fold conformation was found to be dominant in many plant cell walls based on solid-state NMR analysis (Simmons et al., 2016; Kang et al., 2019). Thus, an acetylation profile generated by acetylation and deacetylation may be indispensable for the formation of the two-fold xylan conformer (Grantham et al., 2017; Zhang et al., 2019). Moreover, two-fold conformers bind onto the cellulose surface, forming non-covalent links between xylan and cellulose, whereas three-fold conformers form a hydrated matrix to contact lignin nanodomains (Simmons et al., 2016; Kang et al., 2019; Zhang et al., 2019). The interaction between two-fold xylan and cellulose is crucial for the construction of an integral load-bearing network. A deficiency in any of these components disturbs the cellulose-xylan link, leading to abnormal plant growth and development (Simmons et al., 2016; Zhang et al., 2019).
Methylesterification, a modification that attaches methyl groups onto the carboxyl groups of uronic acids, is another important modification on wall polymers. This modification mainly occurs on GalA residues of HG. Newly synthesized HG is highly methylesterified and must undergo de-esterification after being deposited into the cell wall (Zhang et al., 2018c; Du et al., 2020). The proper degree of methyl esterification on HG is essential for pollen tube growth, cell adhesion, and meristem maintenance (Jiang et al., 2005; Zhang et al., 2018c; Du et al., 2020), but its role in controlling pectin structure is unknown. Three-dimensional direct stochastic optical reconstruction microscopy (3D-dSTORM) plus cryo-scanning electron microscopy (cryo-SEM) was recently used to analyze HG in muro. This analysis detected a nanofibril-like pectin in the anticlinal wall of the cotyledon. De-esterification of HG collapses this nanofilament structure (Haas et al., 2020), indicating that the expansion capacity of the extracellular matrix, such as pectin and hemicellulose, helps shape the cell. This finding challenges the conventional model in which turgor pressure and constraint by the cell wall are the major forces driving cell expansion (Geitmann and Ortega, 2009). Therefore, further improving nanoimaging technologies could provide new insights into cell wall construction.
CELL WALL REGULATION AND SIGNALING
The cell wall is heterogeneous in terms of both chemistry and structure. The over 40 types of cells in plants exhibit diversity in terms of cell wall composition and organization, leading to cell-type specific wall structures (Farrokhi et al., 2006; Burton et al., 2010; Loqué et al., 2015). Therefore, an elaborate regulatory machinery represents another level of control that establishes the intricate structure and diverse functions of the cell wall.
Transcriptional regulatory network
Transcriptional regulation represents a large-scale control mechanism for the cell wall, since it simultaneously affects multiple metabolic pathways and regulates the transcription of many cell wall-related genes. Many studies have revealed that the regulation of the cell wall, especially SCW formation and construction, occurs via a hierarchical transcriptional network consisting of a series of NAC and MYB transcription factors (Demura and Fukuda, 2007; Zhong and Ye, 2007; Zhong et al., 2008; Taylor-Teeples et al., 2015; Chen et al., 2019). The top-layer master regulators include Vascular-related NAC-domain1 (VND1) to VND7, NAC secondary wall thickening promoting factor1 (NST1), and secondary wall-associated NAC domain protein1 (SND1/NST3) and NST2 (Kubo et al., 2005; Mitsuda et al., 2005; Zhong et al., 2006; Li et al., 2012a; Tan et al., 2018). The knockout of genes for these master regulators leads to the complete loss of secondary wall thickening in specific tissues; misexpressing these genes often results in the ectopic deposition of SCW (Kubo et al., 2005; Mitsuda et al., 2005; Zhong and Ye, 2007). These transcription factors thus function as molecular switches in the regulation of SCW biosynthesis. Moreover, four AP2/ERF transcription factors appear to be master regulators of PCW biosynthesis, as misexpressing either of these genes under the control of a fiber cell-specific promoter induced the production of thickened cell walls with PCW characteristics in fiber cells of the nst1 nst3 mutant, which completely lack native SCW (Sakamoto et al., 2018).
These regulators are in turn positively or negatively regulated via interactions with WRKY12, MYB26, XND1, VNI2, E2Fc, KNAT7, HB15, and MYC2/4, which integrate cell wall biosynthesis with other physiological processes (Wang et al., 2010; Yamaguchi et al., 2010; Taylor-Teeples et al., 2015; Zhang et al., 2018a, 2018b; Wang et al., 2019). The interaction between rice NAC31/NAC29 and the DELLA protein Slender rice1 represents an interconnected nexus that inputs gibberellin signaling into cell wall formation (Huang et al., 2015). Some NAC transcription factors directly target cell wall-synthesizing genes (Zhao et al., 2010). A battery of MYB transcription factors, such as MYB46 and MYB83, constitute the downstream hierarchy of NAC regulators, as they usually directly regulate the expression of cell wall biosynthesis genes (Zhong et al., 2008; Zhou et al., 2009). The kinase PdMPK6 phosphorylates a MYB associated with lignin biosynthesis in Populus, indicating another regulatory manner during SCW formation (Gui et al., 2019). Other groups of transcription factors, such as C3H14, BLH6, and OFP4, as well as epigenetic regulators such as miR397 and miR319a, are also involved in this regulatory process (Lu et al., 2013; Chai et al., 2015; Hou et al., 2020), further enriching our understanding of this regulatory network. Additional efforts should focus on uncovering tissue- or cell-type specific regulatory networks. Several reviews provide a more detailed view on progress in understanding the regulation of cell wall biosynthesis (Zhong and Ye, 2015; Rao and Dixon, 2018).
Cell wall compartmentalization
The cell wall is not uniform, as it possesses plasmodesmata, Casparian strips, pitted and spiral patterns, and so on. During plant growth, the asymmetrical deposition of wall products must be tightly controlled to allow the cell wall to fulfill its many physiological functions, such as determining cell shape, seed dispersion, and nitrate uptake during nodule symbiosis (Feraru et al., 2011; Hofhuis et al., 2016; Wang et al., 2020). Considering the close-knit contacts between the cell wall and PM, the cell wall–PM continuum might be crucial for directing the heterogeneous deposition of cell wall material and determining the behaviors of the cell wall in cell polarization and morphogenesis (Oda and Fukuda, 2012a; Bashline et al., 2014; Liu et al., 2015).
Xylem vessels represent an ideal system for addressing the mechanisms of wall deposition with regiospecificity. Because CSC movement on the PM is guided by CMTs, the proteins that regulate microtubule dynamics at specific PM domains are crucial for cell wall compartmentalization. Based on a series of findings obtained using a metaxylem induction system, the GTPase ROP11 recruits MIDD1 and Kinesin-13A to a specific domain of the PM and disassembles microtubules to inhibit cell wall deposition at the defined region (Oda et al., 2010; Oda and Fukuda, 2012a, 2012b, 2013). IQD13 (an IQ67 domain protein) associates with CMTs and the PM to laterally restrict the localization of an ROP GTPase to shape pits on the vessel walls (Sugiyama et al., 2017). In addition, Wallin (WAL) is recruited to the PM at pit boundaries by Boundary of ROP domain1 (BRD1) to promote actin assembly, thereby shaping pit borders as well (Sugiyama et al., 2019). Furthermore, using long-term live-cell imaging technology in Arabidopsis cells undergoing protoxylem transdifferentiation, the local recruitment of the microtubule-bound nucleation factor KATANIN was observed during the patterning of spiral walls (Schneider et al., 2020). These mechanisms might be applicable to cell wall compartmentalization in other cell types as well, although visualizing this process is still technically challenging.
Cell wall integrity mediates plant growth and stress adaptation
Tension force generated by antagonism between turgor pressure and wall constraint often provides clues about the systematic monitoring of wall integrity during plant growth and development, as well as environmental adaptation. Plants have evolved cell wall integrity (CWI) machinery to balance the extensibility and rigidity of cell walls and sustain growth vigor. Whereas CWI signaling pathways in yeast have been largely elucidated, such pathways are more complex in plants and remain elusive because plants are highly ordered multicellular organisms (Hamann, 2015).
Receptor-like kinases (RLK) located at the PM appear to function as CWI sensors. A screening for genetic suppressors of the Arabidopsis cesa6 mutant procuste1 revealed that a mutation in THESEUS1 (THE1) rescued the growth abnormalities of procuste1, but the mutant exhibited defective cellulose content (Hematy et al., 2007). THE1 is a key component which perceives cell wall damage and regulates plant growth. Several members of the Catharanthus roseus receptor-like kinase1-like (CrRLK1L) family have been identified, including Feronia (FER), Anxur1 (ANX1) and ANX2, and Buddha's paper seal1 (BUPS1) and BUPS2, which are essential for CWI during root cell and pollen tube elongation (Ge et al., 2017; Feng et al., 2018). Feronia interacts with the extracellular glycoproteins Leucine-rich repeat extensins (LRXs) and binds to pectin via its extracellular domain (Feng et al., 2018; Dünser et al., 2019). Moreover, secreted Rapid alkalinization factor (RALF) peptides, such as RALF1, RALF4/19, RALF23, and RALF34, interact with these CrRLK1L family members and LRX proteins, suggesting that they function as ligands to mediate extracellular matrix signaling, for example, changes in redox, pH, and tension stimuli (Mecchia et al., 2017; Moussu et al., 2020). In the presence of homologs of these three components, complex interactions are thought to occur, and trinary/ternary signaling pathways may be diversified to govern reproduction, development, and biotic/abiotic stress responses (Vogler et al., 2019).
Leucine-rich repeat (LRR) kinases are also required for wall damage signaling. Lesions in Fei1 and Fei2 disrupt cell wall synthesis and anisotropic expansion (Xu et al., 2008). Fei2 functions in the perception of cell wall damage via the mechanosensitive Ca2+ channel MCA1, which functions downstream of THE1 (Engelsdorf et al., 2018). MDIS1-interacting receptor-like kinase2 (MIK2) is involved in CWI via a joint action with THE1 (Van der Does et al., 2017). Interestingly, wall damage-elicited signaling pathways can undergo crosstalk with pathways triggered by pattern-triggered immunity (PTI), although the mechanism is still unclear (Engelsdorf et al., 2018). Furthermore, oligogalacturonides (OGAs) released from the plant cell wall can promote damage-associated molecular patterns to activate plant immune responses and plant growth: wall-associated kinase1 (WAK1) functions as a receptor of OGAs during this process (Brutus et al., 2010). Another CWI signaling pathway is mediated by PME inhibitor5 (PMEI5)-RLP44, which functions with the BR co-receptor BAK1 to synergistically control CWI and cell growth (Wolf et al., 2012, 2014; Wolf, 2017). In addition, STRUBBELIG (SUB), an atypical receptor-like kinase, regulates isoxaben-induced cell wall stress responses (Chaudhary et al., 2020). Once additional signaling pathways are identified, perhaps the framework of the plant CWI machinery will be uncovered in the near future.
Plants grow and develop in response to various environmental and internal signals. As the cell wall provides physical support to the cell, stimuli mediated by the cell wall are thought to be largely related to mechanics. Mechanical stimuli derived from cell wall deformation during cell expansion and morphogenesis, wind-induced bending, rain or animal-mediated touches, and so on are sensed by the plant. The relevant signaling pathways are then triggered to adjust plant growth status. Mechanoperception is an emerging research area that is attracting widespread attention (Anderson and Kieber, 2020). New analytical techniques and molecular tools that can measure the strength of these forces at the cell or tissue level are urgently needed to accelerate such studies.
PLANT CELL WALL FUNCTIONS
The plant cell wall, which provides a structural basis for the plant, is largely derived from photosynthetically assimilated products and represents the most abundant renewable resource on Earth. Plants have evolved a way to construct this complex, recalcitrant material in an economical manner. The mechanical properties of the plant cell wall guarantee its involvement in most physiological processes associated with plant growth and adaptation. Therefore, the functions of the plant cell wall are quite diverse. The plant cell wall also has great economical value as lignocellulosic cell wall biomass, offering a wide variety of valuable components for a sustainable economy. The applications of this biomass include but are not limited to fiber, feed, food, and fuel.
Plant morphogenesis and growth
The mechanical force that drives plant cells to undergo anisotropic growth, tip growth, or diffuse growth arises from the balance of turgor pressure and cell wall tension, leading to various cell geometries and organ shapes (Chebli and Geitmann, 2017). The cell wall not only serves as an exoskeleton encasing plant cells, but it also directs cell expansion polarity (Wolf et al., 2012; Du and Jiao, 2020). Among cell wall polymers, cellulose microfibrils and their orientation are the major determinants of cell morphogenesis (Keegstra et al., 1973; Cosgrove, 2001, 2018; Hofhuis et al., 2016). The cylindrical shape of many elongated plant cells is likely achieved through the deposition of transversely oriented cellulose microfibrils (Zhang et al., 2017b). This notion was validated by observing the formation of spiral xylem vessel walls, in which the organized CSC trajectories have been observed (Watanabe et al., 2015). Highly methylesterified pectins are produced and secreted at the tip of the pectin-rich pollen tube to sustain wall flexibility; the methylesterification is then removed by PMEs, and callose and cellulose are incorporated to strengthen the walls at the subapical region (Jiang et al., 2005; Chebli et al., 2012). These actions balance the elasticity and rigidity of the cell wall, facilitating rapid tip growth. Aborting PME activity disturbed tube wall properties and impeded tip growth of the pollen tube (Jiang et al., 2005; Zhang et al., 2018c). Plant grafting is a widely used horticulture technique to modify plant morphogenesis and improve traits. Among many factors that determine the success of grafting combinations, cell wall reconstruction at the graft boundary is vital. A subclade of β-1,4-glucanases were recently identified to mediate this process (Notaguchi et al., 2020), which may enhance plant interfamily grafting techniques.
Plant cell expansion is an important manifestation of plant growth which requires some wall polymers to become loosened and degraded and new wall components to be incorporated to increase cell volume (Chebli and Geitmann, 2017; Cosgrove, 2018). Expansins and multiple endotransglycosylase/hydrolases, such as polygalacturonases and PLs, are involved in wall extension and remodeling (Xiao et al., 2014; Cosgrove, 2015; Rui et al., 2017), leading to so-called turgor-driven expansion (Cosgrove, 2018). However, the molecular mechanisms underlying cell expansion remain to be elucidated, as the proteins involved in this process have not yet been fully characterized, and the dynamic actions of these proteins are unclear. In the recently proposed “expanding beam” model, matrix pectic nanofilaments themselves possess an intrinsic expansion capacity (Haas et al., 2020), complementing the previous cell expansion model. Hence, the impacts of cell walls on cell morphogenesis and growth may be more complicated than previously thought.
Mechanical strength
An upright growth habit is one of the most important characteristics of land plants. This growth habit is conferred by the deposition of thickened SCW in fiber and xylem vessels following cell growth. The emergence of a thickened SCW is considered to be an important evolutionary event during the adaptation of plants to a terrestrial environment (Xu et al., 2014). We summarized SCW biosynthesis and regulation in the above sections. Here, we introduce an in planta view of mechanical strength.
Lodging, a visible manifestation of compromised mechanical support due to disrupted SCW formation, is harmful to crop production. The isolation and characterization of three series of mutants with reduced mechanical strength have led to the identification of many key genes required for SCW formation (Burk et al., 2001; Scheible et al., 2001; Zhang and Zhou, 2011). The mechanisms underlying mechanical strength formation have been extensively investigated. At the microscale level, the Arabidopsis fragile fiber (fra) mutants usually possess interfascicular fiber cells with defective cell walls (Burk et al., 2001; Zhong et al., 2002), while Arabidopsis irregular xylem (irx) mutants have collapsed xylem vessels (Scheible et al., 2001; Taylor et al., 2003; Brown et al., 2007; Wu et al., 2009). The rice mutants, brittle culm (bc) and brittle leaf sheath (bs), have been successfully used to identify genes involved in cellulose and xylan biosynthesis (Zhang et al., 2009; Xiong et al., 2010). Changes in acetylation modification on xylan also lead to disrupted SCW deposition in epidermal fiber and vessel cells (Zhang et al., 2017a, 2019). At the nanoscale level, decreased mechanical strength in plants may arise from the incorrect bundling and orientation of cellulose nanofibrils, as revealed by AFM (Zhang et al., 2019). Therefore, mechanical strength largely relies on the normal packaging of the load-bearing network consisting of multiscale cellulose microfibrils and their interactions with xylan.
Investigation of mechanical force at the cellular level also provides opportunities to detect mechanical stimuli that drive cell differentiation and growth. Biomechanics in plant cells is regarded as a type of force at a “soft surface,” which is generated by the interactions between turgor pressure and cell wall tension (Hamant and Haswell, 2017). The degree of pectin methyl esterification at the meristematic zone is a central factor in determining cell and organ shape (Peaucelle et al., 2011). This esterification is regulated by the actions of PME-PMEI pairs, which trigger mechanical cues that are integrated into signaling pathways during plant development (Qi et al., 2017). Currently, the balance of esterified and de-esterified pectin states was identified as essential for the root clock mediated lateral root initiation in Arabidopsis (Wachsman et al., 2020). Furthermore, the epidermis, which harbors thickened cell walls, usually provides higher resistance to tension than inner tissues and plays a key role in mechanical stimuli-triggered growth (Savaldi-Goldstein et al., 2007). Cell wall damage might also cause mechanical stress and induce CWI signaling pathways to restore plant growth.
Plant adaptation and defense responses
Due to their sessile growth habit, most flowering plants have adapted to their limited terrestrial environments by exhibiting high plasticity in their responses to local conditions including nutrient availability, light levels, and temperature, as well as toxic substances or harmful conditions (Zhao et al., 2020). Remodeling cell wall biosynthesis is a common response to exposure to environmental changes (Endler et al., 2015). As the cell wall often functions at the interface where stress and attack occur, cell wall damage might be the cause and/or effect of plant adaptations (Wolf, 2017). At least two types of responses occur: rapid responses, in which the actions of proteins required for cell wall synthesis are directly altered in a short time (Crowell et al., 2009; Gutierrez et al., 2009; Lei et al., 2015); and long-term responses, in which a number of genes involved in cell wall metabolism are regulated to sustain plant growth under stress (Zhao et al., 2020). In plants under stress, the mobility of CSCs on the PM slows and the CSCs are removed from (or returned to) the PM by endocytosis (or exocytosis) to rapidly modify cellulose synthesis (Bashline et al., 2014; Lei et al., 2015). With the aid of cellulose synthase cofactors (such as CC1/2, which stabilize CMTs), the mobility of CSCs and cellulose synthesis are maintained to sustain growth under long-term saline conditions (Endler et al., 2015).
Plants must contend with a range of abiotic stresses, including osmotic, ionic, oxidative, and cold and freezing stress. In addition to CESAs and their cofactors, other proteins are required for abiotic stress-induced cell wall dynamics. The glycosylphosphatidylinositol-anchored fasciclin-like arabinogalactan (FLA) protein Salt overly-sensivity5 (SOS5) and the nucleotide sugar conversion enzyme Murus4 help plants adapt to salt stress (Shi et al., 2003; Zhao et al., 2019). Several xyloglucan processing enzymes, such as XTH31, are required for root growth in plants under aluminum stress (Zhu et al., 2012, 2014; Wan et al., 2018). Xylan O-acetyltransferase1 (XOAT1/Eskimo1) functions in freezing tolerance (Xin and Browse, 1998; Lunin et al., 2020). Genetic studies revealed that blocking strigolactone biosynthesis completely recovered the abnormal developmental and stress responses caused by a lesion in ESK1/TBL29 (Ramirez et al., 2018; Ramírez and Pauly, 2019), indicating that the polysaccharide O-acetylation modification is crucial for plant adaptation to external stress.
Ultraviolet radiation (UV), a common stress faced by terrestrial plants, damages DNA and leads to the production of reactive oxygen species. As plant cell walls are directly exposed to UV, plants have evolved the crucial compounds phenylpropanoid derivatives to absorb UV (Vogt, 2010). Many plant tissues that are exposed to UV, including stems, leaves, and pollen grains, often contain phenylpropanoid derivatives, but these components vary (Torabinejad et al., 1998). Several phenolic compounds were recently identified in sporopollenin, a structural layer covering pollen grains, using pyrolysis gas chromatography-mass spectrometry and liquid-state NMR (Li et al., 2019; Xue et al., 2020). These studies revealed the importance of lignin biosynthesis in plant adaptation to UV and provided important insights into plant evolution in terrestrial environments.
The cell wall also functions as a physical barrier against pathogens. Based on the “survival of the fittest,” plants have evolved cell walls of various textures armed with functional groups, such as substituents and modifications, to adapt to different biomes (Burton et al., 2010; Xu et al., 2014). For example, the acetyltransferases powdery mildew resistant5 (PMR5), TBR1, and RWA2, which are involved in cell wall acetylation modification, affect pathogenicity via the WAK1-PAD3/OGA defense pathway (Chiniquy et al., 2019). The WAK protein Xa4 confers rice plants with durable resistance against Xanthomonas oryzae while improving multiple agronomic traits without compromising grain yield (Hu et al., 2017). The exocytotic protein Bph6 promotes cell wall reinforcement and contributes to broad resistance to planthoppers in rice (Guo et al., 2018). Furthermore, a pathogen-secreted paralogous decoy protein protects the apoplastic effector PsXEG1 from the glucanase inhibitor GmGIP1 produced by the host in the battle between Phytophthora sojae and soybean (Ma et al., 2017). Hence, the cell wall has much broader effects on the microbes that attack plants than previously thought. Studies on the relevant signaling pathways and crosstalk with jasmonate- or salicylate-mediated defense pathways would increase our understanding of the “molecular battles” between plants and pathogens.
FUTURE PROSPECTS AND CHALLENGES
The plant cell wall, a structure derived from CO2 assimilation products, is ubiquitous in nature, with vast biological significance and commercial importance. In the past decades, genetics and genomics, combined with biochemistry, have proven to be powerful tools for characterizing enzymes involved in the synthesis and modification of the cell wall. As summarized in this review, multiple glycosyltransferases that mediate specific sugar linkages in cellulosic, hemicellulosic xylan, mannan, and glucan, and pectic polysaccharides have been identified; numerous hydrolases, pectin methylesterases, acetyltransferases, and esterases that remodel and modify cell wall polysaccharides have also been reported. These findings have helped uncover the elaborate machinery underlying cell wall formation and remodeling. As increasing numbers of cell wall synthesizing proteins are identified, the synthesis of polysaccharides with commercial and medical value via synthetic biology is becoming possible. Another exciting prospect is the visualization of the cell wall polymer biosynthetic process (Wu et al., 2020). Using cutting-edge physical technologies such as magnetic tweezers, the real-time visualization of polymer growth at the single polymer level has become achievable (Liu et al., 2017). Like DNA and proteins, which can be sequenced, perhaps polysaccharides could someday be analyzed sugar-by-sugar. However, many challenges remain. The presence of multiple homologs in almost all cell wall enzyme families may complicate phenotypic characterization, impeding functional clarification. In addition, enzymatic specificities in terms of localization and reaction substrates, action timing, and companion cofactors increase the difficulty of biochemical characterization.
Cell wall construction (i.e., cell wall composition and organization) is strongly associated with the biological functions of the cell wall. Increasing evidence has revealed that understanding how these cell wall polymers are cross-linked and assembled into a functional cell wall structure is more valuable than simply understanding their composition. Due to the tremendous structural complexity, progress in understanding cell wall construction had been hampered prior to the application of NMR and AFM. Both technologies can reveal the cross-linking of cellulose and lignin with various folding conformations of xylan and uncover the assembly and orientation of distinctive cellulosic microfibrils, addressing many previously unsolved issues. Now, new questions arise. For example, can we examine cell wall structure in one cell type or even in a specific domain? The plant cell wall is heterogeneous, even in a single cell. How is this heterogeneity established? Many biological processes, such as xylem vessel differentiation and the cracking of seed pods, are dependent on the formation of specific structural domains on the cell wall (Feraru et al., 2011; Oda and Fukuda, 2012; Hofhuis et al., 2016). Addressing these processes will be vital for further understanding the nature of plant life.
Due to many in-depth studies, our understanding of cell wall function has been updated. Plants are immobile; plant growth is plastic. Plants have evolved a sophisticated machinery to adapt to changes in environmental conditions. The interdependence of the cell wall with intracellular compartments, such as the PM and cytoskeleton (referred to as the cell wall–PM–cytoskeleton continuum) is crucial for this plastic growth. Although achievements have been made in finding connections between the PM and cytoskeleton, the crosstalk within the cell wall and the PM remains elusive (Feraru et al., 2011; Liu et al., 2015). The cell wall and PM might be closely in contact via turgor pressure (Proseus and Boyer, 2005). Such physical links can be bridged by PM proteins, such as AGPs, FLAs, and WAKs, which anchor to apoplastic polysaccharides at particular domains. Among these proteins, CSCs are the most important, as their actions on the PM mediate the bundling of cellulosic microfibrils (Watanabe et al., 2015). Considering that their functions are related to cell growth, plant immune responses, abiotic stress adaptation, and so on, these proteins might also act as mechanosensors and pass mechanical signals to fundamental plant growth and developmental processes. Finally, 70% of photosynthetic products are used to generate cell wall polymers to help build plants. Cell wall biogenesis is thus equivalent to carbon metabolism and must coordinate with nitrogen metabolism (Gao et al., 2020); this finding has enormous implications for our understanding of carbon-nitrogen balance, as well as other life events.
In summary, the plant cell wall is a natural nanostructure. Therefore, studies of new features of the cell wall can be described as the three “Ms.” Multiscale studies reveal how a cell wall is constructed from the nanoscale to the microscale level, as well as the cellular level and ultimately the organ and whole plant, providing an understanding of how these multiscale structures help the cell wall function in plant growth and development. Accurate analytical techniques, such as AFM, NMR, single molecular imaging, and living cell microscopy, are broadly used in cell wall research. On the other hand, plants produce cell walls via an orderly process involving a series of metabolic and regulatory pathways, which require the orchestration of multiple genes, proteins, and cell wall metabolic products. Multidisciplinary approaches are widely used to characterize the distinct biomacromolecules involved in cell wall formation and organization. Multidimensional knowledge will be obtained, providing us with evolving views on the construction and functions of the cell wall.
ACKNOWLEDGEMENTS
We thank Yan Wang for the careful reading and comments on the manuscript. We regret that many original articles on cell wall construction could not be cited due to space restrictions. Baocai Zhang and Yihua Zhou were supported by the National Nature Science Foundation of China (NSFC, 31922006, 31861133015 and 32030077), Youth Innovation Promotion Association CAS (2016094), and the State Key Laboratory of Plant Genomics.






