Natural hybridization and reproductive isolation between two Primula species
Summary
Natural hybridization frequently occurs in plants and can facilitate gene flow between species, possibly resulting in species refusion. However, various reproductive barriers block the formation of hybrids and maintain species integrity. Here, we conducted a field survey to examine natural hybridization and reproductive isolation (RI) between sympatric populations of Primula secundiflora and P. poissonii using ten nuclear simple sequence repeat (SSR) loci. Although introgressive hybridization occurred, species boundaries between P. secundiflora and P. poissonii were maintained through nearly complete reproductive isolation. These interfertile species provide an excellent model for studying the RI mechanisms and evolutionary forces that maintain species boundaries.
-
Edited by: Meng-Xiang Sun, Wuhan University, China
Natural hybridization is common in plants, and has many evolutionary consequences. Introgressive hybridization increases species diversity and ecological adaptability (Jensen et al. 2005; Abbott et al. 2013), and excessive introgressive hybridization results in gene flow and, eventually, species refusion which blear species boundary (Rieseberg and Ellstrand 1993; Runyeon Lager and Prentice 2000). By contrast, reproductive isolation (RI) blocks the formation of hybrids and promotes species isolation (Rogers and Bernatchez 2006; Baack et al. 2015). Most studies on plant RI have focused on only one or a few particular barriers to limit interspecific gene flow, although there are exceptions (e.g., Scopece et al. 2013; Baek et al. 2016; Ma et al. 2016). To determine how species boundaries are maintained between hybridizing species, it is important to understand both the causes and results of hybridization (Furches et al. 2013) and the reproductive barriers that determine the relationship between species boundaries and hybridization of taxa (Widmer et al. 2009; De hert et al. 2012).
Primula L. is a genus of flowering plants with a heterostylous breeding system and extreme species richness, particularly in the eastern Sino-Himalaya region (between 90° and 100° E and 25° to 30° N) (Richards 2003). Only two cases of natural hybridization have been reported in this region (Zhu et al. 2009; Ma et al. 2014). Interspecific hybridization between P. secundiflora Franchet and P. poissonii Franchet was identified using nuclear internal transcribed spacer (ITS) sequences (Zhu et al. 2009). However, the status of the hybrid individuals and interspecific RI were not mentioned. To explore the consequence of hybridization and the maintenance of species boundaries between these two species, we identified the genetic structure of 110 individuals in the sympatric populations using ten SSR (simple sequence repeats) loci. In addition, we conducted field experiments in Shangri-La to evaluate the contribution of various reproductive barriers (pre-pollination isolation: phonological and pollinator-mediated isolation; post-pollination isolation: seed number, viability and germination) to the total RI between these two species (File S1).
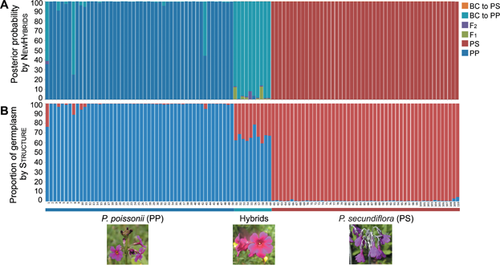
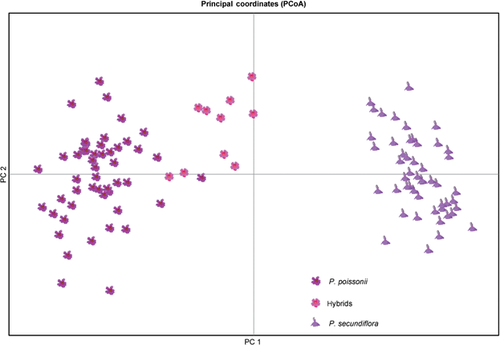
The number of alleles per locus ranged from 5 to 11 (average 7.9); the allele size range and number of alleles per locus are shown in Table S1. Results from the NEWHYBRIDS program suggested that 97 of the 100 morphological parental individuals were pure parental species (with posterior probabilities of ≥90.7%), while the remaining three individuals were backcrosses to P. poissonii. All ten hybrids were backcrosses to P. poissonii (with posterior probabilities of ≥85.8%; Figure 1A). We assigned individuals that had been previously morphologically identified as P. secundiflora to one cluster with high probability (q = 0.993 ± 0.001) using the STRUCTURE software and those that had been previously morphologically identified as P. poissonii to the other cluster with a similarly high probability (q = 0.985 ± 0.005). The mean estimated proportion of P. secundiflora was 0.340 ± 0.017 in the ten hybrids (Figure 1B). P. poissonii and P. secundiflora individuals were separated into two clusters in PCoA (Figure 2).
The total isolation of each species was quite high, i.e., 1.0000 for P. secundiflora and 0.9968 for P. poissonii when it served as mother donor (Table 1). Post-pollination isolation explained 54.70% and 51.76% of the total isolation for P. secundiflora and P. poissonii, respectively, which is a little more than that explained by pre-pollination isolation. Pollinator-mediated barriers and low interspecific seed number contributed the most to the total RI. Post-pollination isolation limited interspecific gene flow when pre-pollination isolation was permeable. Detailed information for each barrier was documented in File S2. Although introgressive hybridization had occurred, species boundaries were maintained by multiple reproductive barriers. As the flowering times of the two species were nearly coincident, flowering time represents only a minor reproductive barrier. Pollinator assemblage mediated barriers contributed an asymmetric moderate isolation, with stronger isolation in P. poissonii, because all the visits to P. secundiflora were from Hymenoptera (bumblebees and Anthophora species), whereas about 30% of visits to P. poissonii were from Lepidoptera (butterflies). These findings suggest that pre-pollination barriers between P. secundiflora and P. poissonii were not complete, in such case, post-pollination barriers would work to restrict hybridization. Here, we showed that interspecific hybridized F1 seed numbers were significantly lower than those for the intraspecific crosses, especially when P. secundiflora was the maternal donor. Furthermore, embryo development failure was common in seeds produced by inter-specific crossing, and the seed viability resulting from hybridization was significantly lower than that in intraspecific crosses, visible under X-ray as empty seeds and stunted embryos. Finally, low germination rate is a known post-pollination barrier preventing hybridization, and similarly, we found low germination rates for hybrid seeds in both P. poissonii and P. secundiflora.
| Components of RI | Absolute contribution to total RI | |||
|---|---|---|---|---|
| Reproductive barriers | P. secundiflora♀ | P. poissonii♀ | P. secundiflora♀ | P. poissonii♀ |
| Phenological | 0.130 | 0.111 | 0.1304 | 0.1111 |
| Pollinator mediated | 0.371 | 0.416 | 0.3226 | 0.3698 |
| Pre-pollination RI | 0.4530 | 0.4809 | ||
| Seed number | 0.980 | 0.704 | 0.5361 | 0.3654 |
| Seed viability | 0.895 | 0.788 | 0.0098 | 0.1211 |
| Seed germination | 0.989 | 0.902 | 0.0011 | 0.0294 |
| Post-pollination RI | 0.5470 | 0.5159 | ||
| Total RI | 1.0000 | 0.9968 | ||
Disturbed habitats might maximize the opportunities for interspecific hybridization (Arnold 1997). A convincing evidence is sunflower hybrid swarms that formed following habitat disturbance due to grazing, and/or trail and road construction (Heiser 1979). In another case, sheep disturbance was believed to be a cause for hybridization of Psidium socorrense and P. sp. aff. Sartorianum (López-Caamal et al. 2014). Grazing activity from livestock is common in the P. secundiflora × P. poissonii populations, and may have created habitat disturbances and favored the formation of hybrids. Once F1s arise, they can backcross to parental species, following a classic pattern of natural hybridization (Arnold 1997; Rieseberg and Carney 1998). The differences between the two parental species in heteromorphic incompatibility might explain the occurrence of backcrosses to P. poissonii. Viable seed was generally set only when pollination occurred between different morphs (termed as “legitimate” crosses in Primula), but in many species illegitimate pollinations (selfs or crosses between plants of the same morph) result in some seed set (Richards 2003). When crosses happened on P. poissonii mothers, more seeds could be produced, while few or no seeds could be formed on P. secundiflora mothers. It is possible that the weak heteromorphic incompatibility system in P. poissonii provided a greater chance for hetero-specific pollen grains to penetrate their stigmas and styles. Similarly, for another pair of Primula species, P. beesiana and P. bulleyana, where the numbers of F1 seeds are substantially lower on P. bulleyana mothers (Ma et al. 2014).
Overall, despite the sympatry, synchronous flowering times and shared pollinators, we found that P. poissonii and P. secundiflora maintained species integrity for long periods of time due to strong RI, reducing the instances of natural hybridization. These naturally hybridizing Primula species, with different incompatibilities, offer a unique chance to understand the evolutionary importance of RI in heterostylous species.
ACKNOWLEDGEMENTS
This research was supported by the National Natural Science Foundation of China (31500194 and U1202261). We would especially like to thank Potatso National Park and the Shangri-La Alpine Botanic Garden for logistical support.
AUTHOR CONTRIBUTIONS
Y. X., X. Z., Y. M., J. Z., and Q. L. designed the research and wrote the manuscript; Y. X., X. Z., and L. L. performed experiments; Y. X. analyzed data and prepared the figures and tables.




