Out of Africa: Miocene Dispersal, Vicariance, and Extinction within Hyacinthaceae Subfamily Urgineoideae
Abstract
Disjunct distribution patterns in plant lineages are usually explained according to three hypotheses: vicariance, geodispersal, and long-distance dispersal. The role of these hypotheses is tested in Urgineoideae (Hyacinthaceae), a subfamily disjunctly distributed in Africa, Madagascar, India, and the Mediterranean region. The potential ancestral range, dispersal routes, and factors responsible for the current distribution in Urgineoideae are investigated using divergence time estimations. Urgineoideae originated in Southern Africa approximately 48.9 Mya. Two independent dispersal events in the Western Mediterranean region possibly occurred during Early Oligocene and Miocene (29.9–8.5 Mya) via Eastern and Northwestern Africa. A dispersal from Northwestern Africa to India could have occurred between 16.3 and 7.6 Mya. Vicariance and extinction events occurred approximately 21.6 Mya. Colonization of Madagascar occurred between 30.6 and 16.6 Mya, after a single transoceanic dispersal event from Southern Africa. The current disjunct distributions of Urgineoideae are not satisfactorily explained by Gondwana fragmentation or dispersal via boreotropical forests, due to the younger divergence time estimates. The flattened winged seeds of Urgineoideae could have played an important role in long-distance dispersal by strong winds and big storms, whereas geodispersal could have also occurred from Southern Africa to Asia and the Mediterranean region via the so-called arid and high-altitude corridors.
Introduction
Plant lineages with disjunct distribution patterns have been the main focus of biogeographical studies. Intercontinental disjunctions could be the outcome of range division (vicariance), range contraction (extinction), and range expansion (geodispersal, long-distance dispersal: LDD) (Mansion et al. 2008). Tectonic movements were involved in the fragmentation of continuous landmasses (such as Pangea fragmentation), which enabled the vicariant origin of disjunct distribution patterns. Gondwana break-up (160–80 Mya; Chatterjee and Scotese 1999) enabled the vicariant origin of disjunct distribution patterns in many plant lineages, as inferred in Crypteroniaceae (Conti et al. 2004; Rutschmann et al. 2004) or Dipterocarpaceae (Ashton and Gunatilleke 1987; Ducousso et al. 2004), and acrodont lizards (Rastegar-Pouyani and Papenfuss 2000). Sometimes tectonic movements are helpful in the elimination of the physical barriers between two separate landmasses (e.g. collision of Afro-Arabian plate with Asia (Li et al. 2009)) and resulted in the formation of land bridges, which assisted geodispersal. The formation of the Gomphotherium Land Bridge between 19 and 18 Mya (Rögl 1998) opened a dispersal route between Africa and Eurasia that enabled extensive faunal and floral exchange via geodispersal mechanism between both areas (Rögl 1998). This geodispersal might have occurred through Balinsky's Arid Corridor (Balinsky 1962; Caujapé-Castells et al. 2001; Linder et al. 2005) as suggested in Androcymbium Willd. (Colchicaceae) and Zygophyllum L. (Zygophyllaceae), or the high-altitude corridor (Linder et al. 2005; Devos et al. 2010) mentioned in Euryops Cass. (Asteraceae). Another explanation of overland expansion during Eocene optimum 52–50 Mya via boreotropical forests has been suggested in some plant groups (Weeks et al. 2005; Muellner et al. 2006). However, diverse studies on disparate plants and animal lineages (Baum et al. 1998; Renner et al. 2000; Waters et al. 2000; Vences et al. 2001; Buckley et al. 2002; Winkworth et al. 2002; Bremer et al. 2004; Janssen and Bremer 2004; Sanmartín and Ronquist 2004; Anderson et al. 2005; Davis et al. 2005; Knapp et al. 2005; Ali et al. 2012; Buerki et al. 2012) have revealed that diversification ages for Afro-Madagascan, Afro-Asian, and Afro-Mediterranean disjunctions are too young to be explained by Gondwana fragmentation. These studies highlight the role of LDD over existing barriers (Lomolino et al. 2006) in disjunct distribution patterns, contrary to the concept of rafting via India and dispersal through boreotropical forests.
Biogeographic studies suggest different mechanisms to understand the current disjunct distributions in various plant lineages. A vicariance event (aridification of Africa) was suggested to explain current disjunct patterns in Adenocarpus DC. (Sanmartín et al. 2010), whereas dispersal was the main mechanism involved in Androcymbium (del Hoyo et al. 2009) and Campanulaceae (Roquet et al. 2009). Dispersal from the Mediterranean region to Southern Africa was proposed by some authors (Roquet et al. 2009; Sanmartín et al. 2010), whereas dispersal from Southern Africa to the Mediterranean region was suggested by other authors (Galley et al. 2007; Sanmartín et al. 2010). Moreover, Zhou et al. (2012) presumed dispersal from Africa to Asia and Madagascar.
Subfamily Urgineoideae consists of over 100 species (Manning et al. 2004) and is distributed in temperate and tropical regions in Africa, Madagascar, the Mediterranean region, and Asia (Mwafongo et al. 2010). Generic circumscription in Urgineoideae is still a matter of controversy. Manning et al. (2004) accepted three genera (Bowiea Harv., Drimia Jacq., and Igidia Speta) in the subfamily. More analytical treatments accept between 8 and 21 genera, which are more homogeneous in morphology (cf. Speta 1998; Williams 2000; Pfosser et al. 2012), although few of them still remain para- or polyphyletic. More recently, Wetschnig et al. (2007) have demonstrated that Igidia (a monotypic genus from Madagascar) belongs to Ornithogaloideae, a related subfamily of Hyacinthaceae.
Pfosser and Speta (1999) suggested a Southern Gondwana origin in Urgineoideae, although no detailed biogeographic studies were conducted. The current distribution pattern in this subfamily shows evidences of Afro-Madagascan, Afro-Mediterranean, Afro-Indian and indo-Mediterranean disjunctions. In their recent study on Hyacinthaceae, Buerki et al. (2012) suggested that Urgineoideae originated in sub-Saharan Africa approximately 48 Mya. They identified dispersal events from sub-Saharan Africa to the Cape region, the Mediterranean region, and Asia. Interestingly, they mentioned that all dispersal to the Mediterranean Basin took place before 20 Mya, which indicates disruption of the link between sub-Saharan Africa and the Mediterranean Basin before Mid-Miocene Climatic Optimum (MMCO). They also suggested the role of the Sahara Desert in the current disjunct distribution pattern in this group between sub-Saharan African and the Mediterranean region. Moreover, these same authors suggested the important pivotal role of the Middle East area as a crossroad between the Northern Hemisphere areas. However, Buerki et al. (2012) conclude that their study was based on only approximately 25% of taxa of Hyacinthaceae, and similar studies in additional groups are necessary to assess the general validity of these biogeographic patterns.
The aims of the present study are to detect the potential ancestral range of Urgineoideae and to test the dispersal/vicariance hypotheses with Bayesian divergence time estimation.
Results
Bayesian age estimation and biogeographic inferences
Tree topologies of Urgineoideae were generated by BEAST ver. 1.7.5. Node 1 (PP = 1.00; Figure 1) represents the split between Bowiea volubilis Harv., and the remaining members of Urgineoideae, and C (Southern Africa) is the possible ancestral range at this node. Node 2 consists of the members of Urgineoideae from the Mediterranean region and NW Africa/SW Asia (India), and is strongly supported (PP =1.00). This node also indicates the split between NW African/SW Asian and Mediterranean members. Node 3 represents the colonization of E (NW Africa) from C (Southern Africa), and node 4 indicates possible colonization of H (SW Asia) via NW Africa/Eastern Africa, whereas nodes 5, 7, and 9 indicate colonization of D (Eastern Africa) from C (Southern Africa; Figure 1). Node 6 suggests a second colonization of the Western Mediterranean region and it is well supported (1.00), and further expansion occurred towards Eastern Mediterranean region. Node 8 (PP = 1.00) consists of all Madagascan members of Urgineoideae.
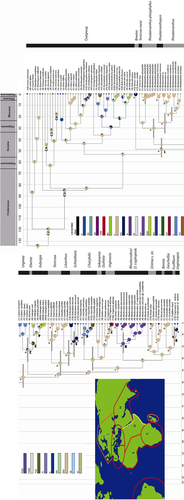
Bayesian posterior probabilities of 95% are shown by asterisks, whereas those between 85% and 94% are indicated by stars. Gray bars indicate 95% highest posterior density interval (see Table 1 for details). Legend color respresents possible ancestral ranges at different nodes (other (black color) means, a number of ancestral ranges with less than 0.001% marginal probability). Inset: Biogeographical areas used in BBM analysis. A, South America; B, Madagascar; C, Southern Africa; D, Eastern Africa; E, NW Africa; F, Western Mediterranean; G, Eastern Mediterranean; H, SW Asia and I, Europe. Nodes 1–9 are discussed in the text. The generic delimitation (Speta 1998) is shown along the vertical bar. Node C 1 to C 6 indicates the calibrated node; C 1 calibrated at 134 Mya (the root node, represent the split between Acorus and remaining monocots), C 2 at 119 Mya (the oldest age of Asparagales and consistent with oldest monocot fossil, see Ramírez et al. 2007 for details), C 3 at 85 Mya (geological constraint representing upper bound age, for Protarum sechellarum Engl., consistent with the age of a geological event, the formation of Seychelles archipelago (Braithwaite 1984)), C 4 at 45 Mya (Colocasieae, lower bound fossil constraint), C 5 at 32 Mya (subtribe Goodyerinae, lower bound fossil constraint) and C 6 at 18 Mya (Arisaema triphyllum (L.) Schott. lower bound fossil constraint).
The mean ages, ancestral ranges, marginal probability, and 95% highest posterior density (HDP) intervals (95% HDP upper and lower bound) are indicated in Table 1. The coefficient of variation is 0.81 (95% HDP interval, 0.49–1.16), which suggests the substitution rate heterogeneity across the tree and favors a relaxed clock approach to the data. The mean rate of evolution is 0.001 substitutions per site per million years (95% HDP: 7.4 × 10−4 to 1.3 × 10−3). Yule speciation rate (birth rate) is 0.11 (95% HDP: 0.07–0.16).
| Nodes | Age estimation (Mya) | BBM | DEC | Support (PP) | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | 95% HPD lower | 95% HPD upper | AR | MP (%) | AR | MP | ||
| 1 | 48.9 | 38.7 | 56.2 | C | 86.57 | C | 100 | 1.00 |
| 2 | 21.6 | 11.7 | 33.0 | C | 55.60 | EF | 60 | 1.00 |
| 3 | 16.3 | 07.6 | 26.1 | E | 60.34 | E | 64 | 0.75 |
| 4 | 07.6 | 02.0 | 16.0 | H | 89.60 | EH | 42 | 1.00 |
| 5 | 14.5 | 07.2 | 26.4 | CD | 82.61 | C | 78 | 1.00 |
| 6 | 08.9 | 02.7 | 18.2 | F | 91.73 | F | 64 | 1.00 |
| 7 | 05.2 | 01.1 | 10.3 | C | 86.52 | C | 46 | 1.00 |
| 8 | 16.5 | 08.2 | 24.6 | B | 92.74 | B | 83 | 1.00 |
| 9 | 11.3 | 03.2 | 18.2 | C | 65.25 | C | 100 | 0.60 |
- Ancestral ranges (AR) and marginal probabilities (MP) are obtained by Bayesian binary method (BBM) and dispersal–extinction–cladogenesis model (DEC) analyses.
The biogeographic reconstructions obtained by the Bayesian binary method (BBM, Yu et al. 2011; Figure 1) and the dispersal-extinction-cladogenesis model (DEC, Ree et al. 2005; Ree and Smith 2008) (Figure S1) analyses show the possible ancestral ranges at each node in the phylogeny of Urgineoideae (Figures 1, S1). The present distribution pattern of Urgineoideae could be explained by 50 (BBM) or 45 (DEC) dispersal events.
Urgineoideae originated in Southern Africa (C) with an 86.57% marginal probability value approximately 48.9 Mya (95% HDP: 56.2–38.7) in Eocene, as evident from the ancestral state reconstructions at node 1 (Figure 1), and this node is strongly supported (PP = 1.00). After in situ diversification in Southern Africa, dispersal occurred to Eastern Africa, the Mediterranean region, Madagascar, and SW Asia (indicated by nodes 2–9). One dispersal event is identified at node 1, from Southern Africa to Eastern Africa, and this dispersal event occurred between 49 (stem node) and 0.74 Mya (crown node). The most favored ancestral ranges at nodes 2, 3, and 4 are C (Southern Africa), E (NW Africa), and H (SW Asia), respectively. The frequency of occurrence of these ranges is 55%, 60%, and 100%, respectively (Table 1). The inferred ancestral ranges at nodes 2, 3, and 4 postulate dispersal from C to E, F, and H between 21.7 (95% HDP: 33.0–11.7) and 7.6 Mya (95% HDP: 16.0–2.0). The result of Lagrange analysis suggests that the favored ancestral ranges at nodes 2, 3, and 4 are EF, E, and EH, respectively. Lagrange analysis indicates an early dispersal from C to EF between 42 and 21 Mya. Thus, the possible geodispersal route from Southern Africa to the Western Mediterranean region and SW Asia is, C → D → E → F and E → H. The E → H dispersal occurred between 16 and 8 Mya, probably via the Gomphotherium Land Bridge. A vicariance event at node 2 divided the Mediterranean and NW African/Asian lineages shown by time–event curve (TEC) analysis (Figure 2). An extinction event in Southern Africa/Eastern Africa was indicated by TEC analysis between 21 and 16 Mya (Figure 1). At node 6 after the colonization of the Western Mediterranean region, dispersal was identified towards the Eastern Mediterranean region approximately 6.5 (95% HDP: 13.2–1.9) and 4.0 Mya (95% HDP: 10.3–0.4). Dispersal from Southern Africa to Eastern Africa are identified at nodes 5, 7, and 9 (between 15.0 (95% HDP: 26.5–7.2) and 5. Mya (95% HDP: 10.3–1.1)).
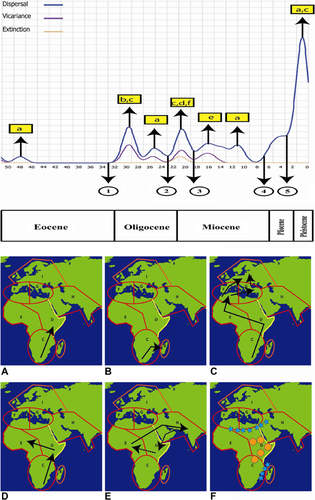
In TECs, X-axes represent ages in Mya, whereas Y-axes indicate dispersal/vicariance/extinction events. (1–2) Direction of oceanic currents from Africa to Madagascar. (2–4) Formation of Gomphotherium land, uplift of the East Africa/Tibet, aridification of Africa/Central Asia/North China, and Miocene thermal maximum. (4) Messinian salinity crisis. (5) Palaeogeographic reorganizations in the circum-Mediterranean region. Legend color represents dispersal, vicariance, and extinction events.
(A) Dispersal to Eastern Africa, (B) to Madagascar, (C) to the Western Mediterranean and then to Eastern Mediterranean region and Europe, (D) to Eastern and NW Africa, and (E) to Asia and back to Eastern and NW Africa. Vicariance and extinction events (F) are indicated with blue stars and orange polygons, respectively. Yellow rectangle indicates dispersal scenarios shown in world maps above and below the TEC.
At node 8 (Figure 1), the possible ancestral state is B (Madagascar) and this probably indicates a single trans-oceanic dispersal event from Southern Africa (C) to Madagascar with high posterior probability support (1.00). This dispersal took place between 30.6 (95% HDP: 39.5–22.9) and 16.6 Mya (95% HDP: 24.7–8.2), as shown in Figure 1.
TEC
Time–event curve analysis (Figure 2) indicates two phases of dispersal events before and after MMCO (16 Mya). In the first phase (before MMCO), dispersal and vicariance events are identified during Oligocene/Early Miocene, and these dispersal events occurred from Southern Africa to NW Africa, the Western Mediterranean region, Asia, and Madagascar. In this time, vicariance events separated Afro-Mediterranean and Afro-Madagascan lineages, respectively. An extinction event is also identified in this phase in Southern and Eastern Africa. In the second phase (after MMCO) during Late Miocene/Pliocene/Pleistocene, only dispersal events are identified. In this phase, dispersal occurred from Southern Africa to Eastern Africa and from SW Asia to Eastern and NW Africa, whereas dispersal occurred from the Western Mediterranean region to the Eastern Mediterranean region and Europe. This method also indicates the role of Miocene geological and climatic changes in the expansion and disjunction in Urgineoideae. Dispersal to the Mediterranean region and Asia occurred between 22 and 14 Mya (Figure 1), which is consistent with the Mid-Miocene thermal maximum (17–15 Mya) and formation of the Gomphotherium Land Bridge (19–18 Mya). A vicariance event (Figure 2) took place approximately 15–13 Mya due to increasing aridification in Africa-Asia and it is consistent with the Late Miocene cooling. The TEC method indicates an extinction event approximately 21–19 Mya (Figure 1) in Southern and Eastern Africa.
Discussion
Processes, such as range division (vicariance), range expansion (geodispersal), and range contraction (extinction) are linked to tectonic plate movements and numerous studies highlighted the role of these processes in the creation of the disjunct distribution patterns. The separation of continuous landmasses termed as “range division” (such as fragmentation of Gondwana) enables the vicariant origin of disjunct distribution pattern in diverse plant and animal lineages. Furthermore, the movements of tectonic plates sometimes help to reduce or eliminate the physical barriers between two land masses (formation of the Gomphotherium Land Bridge) and enables dispersal origin of disjunct distribution pattern, a process that was termed “geodispersal” by Lieberman (2000). The current disjunct distribution pattern in Urgineoideae could not be the outcome of either Gondwana fragmentation or dispersal through boreotropical forests due to younger age estimation in this study (49 Mya), as well as in the study of Buerki et al. (2012, 48 Mya).
Diversification in Southern Africa
The in situ diversification in Southern Africa during Oligocene and Miocene resulted in many Southern African subclades such as Bowiea, Drimia, Fusifilum Raf., Geschollia Speta, Litanthus Harv., Rhadamanthus Salisb., Rhadamanthus platyphyllus B. Nord., Rhadamanthopsis (Oberm.) Speta, Sekanama Speta, Schizobasis Baker, Tenicroa Raf., Tenicroa nana Snijman, Urginavia Speta, and Urgineopsis Compton. After dispersal from Southern Africa, the descendant underwent local diversification in Central Africa (Ebertia Speta and Duthiea Speta), the Western Mediterranean region (Urginea Steinh. and Charybdis Speta), SW Asia (India) (Indurgia Speta), and Madagascar (Rhodocodon Baker). These dispersal events and subsequent local diversification resulted in Afro-Mediterranean, Afro-Asian (Indian), and Afro-Madagascan disjunct distribution patterns in Urgineoideae.
Dispersal from Southern Africa to Eastern and Western Africa
The most recent common ancestors of subclades Bowiea, Schizobasis, and Urgineopsis are distributed in Southern and Eastern Africa. Sanmartín et al. (2010) suggested that Southern Africa has the highest dispersal rate with Eastern Africa. Galley et al. (2007) proposed dispersal from Southern Africa to high-altitude (Afromontane) tropical-latitude regions via the Drakensberg Mountains in Moraea Mill. (Iridaceae). Our results indicate eight independent dispersal events from Southern Africa to Eastern Africa between 48.9 (95% HDP: 56.2–38.7) and 0.7 Mya (95% HDP: 2.6–0.1). These dispersals occurred in two phases, before and after MMCO (16 Mya). The dispersal events before MMCO penetrated up to the Western Mediterranean region as indicated by two dispersal events suggested by DEC (40–30 Mya) and BBM (30–21 Mya) analyses. The dispersal events after 5 Mya (after MMCO) occurred to Eastern Africa and two dispersal events (at nodes 4 and 7) ran up to NW Africa. However, further penetration to the Mediterranean region is not identified in this study and formation of the Sahara Desert approximately 7 Mya (Schuster et al. 2006) could explain this disruption.
Sanmartín et al. (2010) mentioned a lower rate of biotic exchange between Southern and Northern Africa via Western Africa and an older disjunction between these areas, compared to Eastern and NW African ones. Our result indicates a dispersal event from Southern Africa to NW Africa from 21.4 (stem node, 95% HDP: 33.0–11.7) to 16.3 Mya (crown node, 95% HDP: 26.0–7.6). Dispersal to NW Africa occurred through two routes, either directly from Southern Africa or via Eastern Africa. If direct dispersal between Southern Africa and NW Africa occurred, then low biotic exchange and earlier disjunction between these areas invoke LDD to explain the presence of Urgineoideae in NW Africa. An alternative explanation suggests dispersal from Southern Africa to NW Africa via Eastern Africa. However, this alternative route of dispersal is more plausible due to recent dispersal events (between 5 and 1 Mya) as indicated by nodes 5, 7, and 9 (Figure 1). The route of dispersal suggested at node 7 is C → D → E, whereas another dispersal running towards NW Africa via Eastern Africa is indicated at node 4 (leading downwards), corresponding to H → D → E.
Dispersal from Southern Africa to the Western Mediterranean region and Asia
The warm and dry climatic conditions, which prevailed in the Western Mediterranean region compared with the humid conditions in the Eastern Mediterranean region during Miocene (Kovar-Eder 2002), favored open xeric vegetation in the Western Mediterranean, which were suitable for colonization by members of Urgineoideae. Dispersals have been suggested from sub-Saharan Africa to the Mediterranean Basin (Ali 2012; Buerki et al. 2012) during Early Miocene. Buerki et al. (2012) suggests that these dispersal events took place before 20 Mya, whereas Ali et al. (2012) suggest a single dispersal event from sub-Saharan Africa to the Mediterranean region between 19 and 17.92 Mya in Hyacinthoideae.
We suggest that the Western Mediterranean, and not the Eastern Mediterranean, was first colonized by Southern African lineages via two independent dispersal events, as suggested by the ancestral reconstructions at nodes 2 and 6 (Figure 1), which are strongly supported (PP = 1.00). Pfosser and Speta (2004) suggested a similar pattern in Hyacinthaceae, the members of which reached the Western Mediterranean region via NW Africa during Oligocene–Miocene, based on the existence of diploid populations (probably ancestral) of Urginea fugax (Moris) Steinh. and Charybdis in the Western Mediterranean. Similarly, the members (Albuca L., Battandiera Maire, Dipcadi Medik., Nicipe Raf., and Stellarioides Medik.) of the sister group Ornithogaloideae are disjunctly distributed in Eastern and NW Africa, as well as some representatives of Battandiera, Dipcadi, and Stellarioides in Morocco and Algeria (cf. Martínez-Azorín et al. 2010; 2011), suggesting a dispersal route connecting Southern Africa and the Western Mediterranean region through Eastern and NW Africa. To understand these routes of dispersal, extensive sampling from Eastern and NW Africa is necessary. The dispersal event indicated by ancestral reconstructions at node 2 occurred between 21.5 (stem node) and 8.5 Mya (crown node), whereas the dispersal suggested at node 6 occurred between 30.0 (stem node) and 9.0 Mya (stem node). Many recent studies suggested similar dispersal patterns (Coleman et al. 2003; del Hoyo et al. 2009; Sanmartín et al. 2010; Ali et al. 2012; Buerki et al. 2012).
Bayesian binary method analysis at nodes 2 and 3 pointed out two alternative scenarios to explain the possible geodispersal routes between Southern Africa, the Western Mediterranean region, and NW Africa/SW Asia. These alternative scenarios are: (i) C → E and then F ← E → H; and (ii) C → D → E→ and then F ← E → H. The early divergence time estimates for NW Africa/SW Asia compared to the Western Mediterranean region indicates the northward route of dispersal from Southern Africa to the Western Mediterranean region probably via Eastern and NW Africa. Our analysis suggests the highest dispersal events (eight dispersals) from Southern Africa to Eastern Africa, and it is consistent with the higher dispersal rates between Southern Africa and Eastern Africa proposed by Sanmartín et al. (2010). The same authors also mentioned the highest dispersal rates between Eastern and NW Africa.
The congruence between geological and dispersal events highlight the geodispersal explanation of disjunct distribution patterns, and incongruity invokes the LDD explanation. The dispersal, vicariance, and extinction events during Early Miocene, between 20 and 16 Mya (Figure 2), are congruent with the geological and climatic events, such as the uplift of the East African mountains/Himalaya, and the formation of the Gomphotherium Land Bridge, and thus invoke a geodispersal mechanism to explain the current disjunct distribution pattern in Urgineoideae. This dispersal probably occurred via the Arid Corridor (Caujapé-Castells et al. 2001; Linder et al. 2005; Bellstedt et al. 2012) and the High-Altitude Corridor (Linder et al. 2005; Devos et al. 2010), as inferred in Androcymbium and Euryops, respectively. An extinction event in Early Miocene, between 20 and 16 Mya in Southern Africa and Eastern Africa, diminishes the importance of this dispersal route, which connected Southern Africa and the Western Mediterranean via Eastern Africa. However, two dispersal events (nodes 4 and 7) from H to E (node 4) and C to E (node 7) via D during Pliocene and Pleistocene evidence the important role of Eastern Africa as a dispersal corridor to the north.
The ancestral reconstruction at nodes 2, 3, and 4 suggests a possible dispersal event from Southern Africa to SW Asia (India) via NW Africa or Eastern Africa between 16.3 (stem node) and 8.0 Mya (crown node), and this dispersal event is consistent with the formation of the Gomphotherium Land Bridge (Rögl 1998, 1999) during Early Miocene, due to the collision of the Afro-Arabian plate with Asia (Li et al. 2009), which enabled floristic exchange between Africa and Eurasia. Recently, Zhou et al. (2012) observed a similar pattern of African origin and dispersal to Asia (between 18 and 16 Mya) in Uvaria L. (Annonaceae). Dispersal has been suggested from Asia to Africa in Bridelia Willd. (Phyllanthaceae) (Li et al. 2009), Macaranga Thouars and Mallotus Lour. (Euphorbiaceae) (Kulju et al. 2007). A dispersal event from SW Asia (India) to Eastern and then NW Africa (node 4) is also identified between 5 and 1 Mya.
Time-event curve analysis also suggests vicariance and an extinction event before MMCO, which on the one hand separated the Mediterranean and Asian/NW African lineages, on the other hand NW African and SW Asian (Indian) lineages. Volcanism linked to African mantle plume activity (African superswell) between 40 and 25 Mya (McDougall and Brown 2009), uplift of Eastern Africa and formation of East African Rift System (EARS) between 45 and 18 Mya (McDougall and Brown 2009), slow and progressive aridification of Africa during Miocene (Bellstedt et al. 2012), and formation of the Sahara Desert (7 Mya (Schuster et al. 2006)) could explain vicariance and extinction events.
Dispersal from the Western Mediterranean to Eastern Mediterranean
The presence of diploid populations (ancestral population) of Charybdis and Urginea in Morocco suggests that the members of Urgineoideae invaded the Western Mediterranean during Early Miocene (Pfosser and Speta 1999). Closure of the Gibraltar Straight (Messinian salinity crisis (Krijgsman et al. 1999)) and a series of palaeogeographic reorganizations in the Mediterranean region (Scotese et al. 1988, separation of the Red Sea from the Mediterranean; Rögl 1998) facilitated the formation of new dispersal routes in the Pliocene (5 Mya) between Africa and Eurasia (Thompson 2000; Fernandes et al. 2006). Extensive examples of dispersal events through this route have been suggested in Campanulaceae (Roquet et al. 2009), Hyacinthoideae (Ali et al. 2012), Hyacinthaceae (Buerki et al. 2012), and other plant groups (Mummenhoff et al. 2001; Mansion et al. 2008; Désamoré et al. 2011). Most of the dispersal events between Western and Eastern Mediterranean regions occurred between 6 and 1 Mya (Figure 1, node 6), which is consistent with the palaeogeographic changes in the Mediterranean basin.
Dispersal from Southern Africa to Madagascar
The members of Urgineoideae (node 8) in Madagascar form a well-supported (PP = 1.00) subclade (Figure 1) as also shown by Pfosser et al. (2012). The presence of Urgineoideae in Madagascar could be explained either by a single dispersal or vicariance event as suggested in Didieriaceae, Sarcolaenaceae, Spherosepalaceae, Asteropeiaceae, and Physenaceae (Yoder and Nowak 2006). The vicariance explanation is not probable because the mean age estimated for the Madagascan subclade (node 8) is 16.6 Mya, which is much younger than the African and Madagascar separation (121 Mya; Rabinowitz et al. 1983). The incongruity between geological events and origin of species invoke LDD to explain the present distribution patterns.
Diverse studies focusing on dispersal between Africa and Madagascar suggest a different time window for these dispersals. Zhou et al. (2012) pointed to a dispersal event in Uvaria from Africa to Madagascar between 17 and 12.1 Mya. Other studies such as on frogs (Vences et al. 2003, 2004), chameleons (Raxworthy et al. 2002), snakes (Nagy et al. 2003), lemurs (Yoder and Yang 2004), and freshwater crabs (Daniels et al. 2006; Cumberlidge et al. 2008; Klaus et al. 2006) suggest LDD between Africa and Madagascar. Daniels et al. (2006) commented that freshwater crabs reached Madagascar between 43.5 and 39 Mya, whereas Klaus et al. (2006) submitted that this dispersal took place approximately 28 Mya. Bayesian divergence time estimation, BBM, and DEC analyses indicate that this dispersal event occurred in Urgineoideae between 30.6 (95% HDP: 39.5–22.9, age of stem node) and 16.6 Mya (95% HDP: 24.7–8.2, age of crown node), it being similar to the dispersal time estimated for Uvaria (Zhou et al. 2012).
Ali and Huber (2010) suggested a strong anticlockwise gyre directed from the African coast eastwards to Madagascar rather than southwards through the Mozambique channels, towards the Agulhas current, as it occurs today. They mentioned an enhanced eastward flow (velocities > 20 cm/s) from NE Mozambique and Tanzania to the northern coast of Madagascar, it occurring for 3 and 4 weeks in a century, and which could cross the distance in 25–30 days. The direction of these oceanic currents enabled LDD via rafting on large vegetation mats (Ali and Huber 2010; Pfosser et al. 2012; Zhou et al. 2012) from the mainland to Madagascar. However, in Early Miocene, the current was directed from Madagascar towards Africa and made the journey of mammals to Madagascar difficult (Ali and Huber 2010). McCall (1997) suggested the existence of a nearly continuous land bridge between Madagascar and Africa from the 45 to 26 Mya. Thus, dispersal to Madagascar could have occurred through birds, strong winds, oceanic currents, and land bridges in different times.
Role of seed morphology in dispersal efficiency in Urgineoideae
Long-distance dispersal over existing barriers (Lomolino et al. 2006) has been suggested for Melastomataceae (Renner 2004) and Simaroubaceae (Clayton et al. 2009) by winds and birds. Zhou et al. (2012) mentioned the role of birds (Hornbills and the Trumpet Manucode bird of paradise) in Uvaria seed dispersal, however, the dispersal via wind and water are marginal in this genus. In Urgineoideae, dispersal via birds could be considered as a rare phenomenon and it may only occur in particular cases, because no special adaptations to ornithochory are observed in their diaspores. For instance, seeds of urgineoid species growing in wetlands or near these habitats could be mixed with mud and could be attached to feathers or legs of birds, this allowing occasional dispersal over long distances.
After in situ diversification in Southern Africa, members of Urgineoideae colonized the Western Mediterranean region, Asia (India), NW Africa, and Madagascar. Seeds in Urgineoideae (Urginea, Indurgia, and Rhodocodon) are light, very thin, flattened, and somewhat winged (Figure 3). Anemochory must be understood as the most plausible dispersal mechanism, because strong winds and big storms could move seeds over long distances. A similar pattern of dispersal is also observed in Ornithogaloideae, in which tribes Albuceae and Dipcadieae show flattened and winged seeds that favor LDD by winds, and that is probably the reason why four different lineages (Albuca, Battandiera, Dipcadi, and Stellarioides) colonized the Northern Hemisphere independently (Martínez-Azorín et al. 2010).

(A) Seed morphology of taxa has distribution in the Mediterranean region and Madagascar.
(B) Seed morphology of taxa having distribution in Southern, Eastern, and NW Africa.
(C) Seed morphology of taxa having distribution in Southern Africa (Ilg 2010).
Materials and Methods
Taxonomic sampling
The material of Urgineoideae published in Wetschnig et al. (2007), Buerki et al. (2012), Pfosser et al. (2012), our own data (mentioned in Table S1), and obtained from the European Molecular Biology Laboratory database were used to test biogeographic hypotheses. This study is based on approximately 45% of taxa of Urgineoideae, which represents all the clades and considerably increases the available information on the subfamily Urgineoideae. The analyses were based on the combined data matrix (trnCGCA-ycf6 intergenic region + trnL-intron + trnL-F intergenic spacer IGS + rbcL + matk) of 121 taxa (79 ingroup and 42 outgroup). Twenty-nine members of closely related subfamilies and 13 taxa (for node calibration) were selected as the outgroup. The generic delimitation (Speta 1998) is shown in Figure 1 along the vertical bar. Information on the distribution areas of terminal taxa was obtained from previous studies (Pfosser and Speta 1999; Pfosser et al. 2003, 2006, 2012; Manning et al. 2004; Buerki et al. 2012).
Phylogenetic analysis and divergence time estimation
The alignment of the DNA sequences was performed with Clustal X (Jeanmougin et al. 1998) by applying pairwise multiple alignment parameters. On average, less than 1% of data matrix cells were scored as missing data and ambiguously aligned sequences were excluded.
BEAST ver. 1.7.5 (Drummond et al. 2002, 2012) was applied to obtain the tree topology, substitution rates, and divergence time estimation with Yule tree prior (Yule 1924) and uncorrelated lognormal distribution, under the GTR + I + G model, it being selected by jmodeltest ver. 0.1.1 (Guindon and Gascuel 2003; Posada 2008). Two independent Markov chain Monte Carlo analyses were conducted for one hundred million steps. Trees were sampled every 2,000 generations. Convergence of the stationary distribution and sampling adequacy was checked on Tracer ver. 1.5 (Rambaut and Drummond 2009). When the effective sample size of all parameters was above 200, the result was considered reliable. Logcombiner ver. 1.7.5 was used to combine these two independent runs. After discarding the first 5,000 trees as burn-in, the samples were summarized in the maximum clade credibility tree using TreeAnnotator ver. 1.7.5 (Drummond et al. 2012) with the posterior probability, substitution rates, mean node age, and 95% highest posterior density intervals. The results were visualized using Fig.tree ver. 1.4 (Rambaut 2012). The obtained Bayesian tree is shown in Figure 1.
Node calibration
The phylogenetic tree is calibrated on the basis of fossil records, geological events, and secondary calibration (Forest 2009). Fossil calibration is by far the best method to transform the relative time estimates into absolute ages (Magallón 2004), using single or multiple calibration points, though the latter is preferred (Forest et al. 2005).
The split between Acorus L. and other monocots is estimated to be 134 Mya (Bremer 2000) or between 127 and 141 Mya (Wikström et al. 2001) based on multiple fossil calibrations (Meng et al. 2008). In the absence of fossil records in Urgineoideae, we calibrated the crown age of monocots as 134 Mya, which was also adopted in various other studies (Janssen and Bremer 2004; Good-Avila et al. 2006; Meng et al. 2008). We constrained the minimum crown node (C 2) age of Asparagales at 119 Mya based on the oldest age of the order (see Janssen and Bremer 2004; Meng et al. 2008), and this age is also congruent with the oldest known monocot fossil (see Ramírez et al. 2007). We also used three lower bound fossil constraints to calibrate node C 4 (Colocasieae, minimal age 45 Mya; see Renner and Zhang 2004 for details), node C 5 (subtribe Goodyerinae, minimal age 32 Mya; see Ramírez et al. 2007 for details) and C 6 (Arisaema triphyllum (L.) Schott., minimal age 18 Mya; see Renner and Zhang 2004 for details). One geological constraint was used to calibrate the node C 3 (Protarum sechellarum Engl. maximum age 85 Mya; see Renner and Zhang 2004 for details, consistent with the age of a geological event, the formation of the Seychelles archipelago (Braithwaite 1984)).
TEC
In TEC analysis, events of extinction, dispersal, and vicariance are assigned to a time frame, in which peaks represent dispersal, vicariance, and extinction events, whereas valleys represent geological events. The TEC was obtained by using the tree file generated by BEAST ver. 1.7.5, and applying the TEC method in RASP (Figure 2).
Biogeographic analyses
The distribution range of Urgineoideae and Oziroe Raf. was divided into nine areas, on the presence of one or more endemic species. These areas are: A (South America), B (Madagascar), C (Southern Africa), D (Eastern Africa), E (NW Africa), F (Western Mediterranean region), G (Eastern Mediterranean region), H (SW Asia), and I (Europe) as shown in Figure 1 and are based on the current distribution of Urgineoideae.
The model-based approaches, BBM (Yu et al. 2011) and DEC (DEC, Ree et al. 2005; Ree and Smith 2008), were used to infer the biogeographic histories of Urgineoideae. In the BBM method, binary character states are used to code the geographic range of the terminal taxa (OTUs) as being present (1) or absent (0) in each unit area. For instance, if OUT “x” occurs in area AC and the total range of all terminal taxa is ABCD, then the geographic range of “x” is 1 010. The process of inferring ancestral states was as same as which in Mrbayes ver. 3.12. We enforced a topological constraint corresponding to each node using our Bayesian tree and then ancestral states for each node were reported. Two independent runs of 10 chains with a temperature of 0.1 were run for 1 million generations and sampled every 100 generations. A distance between runs of less than 0.01 was used as an indicator of convergence (Moyle et al. 2012). In the DEC model (Lagrange), branch length information of a given tree is required. Maximum clade credibility tree was used as input for both BBM and DEC analyses. Lagrange analysis was conducted using M0 and M1 models. In M0 (not shown), area connection and maximum areas per range were unconstrained, while in M1 (Figure S1), a maximum of two areas was allowed in ancestral ranges.
Acknowledgements
We are thankful to Richard M. K. Saunders for valuable suggestions. We thank S. Buerki for providing DNA sequences. Karl Franzens University Graz (Austria) and Biocenter Linz (Austria) kindly provided technical support and working facilities. We are also thankful to the Higher Education Commission of Pakistan for providing financial support for the study.




