Beyond a vestigial organ: effects of the appendix on gut microbiome and colorectal cancer
Abstract
The role of appendectomy in the pathogenesis of colorectal cancer (CRC) is a recent topic of contention. Given that appendectomy remains one of the most commonly performed operations and a first-line management strategy of acute appendicitis, it is inherently crucial to elucidate the association between prior appendectomy and subsequent development of CRC, as there may be long-term health repercussions. In this review, we summarize the data behind the relationship of CRC in post-appendectomy patients, discuss the role of the microbiome in relation to appendectomy and CRC pathogenesis, and provide an appraisal of our current understanding of the function of the appendix. We seek to piece together the current landscape surrounding the microbiome and immunological changes in the colon post-appendectomy and suggest a direction for future research involving molecular, transcriptomic, and immunologic analysis to complement our current understanding of the alterations in gut microbiome.
Introduction
Colorectal cancer (CRC) is the third most common cancer worldwide and plays a significant and increasing role in cancer mortality, in both developing and developed populations.1 While genetics play a significant role in contributing to the development of CRC, environmental and lifestyle factors additionally constitute a major component of risk.2 Appendicitis is a common cause of acute abdomen with an estimated lifetime risk of 7–8%, comprising a substantial number of annual deaths and years of life lost in developing countries, as well as visits to the emergency department in both developed and developing countries alike.3 The role of appendectomy in the pathogenesis of CRC has been a recent topic of contention, given the preexisting assumption of the appendix as a vestigial organ with little to no usage, and the recommendation of appendectomy as first-line management in cases of appendicitis. Current surgical guidelines recommend appendectomy as an ideal first-line management strategy for most appendicitis presentations, although in recent years conservative medical management with antibiotics has been considered as a viable substitute, especially in patients with uncomplicated, simple appendicitis (without phlegmon, abscess, perforation, or appendicolith).4 Even in cases whereby medical management with antibiotics was successful, interval appendectomy is still considered as a means of definitive treatment and reducing the rate of subsequent recurrences.5 As one of the most commonly performed operations, it is inherently crucial to elucidate the crux of the association between prior appendectomy and subsequent development of CRC, if any, as there may be long-term health repercussions.
In this review, we summarize the data behind the relationship of CRC in post-appendectomy patients, discuss the role of the microbiome in relation to appendectomy and CRC pathogenesis, and provide an appraisal of our current understanding of the function of the appendix. We seek to piece together the current landscape surrounding the microbiome and immunological changes in the colon post-appendectomy and suggest a direction for future research involving molecular, transcriptomic, and immunologic analysis to complement our current understanding of the alterations in gut microbiome.
Epidemiological observation of the relationship between previous appendectomy and CRC
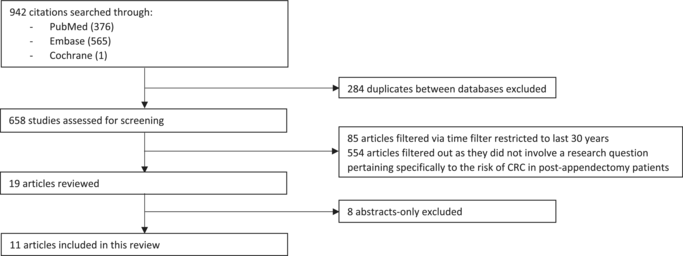
We performed a literature review to appraise the current literature regarding the risk of CRC in post-appendectomy patients. We searched the Medline, EMBASE, and Cochrane databases and consolidated a list of papers which fit our research question; only peer-reviewed full publications were considered; abstracts and conference submissions were excluded from our search. To maintain relevancy, papers older than 30 years were also excluded from our review. We were able to collate and review 11 selected papers, which consisted of case series and cohort studies, as well as a recent meta-analysis. A list of the search terms utilized and a flowchart of the review can be viewed below under Appendix A.
Our review showed a general trend toward an increased risk of CRC in post-appendectomy patients. An increased risk of CRC in post-appendectomy populations were statistically significant in five out of nine studies comprising of case–control and cohort studies that yielded data on observed CRC cases between control and post-appendectomy populations, with hazard ratios (HR) for CRC ranging from 1.14 to 2.99 in the post-appendectomy cohorts as compared to control.6-10 In two studies, this increased risk was found to be even more apparent in subgroups consisting of patients with an older age, defined by Shi et al.8 as an age of 50 and older and by Wu et al.7 as an age of 60 and older at the time of appendectomy and corresponding to an HR of 2.02; 1.71–2.40 and 1.24; and 1.06–1.45, respectively. Two studies (Wu et al.,7 Lee et al.9) identified that the risk of CRC was highest in the first 3 years post-appendectomy, and it seems that after which the risk for CRC returns to baseline. The cohort whereby an incidental appendectomy was performed was also identified by Wu et al.7 to be associated with the highest increased risk for CRC with a HR of 2.90; 2.24–3.75 (P < 0.001). A final study performed by Song et al.10 showed no significant increase in CRC risk for the combined population of post-appendectomy patients, but found an increase in CRC risk in a subgroup analysis of patients at 5–14 years post-appendectomy (HR 1.12; 1.05–1.20). Oddly, no increased risk of CRC was identified in the subgroup of patients identified 1–4 years post-appendectomy (HR 1.05; 0.94–1.17), which is conflicting with the findings of the previously mentioned studies.
The remaining four studies showed differing results. A recent case–control study performed by Mandi et al.11 revealed no significant increase in the risk of CRC in a post-appendectomy cohort. Rothwell et al.12 further identified that appendectomy was associated with a decreased risk of CRC instead, specifically regarding colon cancer (excluding rectal cancer) with a HR of 0.90; 0.81–0.99 and distal colon cancer (HR 0.77; 0.65–0.90), though comparative analyses did not achieve statistical significance in pooled analyses factoring in the entire population, and their findings did not differ during subgroup analyses stratifying patients by age. A study performed by van den Boom et al.13 corroborated these findings with a decreased risk of all types of cancer post-appendectomy (HR 0.86; 0.75–0.98, P = 0.005), including a decreased risk in colon cancer specifically post-appendectomy (HR 0.65; 0.43–0.97). They additionally found no evidence for an increased risk of cancer in the immediate years post-appendectomy, with only three of the 273 incident malignancies occurring within the first 4 years after appendectomy. An additional cohort study by Cope et al.14 following up on appendectomy within a pediatric population also showed no increase in overall cancers or colon cancers post-appendectomy, although an increased risk of stomach cancers and NHLs in the post-appendectomy cohort was observed. This risk was statistically significant for stomach cancer in the cohort of patients who underwent appendectomy between ages 15 and 19.
A recent systematic review and meta-analysis performed by Liu et al.15 described findings similar to the trends described above, finding an overall OR of 1.31; 1.05–1.62 for CRC in the combined post-appendectomy populations. They further conducted a subgroup analysis based on geographical populations and concluded that the trend toward increased OR for CRC post-appendectomy was significant in American and Asian populations (OR 1.68; 1.15–2.44 and OR 1.46; 1.04–2.05, respectively), but not significant in European populations (OR 0.94; 0.87–1.02). While the apparent drivers for these ethnic or geographical differences are not well elucidated, Liu et al. attributed these findings to advanced socioeconomic status within the region and strong support for regular colonoscopy screening and earlier intervention of precancerous lesions. We recognize the robust data that Liu et al. have summarized in their comprehensive paper and build upon their analysis through further scrutinization of the specific subgroups outlined in each paper, with special reference to the available data outlining the specific risk of CRC at specific intervals post-appendectomy, as well as available trends on the predominance of left-sided or right-sided CRC in post-appendectomy patients. In particular, the temporal relationship between the increased risk of CRC and appendectomy and the incidence of right-sided tumors are of particular interest to us, possibly hinting at mechanisms of tumorigenesis that are important targets for research. These specific details are summarized under Table 1, which hosts a consolidated summary of the key takeaways within the reviewed articles.
| Authors | Title | Year | Type of study | Population (n) | Country/demographic | Summary |
|---|---|---|---|---|---|---|
| Shi et al.8 | Altered gut microbiome composition by appendectomy contributes to colorectal cancer | 2022 | Cohort study | 129 155 | China | CRC was increased in post-appendectomy subjects HR 1.730 and 95% CI: 1.490–2.010, especially in patients with appendectomy >50 years. Appendectomy cases also had increased proximal CC as compared to distal CC and RC |
| van den Boom et al.13 | Appendectomy and the subsequent risk of cancer: a prospective population-based cohort study with long follow-up | 2022 | Cohort study | 7983 | Amsterdam | Overall decreased risk of cancer post-appendectomy (HR 0.86; 0.75–0.98, P = 0.005), specifically in gastrointestinal and colon cancers. GI cancers post-appendectomy 0.75 (0.56–0.99); colon cancer 0.65 (0.43–0.97). No increased risk of cancer in the few years directly after appendectomy |
| Rothwell et al.12 | Colorectal cancer risk following appendectomy: a pooled analysis of three large prospective cohort studies | 2022 | Pooled analysis of 3 cohort studies | 4827 | Europe (UK/France) | Appendectomy associated with decreased risk of colon cancer and distal colon cancer in subgroup analyses |
| Lee et al.6 | Long-term impacts of appendectomy associated with increased incidence of inflammatory bowel disease, infection, and colorectal cancer | 2021 | Cohort study | 914 208 | Korea | Odds ratio of CRC in post-appendectomy patients were 2.99 (2.75–3.26) as compared to control |
| Lee et al.9 | The risk of colorectal cancer after cholecystectomy or appendectomy: a population-based cohort study in Korea | 2018 | Cohort study | 707 663 | Korea | HR of CRC was elevated in the first year after appendectomy (HR 4.22; 2.87-6.20) and in the 2nd-3rd year after appendectomy (HR 2.34; 1.36-4.03). This was significant even after adjusting for sex and comorbidities, after which HR of CRC was no longer statistically significant (HR 0.77; 0.51-1.16). Specifically, HR for rectal carcinoma was never increased in all subgroup analyses |
| Song et al.10 | Risk of gastrointestinal cancers among patients with appendectomy: a large-scale Swedish register-based cohort study during 1970-2009 | 2016 | Cohort study | 480 382 | Sweden | No significant increase in CRC for the post-appendectomy cohort versus control. However, the risk for CRC at 5–14 years post-appendectomy was statistically significant (HR 1.12; 1.05–1.20), especially for right-sided CRC during this time period (HR 1.15; 1.05–1.26), whereas left-sided CRC was not significant (HR 1.02; 0.91–1.15). Esophageal adenocarcinoma was also increased after appendectomy |
| Wu et al.7 | Association between appendectomy and subsequent colorectal cancer development: an Asian population study | 2015 | Cohort study | 75 979 | Taiwan | HR of CRC overall was 1.14 (1.02–1.28) in post-appendectomy patients (P < 0.05), and HR of CRC was only significant in the population whereby appendectomy was performed ≥60 years of age at 1.24 (1.06–1.45) (P < 0.01). Furthermore, HR of CRC in appendectomy performed ≥60-year-old cohort compared to appendectomy performed <60-year-old cohort was 12.8 (11.6–14.1) (P < 0.001). HR of CRC was highest in cohort that conducted incidental appendectomy at 2.90 (2.24–3.75) (P < 0.001) and was highest in the following 1.5–3.5 years post appendectomy at 2.13 (1.63–2.77) (P < 0.001) |
| Cope et al.14 | Appendectomy during childhood and adolescence and the subsequent risk of cancer in Sweden | 2003 | Cohort study | 106 763 | Sweden | Study was conducted on appendectomy performed in a pediatrics population. No increase in overall cancers nor colon cancer but increased risk of stomach cancers and NHLs in the post-appendectomy cohort. Statistically significant risk in stomach cancer was observed in the cohort of patients who underwent appendectomy between ages 15–19 |
| Mándi et al.11 | The role of appendectomy and cholecystectomy in the pathogenesis of colorectal carcinomas | 2021 | Case–control study | 458 + 444 | Hungary | No significant increase in post-appendectomy patients for the cohort with CRC versus control |
| Liu et al.15 | Risk of colorectal cancer after appendectomy: a systematic review and meta-analysis | 2023 | Systematic review, meta-analysis | 22 studies | Worldwide | Worldwide risk of CRC post-appendectomy was increased, as well as in American and Asian populations. However, this was not significant in European populations |
| Ábrahám et al.22 | Evaluating the distribution of the locations of colorectal cancer after appendectomy and cholecystectomy | 2020 | Case series | 604 | Hungary | In patients with previous appendectomy, right-sided CRC was higher (P = 0.0007) |
- CC, colon cancer; CRC, colorectal cancer; HR, hazard ratio; NHLs, non-Hodgkin lymphomas; RC, rectal cancer.
We further reviewed literature regarding the anatomical location of colorectal tumors in post-appendectomy CRC patients. Left-sided CRC is defined as colorectal tumor originating from the regions of the colon distal to the left colic (splenic) flexure, including the descending colon, sigmoid colon, and rectum; right-sided CRC is defined as colorectal tumor originating proximal to the left colic flexure, including the cecum, ascending colon, and transverse colon.16 Studies have shown that left-sided and right-sided CRC exhibit distinct molecular and clinicopathological features.17, 18 Certain biomarkers such as BRAF and dMMR/MSI-H have been shown to have a predisposition for right-sided CRC, whereas APC, KRAS, and p53 mutations are more frequently encountered in left-sided tumors.19-21 In our review, we identified three articles that described an increased incidence of right-sided CRC in post-appendectomy patients; in the Song et al. study, this increased prevalence of right-sided CRC was noted to be observed most strongly during 5–14 years post-appendectomy.8, 10, 22 This is particularly significant as ascending CRC has classically been documented to be associated with a worse prognosis for patients.23, 24 The rationale behind this observation is not yet known, but given the highlighted differences in driver mutations between left-sided and right-sided CRC reported in the literature, we hypothesize that appendectomy might play a role in regulating the driver mutations, which characteristically give rise to right-sided CRC.
The microbiome's central role in the pathogenesis of CRC
The current literature relating to the role of the appendix and the increased incidence of CRC post-appendectomy mainly revolves around the hypothesis that appendectomy causes perturbations within the gut microbiome. As the intestinal microbiome impacts physiological functions relating to epithelial cell proliferation, differentiation, as well as the dynamic interplay between intestinal immunity and the composition of gut microorganisms, studies have proposed that a disruption to normal functioning within these physiological parameters can lead to a failure of immune regulation and eventual tumorigenesis.25
Alterations in gut microorganism species in the pathogenesis of CRC
The pathogenesis of CRC has been long hypothesized to be linked to an alteration (termed dysbiosis) in the abundance of microorganisms which colonize the gut in patients with CRC. Studies have identified a general shift in composition of gut microbiota in CRC patients, reflecting an altered gut ecological microenvironment, which is characterized by increased strains of “pathogenic” bacteria and reduced strains of “protective” species. The critical species involved in this ecological shift have been summarized in a recent review. Increased abundance of Bacteroides fragilis, Fusobacterium nucleatum, Parvimonas micra, Porphyromonas asaccharolytica and Prevotella intermedia in addition to Escherichia-Shigella were found to be increased in stool and tumor samples of CRC patients.26-28 Protective species that were identified included bacteria such as Clostridium butyricum and Streptococcus thermophilus as well as members of the Firmicutes phylum, which have been observed to decrease in number within samples obtained from CRC patients.27-29
These specific trends in gut microbiota alterations have also been observed in patients with a significant smoking history and alcohol intake, which are factors classically known to promote CRC carcinogenesis; of note, the relative abundance of these protective microorganisms were also seen to return to normal levels after stopping these inciting risk factors, indicating possible regeneration of gut dysbiosis following lifestyle modifications.30-32 These findings lay the groundwork implicating dysbiosis and its role in CRC carcinogenesis.
Biofilm dysregulation, immune pathways, and inflammation in CRC
The gut biofilm is an extrapolation of the concept of microbiota composition and refers to an overarching three-dimensional mucoid structure comprising of extracellular polymeric substances, which integrates the microorganism colonies found in the gut alongside their produced lipids, polysaccharides, proteins, nucleic acids, and ions. This structure further functions to regulate important functions of the gut and plays an important role in gut immunity, barrier function, immune defenses, and complex cell signaling.33
In a recent study by Dejea et al.,34 bacterial biofilms were found to be increasingly prevalent in right-sided CRC tumors and adenomas as compared to left-sided lesions. These biofilm-positive tumors were also identified to be associated with significant downstream changes involving a loss of E-cadherin, a molecule involved in cell adhesion and indicating increased membrane permeability, alongside increases in IL-6 and STAT3 activation, which appears to be the pathogenic signal driving enhanced cellular proliferation and reduced apoptosis favoring CRC tumorigenesis. Sobhani et al. further elucidated changes in immune regulation such as an increase in IL-17-positive cells in CRC patients, which was significantly associated with the quantitative load of Bacteroides species, a microorganism that is known to be positively associated with CRC carcinogenesis.35 This is consistent with the findings under Sears and Pardoll's “alpha-bug” theory, which featured enterotoxigenic B. fragilis as an activator of STAT3, which activates downstream signaling of IL-17 and IL-23 leading to the promotion of chronic inflammation, tumor cell survival, and overall tumorigenesis.36
We further explored recent studies which displayed a direct genotoxic effect of certain species of ‘pathogenic’ gut colonizers such as Enterococcus faecalis and adherent-invasive Escherichia coli. These strains were shown to promote chromosomal instability and the production of a polyketide-peptide genotoxin, colibactin, which promotes p53 expression and tumor cell proliferation.37 The disruption of surface barrier function as evidenced by the reduction of E-cadherin additionally allows for the influx of otherwise neutral commensal bacteria and their products into the tumor stroma, further escalating tumor-associated inflammation, in what has been described as a “driver/passenger model” of bacterial colonization in promoting CRC. In addition, this underlying inflammation is found to promote the secretion of reactive oxygen species by macrophages, dendritic cells, natural killer cells, and neutrophils within the tumor microenvironment, further inciting DNA damage.38 It is also interesting that these cancer-promoting species were also identified to be selectively enriched in CRC populations, which suggests that a positive-feedback loop for the pathogenesis of CRC is present.
These various inciting factors and consequent pathways thus conglomerate into a model for the pathogenesis of CRC generated through various channels, primarily driven by primary dysbiosis of the gut microbiome.
Specific pathways of microbiome dysregulation post-appendectomy
Having established a complex and elaborate link between the gut microbiome and the pathogenesis of CRC, we now turn our attention toward the potential role of the appendix in microbiome regulation, and mechanisms of dysregulation in post-appendectomy patients.
The role of the appendix in providing a “safe harbor” for gut commensals was investigated by Sanders et al. in the setting of Clostridioides difficile colitis, coming to a conclusion that the appendix might indeed hold some value in regulating the general microbiome of the gut but might fail to regulate the microbiome in certain cases whereby the gut has been treated with antibiotics, resulting in C. difficile colitis.39 Prior appendectomy was also illustrated to be associated with worse outcomes in patients with C. difficile infection, possibly explained by a dysregulation to gut microbiota.40
A study by Cai et al.41 further investigated the changes in the gut microbiome species in a case–control study of healthy patients with and without prior appendectomy, illustrating that appendectomy did significantly alter gut microbiome composition, increasing the levels of Escherichia, Shigella, Veillonella, Klebsiella, Megasphaera, Flavonifractor, and Streptococcus in the post-appendectomy population, and reducing levels of Roseburia, Barnessiella, Butyricicoccus, Odoribacter, and Butyricimonas. These alterations in bacterial strains between pre-appendectomy and post-appendectomy states suggest a correlation with previous studies regarding the alterations of specific bacteria types observed in the microbiome of CRC patients. An interesting observation from this study was that the alterations in microbiome composition seemed to “regenerate” back to a pre-appendectomy composition over time (>2 years after appendectomy in the study), which appears to indicate that colonic microbiome dysregulation might be of a transient nature. This also correlates well with the findings from previous studies discussed earlier (Wu et al.,7 Lee et al.9), which indicate an increased risk of CRC post-appendectomy immediately after appendectomy, with subsequent reduction in risk with the number of years post-appendectomy, and opens up the possibility of an untapped treatment window during this period whereby probiotics or fecal transplants might be more effective in restoring gut microbiome back to a “pre-susceptible” state. We would, however, also like to raise a point that these two studies were conducted as retrospective cohort studies, giving rise to the possibility of a surveillance bias in the post-appendectomy arm secondary to increased surveillance colonoscopy, which might be a protocol of certain institutions following prior appendectomy, accounting for an increased incidence in CRC detected in the period immediately following an appendectomy.
Shi et al.8 comprehensively delineated two distinct groups of bacteria that were altered post-appendectomy, with one group being significantly enriched and the other depleted post-appendectomy. A majority of pathogens identified to promote tumorigenesis of CRC was significantly enriched in the post-appendectomy population, consisting of Bacteroides vulgatus, B. fragilis, Veillonella dispar, Prevotella ruminicola, Prevotella fusca, Prevotella dentalis, and Prevotella denicola; conversely, five species recognized as protective bacteria were depleted, such as Blautia sp. SC05B48, Collinsella aerofaciens, Lachnospiraceae bacterium Choco86, Enterococcus hirae, and Blautia sp. YL58. These separate enriched and depleted groups of bacteria were further identified to form a microbiome ecological network, which yielded negative correlations with each other. The pathways involving dysregulations in intestinal barrier function and loss of E-cadherin and other adherent junction proteins was also identified in this study, which corroborates with the previous studies mentioned above. For added clarity, a detailed table can be found under Table S1 detailing our current understanding of the bacteria species involved in post-appendectomy and CRC changes.
Bollinger et al.42 finally elaborated on the correlation between the appendix and the maintenance and regulation of gut biofilm, especially pertaining to the proximal (right-sided) end of the large bowel, indicating that biofilm dysregulation might be most apparent in the proximal colon post-appendectomy. While this also correlates well to the increased finding of right-sided colon cancer presented above, further studies directly addressing this potential correlation are still required to further our understanding of the pathophysiology behind this proposed association. For reference, a detailed figure detailing these pathways involving the dysregulation of gut microbiome in the pathogenesis of CRC can be found under Figure 1.
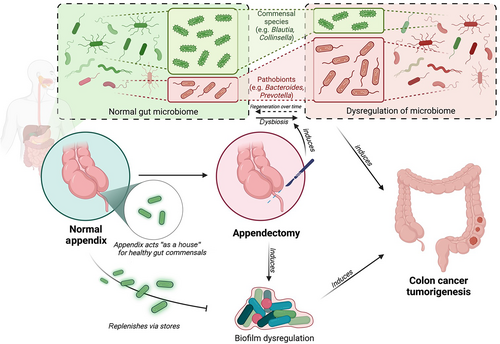
Present role of the appendix: vestigial organ or important regulator of gut immunity?
The human appendix vermiformis has long been considered an evolutionarily vestigial organ, although it has presumptive immune system functions and appears to support beneficial bacterial gut microbes. The comparative anatomy of the mammalian appendix reveals a significantly longer appendix in herbivores than in carnivorous animals.43 The lengthier appendix in herbivores has been associated with the presence of cellulose-digesting bacteria that colonize the structure, which serves as a fermentation chamber. In contrast, in carnivorous or omnivorous animals including all hominoids, the appendix is often absent or very small, suggesting its relatively minor physiological role in their digestive systems.
Nevertheless, studies have suggested that the appendix may play a key role in our immunity.44 The appendix is especially abundant in gut-associated lymphoid tissue (GALT) and biofilms that support the theory that it is colonized by commensal bacteria, compared to other parts of the colon.42, 45 The human appendix is also a major site of IgA production.46, 47 The appendix has been hypothesized to acts as a reservoir from which symbiotic gut microbes can be established.42 When the intestine is infected by pathogenic bacteria, the appendix may shed a healthy microbiome to re-inoculate the gut with its normal flora. Such rapid restoration of microbiome is especially important for survival in environments where diarrheal illness can be life-threatening, especially for young children who may also suffer from a lack of access to clean water.42 Notably, evolutionary studies showed that the appendix has evolved independently at least twice and is correlated with increased maximal longevity in mammals.43, 48 These support the notion that the appendix has a vital biological function contrary to popular opinion, playing a role in maintaining the immunological balance of commensal bacteria. This hypothesis is consistent with clinical observations reporting associations between appendectomy and inflammatory diseases, such as ulcerative colitis and rheumatoid arthritis.49, 50
Indeed, recent literature had shed further light on the effects of the appendix on the immunological functions of the human colon. The appendix appears to be intricately linked to immunological functions of the colon—prior appendectomy has been associated with a decreased risk of ulcerative colitis as well as a reduced risk of colectomy in patients subsequently diagnosed with ulcerative colitis, presumably as a result of immunological changes mediated by appendectomy.51 The appendix also appears to play a vital role in the priming of T cells as part of innate anti-tumoral immunosurveillance via the attraction and retention of lymphocytes to the gut via α4β7 integrin, and post-appendectomy levels of CD3+ and CD8+ T-cell infiltration within the gut have been observed to be significantly decreased on immunohistochemistry staining.52 This post-appendectomy “immune switch” further comprises of molecular changes such as a reduction in PD1 expression in CD4+ and CD8+ T cells post-appendectomy and a decreased ratio of T effector memory to T central memory cells. We further postulate that the molecular profile of CRC, namely, that of MSI-H/dMMR CRC, might also be affected by appendectomy given that the proximal pattern of presentation of CRC post-appendectomy appears to correlate with the right-sided preponderance of MSI-H/dMMR CRC, alongside recent reports that the microbiome in MSI-H/dMMR CRC is altered with increased species of Bacteroides, Prevotella, and Fusobacterium, which are species identified to be both CRC-inducing and upregulated in the post-appendectomy gut microbiome.8, 21, 53
We thus hypothesize that the immunology of the colon, particularly that of the proximal colon, is altered post-appendectomy, favoring tumorigenesis in part due to reduced immune regulation and surveillance. We propose that further studies conducted should not solely aim to investigate the location of tumors post-appendectomy and advocate taking further steps in characterizing tumors through an identification of molecular and transcriptomic profiles (MSI-H/dMMR, BRAF), cell-surface proteins, and immune signaling (PD1, PD-L1, IL-17A). This will help us not just in broadening our understanding of the pathways implicated in post-appendectomy tumorigenesis but also in the identification of therapeutic biomarkers for personalized therapy. Possible applications for personalized therapeutics include pembrolizumab, a PD-1 immune checkpoint inhibitor that displays increased oncologic response in MSI-H/dMMR CRC as well as in microbiomes reflecting a low level of Akkermansia muciniphila.54, 55
The current evidence regarding the role of the appendix in the pathogenesis of CRC are still modest, but new research seems to suggest that the appendix may play a more important role than previously thought and that preserving the appendix may have a biological role. Considering this, it might be prudent for clinicians to reconsider the various alternatives to appendectomy for the management of acute appendicitis in a move toward medical, appendix-conserving management where possible. The 2020 update of the World Society of Emergency Surgery (WSES) Jerusalem guidelines presents non-operative management of appendicitis with antibiotics as a safe alternative to surgery in both adult and pediatric populations in selected patients with uncomplicated acute appendicitis and an absence of appendicolith.56 Despite this, we acknowledge recent trials and meta-analyses that inform that population groups subjected to medical management only with antibiotics might yield an increased complication and recurrence rate, although antibiotics-only management appears to be non-inferior to surgical management for quality of life outcomes at 30 days.57-59
We also acknowledge that some studies have shown that there is still an increased risk of malignancy after nonsurgical treatment of appendicitis, attributed to the possibility that the appendiceal inflammation was secondary to a neoplastic lesion, and thus, surgical intervention can play a role in the definitive management of a possible pre-malignant lesion.60, 61 In a parallel analogy to the hypothesis behind a precancerous or early neoplastic lesion predisposing to diverticulitis and diverticular disease in the possible association behind CRC and diverticulitis, we propose an extension of this concept towards our current understanding of appendicitis and CRC.62, 63 We hypothesize that in a similar manner, the first presentation of CRC can occur as appendicitis secondary to obstruction of the blind-end appendix via a precancerous or early lesion and that appendicitis should thus be evaluated as a possible complication of early CRC instead of a standalone entity entirely. Interval appendectomy for complicated appendicitis was also found to successfully remove appendiceal tumors in patients who might otherwise have suffered from undiagnosed appendiceal cancer.64 A recent study has also demonstrated that patients presenting with acute appendicitis are at higher risk of colon cancer in the subsequent year, though it is unclear if this is attributed to the hypothesized post-appendectomy/appendicitis dysregulation of gut microbiome or to appendicitis being an early manifestation or “herald sign” of early colon cancer.65 In view of the current conflicting evidence, the decision for therapy for management of acute appendicitis will still have to be weighed by the surgeon for formulation of a personalized management plan, taking into account disease, surgeon, and patient factors.
The evidence behind the role of the appendix in alterations of the microbiome in disease states is gradually emerging, but there is still a lack of sufficient data going forward for evidence-backed clinical decision making. It suffices to say that the appendix may not be a purely vestigial organ, and that it does play a role in a dynamic, complex interplay in regulation of the gut microbiome and its consequent disease states. We, however, do not yet have sufficient information to weigh in on the full impact of the consequences of post-appendectomy dysbiosis for use in making a clinical decision or recommendation regarding optimal management of appendicitis. Further developments in our understanding of the gut microbiome can additionally improve our ability to detect and screen for CRC for early intervention, advance our understanding of tumorigenesis post-appendectomy, and identify predictive and prognostic biomarkers for personalized therapy.66 The current data and understanding that we have surrounding the gut microbiome is promising, and new data and findings will be imperative in establishing a breakthrough of our current understanding of this complex phenomenon.
Appendix A: Search terms used for the literature search and PRISMA flowchart of the number of studies evaluated at each stage of the review
- ((colorectal cancer) OR (Colorectal Neoplasms[MeSH Terms])) AND ((appendectomy) OR (appendicitis) OR (Appendectomy[MeSH Terms]))
- (colorectal cancer and (appendectomy or appendicitis)).mp. [mp = title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword heading word, floating subheading word, candidate term word]
- (colorectal cancer) AND ((appendectomy) OR (appendicitis))
Open Research
Data availability statement
The original contributions presented in the study are included in the article/Supporting Information. Further inquiries can be directed to the corresponding author.




