Upper gastrointestinal involvement of Behçet's disease in Japan: endoscopic findings and clinical features
Abstract
Aim
Behçet's disease (BD) can involve any gastrointestinal (GI) tract site. We analyzed the characteristics, risk factors, and treatment responses to upper GI (UGI) involvement in patients with BD.
Methods
This retrospective cohort study analyzed UGI findings in 101 patients with BD who underwent endoscopy between April 2005 and December 2022 at the University of Tokyo Hospital. The patients were divided into two groups based on the presence or absence of UGI findings. Patient backgrounds, clinical symptoms, colonoscopy (CS) findings, and blood test findings were compared between the groups.
Results
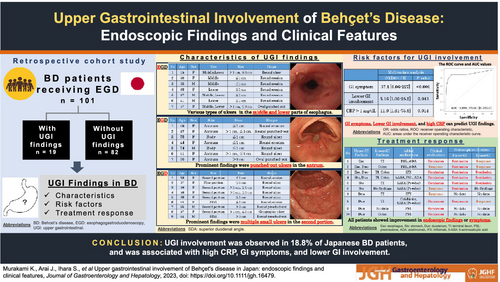
In total, 18.8% (19/101) of the patients had UGI lesions. The prevalence rates in the esophagus, stomach, and duodenum were 6.9%, 6.9%, and 8.9%, respectively. Of these 19 patients, BD treatment were intensified in 10 (52.6%) patients after esophagogastroduodenoscopy (EGD), and all showed improvement in symptoms or endoscopic findings. In the multivariate analysis, symptoms (OR: 37.1, P < 0.001), CRP > 1 mg/dL (OR: 11.0, P = 0.01), and CS findings (OR: 5.16, P = 0.04) were independent predictors of UGI involvement in BD patients. The prediction model for UGI involvement using these three factors was highly accurate, with an AUC of 0.899 on the ROC curve. In the subgroup analysis of intestinal BD, symptoms (OR: 12.8, P = 0.01) and ESR > 20 mm/h (OR: 11.5, P = 0.007) were independent predictors.
Conclusions
EGD should be conducted in BD patients with high CRP, GI symptoms, and lower GI involvement, which leads to better management of BD in terms of improving symptoms and endoscopic findings.
Graphical Abstract
Introduction
Behçet's disease (BD) is a refractory systemic inflammatory disease characterized by a set of clinical manifestations, including recurrent oral and genital ulcers, uveitis, cutaneous lesions, and multisystem involvements.1 The cause of BD has not been fully elucidated; however, there is a genetic predisposition associated with human leukocyte antigen (HLA) -B51 and various immune cells, and some exogenous and environmental factors are thought to be involved in its pathogenesis.2, 3 The prevalence of BD is relatively high in Mediterranean countries and East Asia, including Japan, and its estimated prevalence in Japan is reported as 13.5 out of 100 000 people.1
Gastrointestinal (GI) findings, also known as intestinal BD, are important manifestations of BD. In Japan, approximately 16% of the patients with BD have GI manifestations. The number of BD patients with GI manifestations in Japan is higher than that in other countries.2, 4 Although the ileocecal region is the most affected site of the GI tract, involvement of the entire and upper GI (UGI) tracts has also been reported.5, 6 As some case reports have also shown that UGI inflammation in patients with BD can cause serious complications such as bleeding, perforation, or fistulas,7, 8 recognizing the presence of UGI involvement is essential to control disease activity. However, the typical characteristics, risk factors, and treatment responses are yet to be estimated, as BD with UGI involvement is very rare.
To address these issues, we performed a single-center retrospective cohort study to investigate UGI involvement's prevalence, clinical characteristics, and treatment response in Japanese patients with BD.
Methods
Study design, setting, and patients
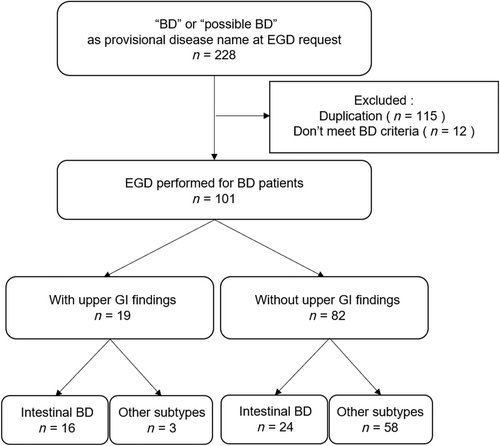
Medical records of patients diagnosed with BD who underwent esophagogastroduodenoscopy (EGD) were retrospectively extracted from the endoscopic databases of the University of Tokyo Hospital. Patients who underwent EGD and whose provisional disease names at EGD were “BD” or “possible BD” between April 2005 and December 2022 were included. Patients who did not meet the criteria suggested by the International Study Group for BD in 19909 or the BD Research Committee of Japan in 200310 were excluded from the chart review (Fig. 1). Finally, 101 patients were divided into two groups: a group of patients with or without UGI findings. Patients who had round or oval punched-out ulcers in the ileocecal region in previous colonoscopy (CS) and were diagnosed with intestinal BD allocated to the intestinal BD subgroup.
This study was conducted using the opt-out method. This study was approved by the Institutional Review Board of the University of Tokyo Hospital (registration no. 11649-(3); date of approval: September 7, 2017) and conformed to the provisions of the Declaration of Helsinki (as revised in Fortaleza, Brazil, October 2013). Patient records were anonymized prior to analysis. Written informed consent was obtained from all the patients.
Variables and outcomes
We evaluated the following clinical factors and background characteristics of patients with BD as possible predictors for the presence of UGI tract involvement: age, disease duration, sex, subtypes of BD (complete, incomplete, and suspicious), extraintestinal manifestations (oral ulcer, skin lesion, ocular lesion, genital ulcer, arthralgia, vasculitis, and neurologic lesion), Helicobacter pylori infection (current infection, previous infection, and no infection), medications (NSAIDs, proton pump inhibitor [PPI], colchicine, 5-aminosalicylic acid [5-ASA], salazosulfapyridine [SASP], azathioprine [AZA], prednisolone [PSL], and anti-tumor necrosis factor-alpha [TNF-α] agents), GI symptoms (chest or abdominal pain, nausea, melena, anemia, dysphagia, and heartburn), lower GI involvements, and laboratory data (C-reactive protein [CRP], serum albumin, erythrocyte sedimentation rate [ESR], and peripheral blood count). We also investigated the timing of PPI administration and treatment duration. We defined “treatment duration” as the period from the initial PPI prescription to the date of the EGD.
The primary outcome was UGI involvement in patients with BD. UGI ulcers and erosions were regarded as the UGI involvement. Findings attributable to other causes, such as reflux or infectious esophagitis, were excluded through comprehensive assessments, including endoscopy, medications, blood tests, and histopathology.
Assessment of the clinical and endoscopic efficacy of treatment
We evaluated the efficacy of BD treatment in patients with UGI involvement. We collected patient histories, including laboratory data, GI symptoms, CS findings simultaneously, and follow-up endoscopy findings. BD treatment were intensified in 10 patients after EGD, and seven of them underwent follow-up endoscopy. Clinical and endoscopic responses were assessed. Three patients did not undergo follow-up endoscopy because of hospital transfers or limitations in their activities of daily living.
Clinical remission was defined as the complete disappearance of GI symptoms, including abdominal pain, anemia, and bleeding. Clinical response was defined as an improvement in GI symptoms. Similarly, endoscopic remission was defined as the complete disappearance of ulcers or erosions on follow-up endoscopy. Endoscopic response was defined as an improvement in findings on follow-up endoscopy.
Statistical analysis
All statistical analyses were performed using EZR,11 which is a graphical user interface for R (R Foundation for Statistical Computing, Vienna, Austria). Categorical variables were analyzed using the chi-squared test or Fisher's exact probability test, and continuous variables were analyzed using the Student's t-test and Mann–Whitney U test, as appropriate. To calculate the odds ratios (ORs) for the likelihood of GI involvement, significant variables in the univariate analysis were entered simultaneously into a multivariate stepwise logistic regression to evaluate their independent predictive values for UGI involvement. Statistical significance was set at P < 0.05, and ORs with 95% confidence intervals (CIs) were determined. We plotted the receiver operating characteristic (ROC) curves and estimated the area under the ROC curve (AUC). The optimal cutoff values of the continuous variables were determined using ROC analysis and published data.
Result
Patient characteristics
A total of 101 patients (50 male and 51 female) with BD were enrolled in this study. All patients were Japanese. Mean follow-up period was 6.9 ± 3.8 years. Nineteen patients were classified into a group of patients with UGI involvement (UGI [+]), and 82 patients were classified into a group of patients without UGI involvement (UGI [−]) (Fig. 1).
Endoscopic findings and treatment efficacy
We found that 18.8% (19/101) of the patients with BD had UGI involvement. The prevalence rates were 6.9% (7/101) in the esophagus, 6.9% (7/101) in the stomach, and 8.9% (9/101) in the duodenum. Various types of ulcers, including single round or multiple oval ulcers have been observed in the middle and lower parts of esophagus. Prominent findings in the stomach were punched-out ulcers in the antrum, and multiple small ulcers were detected in the descending part of the duodenum (Table 1). In the UGI (+) group, BD treatment were intensified in 52.6% (10/19) of patients after EGD. Three patients did not undergo follow-up endoscopy, but all patients showed improvement in their endoscopic findings or symptoms (Table 2); two typical examples are shown in Figure S1. In the UGI (−) group, 14.6% (12/82) of the patients changed their treatment after EGD. BD treatment were intensified more frequently (52.6% vs14.6%, P < 0.001) in the UGI (+) group.
| Sex | Age | Subtype | Cause | Site | Distribution | Size | Shape | Medications | Lower GI | |
|---|---|---|---|---|---|---|---|---|---|---|
| Esophagus | F | 45 | Incomplete | Abdominal pain | Middle, lower | Multiple | >1 cm, ≦3 cm | Round ulcer | PSL, ADA | + |
| F | 38 | Incomplete | Abdominal pain | Middle | Single | ≦1 cm | Round erosion | PSL, ADA | + | |
| M | 35 | Incomplete | Screening | Middle | Single | ≦1 cm | Round erosion | − | + | |
| F | 63 | Suspicious | Screening | Lower | Single | ≦1 cm | Round erosion | − | − | |
| M | 37 | Incomplete | Screening | Middle, lower | Multiple | ≦1 cm | Round erosion | − | + | |
| M | 41 | Incomplete | Heartburn | Lower | Single | ≦1 cm | Round erosion | − | + | |
| F | 57 | Incomplete | Anemia | Middle, lower | Multiple | >1 cm, ≦3 cm | Oval punched-out | IFX | + | |
| Stomach | F | 49 | Suspicious | Abdominal pain | Antrum | Multiple | ≦1 cm | Round erosion | − | + |
| F | 37 | Incomplete | Abdominal pain | Antrum | Single | >1 cm, ≦3 cm | Round punched-out | 5-ASA, PSL, ADA | + | |
| F | 75 | Incomplete | Screening | Body | Single | ≦1 cm | Round ulcer | − | − | |
| F | 44 | Incomplete | Screening | Antrum | Multiple | ≦1 cm | Round erosion | − | − | |
| M | 74 | Incomplete | Abdominal pain | Body | Multiple | ≦1 cm | Round ulcer | − | − | |
| F | 41 | Complete | Anemia | Antrum | Multiple | >1 cm, ≦3 cm | Round ulcer | 5-ASA | + | |
| F | 76 | Complete | Abdominal pain | Antrum | Single | >3 cm | Oval punched-out | 5-ASA | − | |
| Duodenum | F | 59 | Incomplete | Abdominal pain | Second portion | Multiple | ≦1 cm | Round erosion | IFX | + |
| F | 37 | Incomplete | Abdominal pain | First portion | Multiple | ≦1 cm | Round ulcer | 5-ASA, PSL, ADA | + | |
| F | 38 | Incomplete | Abdominal pain | Second portion | Multiple | >1 cm, ≦3 cm | Round ulcer | PSL, ADA | + | |
| F | 56 | Incomplete | Abdominal pain | Second portion | Multiple | >1 cm, ≦3 cm | Round ulcer | Colchicine, 5-ASA | + | |
| F | 36 | Incomplete | Screening | Second portion | Multiple | ≦1 cm | Round ulcer | − | + | |
| F | 44 | Incomplete | Screening | SDA | Multiple | ≦1 cm | Round erosion | − | − | |
| M | 65 | Incomplete | Melena | Second portion | Multiple | >3 cm | Round punched-out | PSL | − | |
| F | 57 | Incomplete | Anemia | Second portion | Multiple | >1 cm, ≦3 cm | Geographic ulcer | IFX | + | |
| M | 64 | Complete | Anemia | SDA | Single | >3 cm | Round punched-out | PSL | + |
- 5-ASA, 5-aminosalicylic acid; ADA, adalimumab; BD, Behçet's disease; GI, gastrointestinal; IFX, infliximab; PSL, prednisolone; SDA, superior duodenal angle.
| Case no. | Upper GI findings | Lower GI findings | Added medications | Clinical evaluations | Endoscopic evaluations | |
|---|---|---|---|---|---|---|
| Upper GI | Lower GI | |||||
| 1 | Esophagus | Terminal ileum | PSL, ADA | Remission | Remission | Response |
| 2 | Esophagus, duodenum | Colon | PSL, ADA | Response | Remission | Response |
| 3 | Esophagus, duodenum | Terminal ileum, colon | IFX | Remission | Remission | Remission |
| 4 | Stomach, duodenum | Terminal ileum, colon | 5-ASA, PSL, ADA | Remission | Remission | Remission |
| 5 | Stomach | Terminal ileum | 5-ASA (powder) | Remission | Remission | Remission |
| 6 | Stomach | No findings | 5-ASA (powder) | Response | Remission | No findings |
| 7 | Duodenum | Terminal ileum | IFX | Response | No data | No data |
| 8 | Duodenum | Terminal ileum | Colchicine, 5-ASA (Powder) | Remission | Remission | Response |
| 9 | Duodenum | No data | PSL | Remission | No data | No data |
| 10 | Duodenum | Colon | PSL | Remission | No data | No data |
- 5-ASA, 5-aminosalicylic acid; ADA, adalimumab; EGD, esophagogastroduodenoscopy; GI, gastrointestinal; IFX, infliximab; PSL, prednisolone.
As for BD treatment and efficacy, medications for BD were intensified in 10 out of 19 patients after EGD (Table 2). PPI had already been used in all 10 patients when UGI involvement was diagnosed. PSL was administered to five patients, which was tapered when their symptoms or findings subsided. Anti-TNF-α agents were used in five patients (adalimumab and infliximab were used in three and two patients, respectively), and 5-ASA and colchicine were administered to four and one patients, respectively. In particular, three patients of them were treated by oral administration of ground 5-ASA powder. Clinical remission was achieved in 70% (7/10) of the patients, and clinical response was achieved in 30% (3/10). Regarding UGI involvement, all patients who underwent follow-up EGD achieved endoscopic remission, and their CS findings also improved (three patients achieved endoscopic remission, and three patients achieved endoscopic response).
Factors associated with UGI findings for BD patients
We compared the clinical features and backgrounds of UGI (+) patients with those of UGI (−) patients. No significant differences were observed in sex, age at endoscopy, age at onset, follow-up duration, or BD subtype. Oral ulcers were the most frequent symptom in both groups, and ocular lesions were less frequent in UGI (+) patients (P = 0.04). No differences were noted in NSAIDs use, H. pylori infection, PPI use, or the other medication use before the examination. The two groups did not differ significantly in the duration of PPI use before undergoing EGD (Table S1). In terms of GI symptoms, 77 patients were asymptomatic. However, in UGI (+) group, 13 patients (68%) suffered from GI symptoms, including abdominal or chest pain, and it was more often compared with UGI (−) group (P < 0.001). Lower GI involvement was more frequent in the UGI (+) group (P < 0.001). The levels of CRP (P < 0.001) and ESR (P < 0.001) were higher, and those of albumin (P = 0.001) and hemoglobin (P < 0.001) were lower in the UGI (+) group (Table 3).
| Characteristics |
UGI (+) group (n = 19) |
UGI (−) group (n = 82) |
Univariate analysis, P value |
|---|---|---|---|
| Male (%) | 6 (31.6) | 44 (53.7) | 0.126 |
| Age at endoscopy (years, mean ± SD) | 52.2 ± 13.9 | 52.1 ± 15.2 | 0.948 |
| Age at onset (years, mean ± SD) | 41.2 ± 11.8 | 36.6 ± 12.6 | 0.200 |
| Duration (years, mean ± SD) | 11.0 ± 12.9 | 15.2 ± 12.8 | 0.100 |
| Type | |||
| Complete (%) | 3 (15.8) | 25 (30.5) | 0.260 |
| Incomplete (%) | 14 (73.7) | 45 (54.9) | 0.197 |
| Suspicious (%) | 2 (10.5) | 10 (12.2) | 1.000 |
| Extraintestinal manifestations | |||
| Oral ulcer (%) | 19 (100) | 77 (93.9) | 0.580 |
| Skin lesion (%) | 17 (89.5) | 70 (85.4) | 1.000 |
| Ocular lesion (%) | 6 (31.6) | 48 (58.5) | 0.043 |
| Genital ulcer (%) | 13 (68.4) | 53 (64.6) | 1.000 |
| Arthralgia (%) | 9 (47.4) | 30 (36.6) | 0.438 |
| Vasculitis (%) | 3 (16.8) | 10 (12.2) | 0.707 |
| Neurologic lesion (%) | 2 (10.5) | 10 (12.2) | 1.000 |
| NSAID user (%) | 10 (52.6) | 25 (30.5) | 0.106 |
| Helicobacter pylori infection status | |||
| Current infection (%) | 0 (0) | 7 (8.5) | 0.342 |
| Previous infection (%) | 6 (31.6) | 34 (41.5) | 0.604 |
| No infection (%) | 13 (68.4) | 41 (50.0) | 0.203 |
| PPI user (%) | 15 (78.9) | 46 (56.1) | 0.070 |
| Colchicine user (%) | 7 (36.8) | 48 (58.5) | 0.100 |
| 5-ASA or SASP user (%) | 1 (5.3) | 11 (13.4) | 0.450 |
| AZA user (%) | 1 (5.3) | 6 (7.3) | 1.000 |
| PSL user (%) | 8 (42.1) | 30 (36.6) | 0.790 |
| Anti-TNF-α agent user (%) | 1 (5.3) | 7 (8.5) | 1.000 |
| GI symptom (%) | 13 (68.4) | 11 (13.4) | <0.001 |
| Abdominal or chest pain (%) | 8 (42.1) | 3 (3.7) | <0.001 |
| Melena or anemia (%) | 4 (21.1) | 5 (6.1) | 0.062 |
| Dysphagia (%) | 0 (0) | 1 (1.2) | 1.000 |
| Heartburn (%) | 1 (5.3) | 2 (2.4) | 0.469 |
| Lower GI involvement (%) | 13 (68.4) | 18 (22.0) | <0.001 |
| C-reactive protein (mg/dL) | 3.82 ± 5.92 | 0.47 ± 1.21 | <0.001 |
| Erythrocyte sedimentation rate (mm/h) | 51.42 ± 27.90 | 23.53 ± 20.56 | <0.001 |
| Albumin (g/dL) | 3.49 ± 0.62 | 4.01 ± 0.44 | 0.001 |
| Hemoglobin (g/dL) | 10.80 ± 2.44 | 12.93 ± 1.87 | <0.001 |
| Platelet (×104/μL) | 32.87 ± 14.21 | 26.90 ± 9.60 | 0.057 |
- P values with statistical significance are shown in bold in the table.
- 5-ASA, 5-aminosalicylic acid; AZA, azathioprine; BD, Behçet's disease; GI, gastrointestinal; NSAIDs, nonsteroidal anti-inflammatory drugs; PPI, proton pump inhibitors; PSL, prednisolone; SASP, salazosulfapyridine; SD, standard deviation; TNF-α, tumor necrosis factor-alpha.
In a multivariate logistic regression model, GI symptoms (OR: 37.1, 95% CI: 6.06–227, P < 0.001), CRP > 1 mg/dL (OR: 11.0, 95% CI: 1.61–75.8, P = 0.01), and lower GI involvements (OR: 5.16, 95% CI: 1.06–25.2, P = 0.04) were independent predictors for the presence of UGI involvements (Table 4). Based on the multivariate analysis, the prediction model using these three factors (GI symptoms, lower GI involvement, and CRP > 1 mg/dL) was highly accurate, with an AUC of 0.899 on the ROC curve (Fig. S2a).
| Multivariate analysis | ||
|---|---|---|
| OR [95% CI] | P value | |
| GI symptom | 37.1 [6.06–227] | <0.001 |
| Lower GI involvement | 5.16 [1.06–25.2] | 0.043 |
| C-reactive protein >1 (mg/dL) | 11.0 [1.61–75.8] | 0.014 |
- P values with statistical significance are shown in bold in the table.
- BD, Behçet's disease; GI, gastrointestinal; OR, odds ratio.
Factors associated with UGI findings for intestinal BD patients
We further investigated the contribution of UGI tract findings in patients with intestinal BD. BD has a broad spectrum of clinical diversity and severity, so we examined whether some intestinal BD phenotypes were associated with UGI findings. Forty patients previously diagnosed with intestinal BD were analyzed. In the univariate analysis, UGI (+) patients more often had UGI symptoms (P < 0.001) and were less likely to be treated with 5-ASA or SASP (P = 0.012). Regarding laboratory data, UGI (+) patients showed higher CRP (P = 0.007), ESR (P < 0.001), and platelet count (P = 0.03), and lower albumin (P < 0.001) and hemoglobin (P = 0.02) levels (Table 5). Multivariate analysis showed that GI symptoms (OR: 12.8, 95% CI: 2.02–80.4, P = 0.01) and ESR > 20 mm/h (OR: 11.5, 95% CI: 1.63–81.0, P = 0.007) were independent predictors (Table 6). Based on multivariate analysis, the prediction model using these two factors (GI symptoms and ESR > 20 mm/h) was accurate, with an AUC of 0.870 on the ROC curve (Fig. S2b).
| Characteristics |
UGI (+) group (n = 16) |
UGI (−) group (n = 24) |
Univariate analysis, P value |
|---|---|---|---|
| Male (%) | 5 (31.3) | 14 (58.3) | 0.117 |
| Age at endoscopy (years, mean ± SD) | 49.3 ± 13.4 | 45.8 ± 12.5 | 0.404 |
| Age at onset (years, mean ± SD) | 39.4 ± 11.7 | 33.6 ± 12.1 | 0.140 |
| Duration (years, mean ± SD) | 9.9 ± 12.8 | 12.2 ± 9.9 | 0.527 |
| Type | |||
| Complete (%) | 2 (12.5) | 6 (25.0) | 0.439 |
| Incomplete (%) | 13 (81.3) | 15 (62.5) | 0.297 |
| Extraintestinal manifestations | |||
| Oral ulcer (%) | 16 (100) | 22 (91.7) | 0.508 |
| Skin lesion (%) | 15 (93.8) | 18 (75.0) | 0.210 |
| Ocular lesion (%) | 5 (31.3) | 13 (54.2) | 0.203 |
| Genital ulcer (%) | 11 (68.8) | 14 (58.3) | 0.740 |
| Arthralgia (%) | 8 (50.0) | 8 (33.3) | 0.339 |
| Vasculitis (%) | 2 (12.5) | 1 (4.2) | 0.553 |
| Neurologic lesion (%) | 1 (6.3) | 2 (8.3) | 1.000 |
| NSAID user (%) | 8 (50.0) | 7 (29.2) | 0.205 |
| Helicobacter pylori infection status | |||
| Current infection (%) | 0 (0) | 0 (0) | 1.000 |
| Previous infection (%) | 5 (31.3) | 10 (41.7) | 0.740 |
| No infection (%) | 11 (68.7) | 14 (58.3) | 0.800 |
| PPI user (%) | 12 (75.0) | 15 (62.5) | 0.503 |
| Colchicine user (%) | 5 (31.3) | 15 (62.5) | 0.105 |
| 5-ASA or SASP user (%) | 1 (6.3) | 11 (45.8) | 0.012 |
| AZA user (%) | 1 (6.3) | 2 (8.3) | 1.000 |
| PSL user (%) | 6 (37.5) | 12 (50) | 0.526 |
| Anti-TNF-α agent user (%) | 1 (6.3) | 4 (16.7) | 0.631 |
| GI symptom (%) | 11 (68.8) | 3 (12.5) | <0.001 |
| Abdominal or chest pain (%) | 8 (50.0) | 2 (8.3) | 0.007 |
| Melena or anemia (%) | 2 (12.5) | 1 (4.2) | 0.553 |
| Heartburn (%) | 1 (6.3) | 0 (0) | 0.400 |
| Lower GI involvement (%) | 13 (81.3) | 19 (79.2) | 1.000 |
| C-reactive protein (mg/dL) | 4.15 ± 6.48 | 0.35 ± 0.71 | 0.007 |
| Erythrocyte sedimentation rate (mm/h) | 48.56 ± 27.30 | 19.67 ± 19.00 | <0.001 |
| Albumin (g/dL) | 3.53 ± 0.61 | 4.14 ± 0.43 | <0.001 |
| Hemoglobin (g/dL) | 11.16 ± 2.29 | 12.74 ± 1.90 | 0.023 |
| Platelet (×104/μL) | 33.71 ± 15.48 | 25.39 ± 8.30 | 0.034 |
- P values with statistical significance are shown in bold in the table.
- 5-ASA, 5-aminosalicylic acid; AZA, azathioprine; BD, Behçet's disease; GI, gastrointestinal; NSAIDs, nonsteroidal anti-inflammatory drugs; PPI, proton pump inhibitors; PSL, prednisolone; SASP, salazosulfapyridine; SD, standard deviation; TNF-α, tumor necrosis factor-alpha.
| Multivariate analysis | ||
|---|---|---|
| OR [95% CI] | P value | |
| GI symptom | 12.8 [2.02–80.4] | 0.014 |
| Erythrocyte sedimentation rate >20 (mm/h) | 11.5 [1.63–81.0] | 0.007 |
- P values with statistical significance are shown in bold in the table.
- BD, Behçet's disease; GI, gastrointestinal; OR, odds ratio.
Discussion
In the present study, UGI involvement was observed in 19 (18.8%) of the 101 Japanese patients with BD. UGI involvement was significantly associated with high serum CRP levels, GI symptoms, and active lower GI tract inflammation; these variables could accurately predict UGI findings. Moreover, we identified the efficacy of BD treatments such as 5-ASA or anti-TNF-α agents.
BD is a chronic and recurrent multi-system inflammatory disorder that can include GI lesions. Ideguchi et al. reported that GI involvement is common in Japan (5–25%) but rare in Mediterranean countries (0–5%).12 The terminal ileum is the most frequently involved part of the GI tract (61.7–76%).13 However, UGI manifestations have not been well elucidated, even in Japan. This is because EGD is not routinely performed in patients with BD. In some studies on UGI involvement, EGD was selectively performed in patients with GI symptoms.14 A recent Korean study of patients with GI symptoms documented UGI involvement in 27.9% (36/129) of patients.15 In a Turkish study, 25% of patients had UGI symptoms, but only 0.5% had esophageal ulcers, 1.6% had gastric ulcers, and 2.1% had duodenal ulcers.16 In our study, 23.8% (24/101) of the patients had symptoms, and 18.8% of the patients had UGI involvement. The prevalence of UGI involvement in BD patients may differ regionally, and it may be associated with the prevalence of intestinal BD.
To our knowledge, features of UGI involvement in BD (e.g. locations or shapes) have not been widely recognized. Previous studies have reported that esophageal involvement is predominantly located in the middle esophagus and reveals various forms.15, 17 Regarding gastroduodenal involvement, several case reports have identified erosions and punched-out ulcers in the gastric antrum6, 18, 19 and the second portion of the duodenum.19, 20 These results are consistent with those of the present study (Table 1). Our data suggest that the gastric antrum and the second portion of the duodenum are common sites of UGI involvement in BD.
Although NSAIDs use and H. pylori infection are recognized as major risk factors for peptic ulcers, we found no significant association between these factors and UGI findings in BD patients, consistent with the previous studies.21, 22 Considering that all patients who underwent intensified BD-specific treatment showed improvements in endoscopic findings or clinical symptoms, GI involvement could have resulted from the pathogenesis of BD. The therapeutic strategy should follow the guidelines for BD as well as those for peptic ulcers, including the inhibition of gastric acid.
Previous studies have demonstrated an association between regular PPI intake and an increased risk of intestinal diseases including inflammatory bowel disease and GI bleeding.23-25 This association may stem from the effect of PPI on gut microbiota. Although our study did not show a significant difference in UGI prevalence between BD patients with and without PPI use, a trend was observed towards a higher prevalence of UGI in BD patients taking PPI (Table 2), suggesting a potential role of PPI in BD pathogenesis. However, the duration of PPI use before EGD tended to be shorter in the UGI (+) group compared with that in the UGI (−) group (Table S1). Therefore, PPI may be prescribed before EGD simply to alleviate UGI symptoms. Further research is needed to elucidate the association between PPI use and UGI findings in BD patients.
Anti-TNF-α agents, PSL, 5-ASA, SASP, and AZA can effectively treat UGI lesions in BD patients.15, 26-29 Particularly, 5-ASA use is associated with lower UGI involvement in patients with intestinal BD in our study. Kinoshita et al. reported the effectiveness of 5-ASA in treating intestinal BD, mainly for lower GI ulcers.30 Moreover, some case reports on UGI involvement in inflammatory bowel disease showed that administering 5-ASA ground to fine powder improved endoscopic abnormalities.31, 32 Our three patients who used fine 5-ASA powder also achieved endoscopic remission (Table 2). Oral 5-ASA powder administration may be effective for UGI lesions in BD patients.
We analyzed the clinical risk factors for UGI involvement. Similar to a previous study analyzing intestinal BD in China,33 we found that UGI involvement was associated with high serum CRP levels in the total cohort and ESR in the intestinal BD cohort. Therefore, UGI tract lesions may reflect BD's disease activity. Moreover, the association of UGI involvement with CS findings and that of the treatment response of UGI involvement with that of lower GI involvement indicates that UGI involvement may represent the state of intestinal BD.
Our results also revealed that UGI symptoms were significantly associated with UGI involvement in the total and intestinal BD cohorts, consistent with the results of a previous study.34 The common clinical symptoms of UGI involvement include substernal or abdominal pain, dysphagia, hematemesis, and melena.14, 15, 35, 36 54.2% (13/24) of the patients with these symptoms in our study were diagnosed with UGI findings on EGD. Unrecognized UGI ulcers can lead to severe bleeding, perforation, stenosis, or fistulas7, 37-39; however, once they are identified, patients can achieve a clinical response via appropriate treatment, as we have shown. Therefore, immediate detection is desirable. Further studies with larger cohorts are warranted for the accurate risk stratification of UGI lesions and strategies for endoscopic screening.
Our study had several strengths. First, we evaluated the detailed patient profiles obtained from medical record reviews. Long-term follow-up enabled us to analyze treatment and prognosis. Second, this study included BD patients who underwent EGD without any abdominal symptoms and estimated the association between the presence of symptoms and the incidence of UGI lesions. Nevertheless, this study had several limitations. First, this was a retrospective, single-center study with potential bias in design. Second, the sample size was small. Third, data on genetic or pathological characteristics such as HLA status are limited (Table S2). However, HLA-A26 is associated with intestinal lesions and may also affect UGI findings.40 Further studies using larger databases are required.
In conclusion, high serum CRP levels, GI symptoms, and active lower GI tract inflammation in BD patients are predictors of UGI findings and EGD may be recommended for BD patients with these factors.
Acknowledgments
There is no acknowledgement.
Open Research
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.