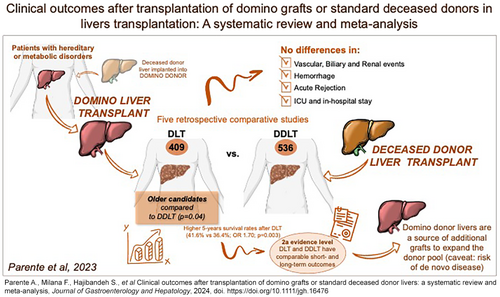
Clinical outcomes after transplantation of domino grafts or standard deceased donor livers: a systematic review and meta-analysis
Abstract
Background and Aim
Domino liver transplantation (DLT) utilizes otherwise discarded livers as donor grafts for another recipients. It is unclear whether DLT has less favorable outcomes compared to deceased donor liver transplantation (DDLT). We aimed to assess the outcomes of DLT compared to DDLT.
Methods
MEDLINE, Embase, and Web of Science database were searched to identify studies comparing outcomes after DLT with DDLT. Data were pooled using random-effects modeling, evaluating odds ratios (OR) or mean difference (MD) for outcomes including waiting list time, severe hemorrhage, intensive care unit (ICU), length hospital stay (LOS), rejection, renal, vascular, and biliary events, and recipient survival at 1, 3, 5, and 10 years.
Results
Five studies were identified including 945 patients (DLT = 409, DDLT = 536). The DLT recipients were older compared to the DDLT group (P = 0.04), and both cohorts were comparable regarding lab MELD, hepatocellular carcinoma, and waitlist time. There were no differences in vascular (OR: 1.60, P = 0.39), renal (OR: 0.62, P = 0.24), biliary (OR: 1.51, P = 0.21), severe hemorrhage (OR: 1.09, P = 0.86), rejection (OR: 0.78, P = 0.51), ICU stay (MD: 0.50, P = 0.21), or LOS (MD: 1.68, P = 0.46) between DLT and DDLT. DLT and DDLT were associated with comparable 1-year (78.9% vs 80.4%; OR: 1.03, P = 0.89), 3-year (56.2% vs 54.1%; OR: 1.35, P = 0.07), and 10-year survival (6.5% vs 8.5%; OR: 0.8, P = 0.67) rates. DLT was associated with higher 5-year survival (41.6% vs 36.4%; OR: 1.70; P = 0.003) compared to DDLT, which was not confirmed at sensitivity analysis.
Conclusion
This meta-analysis of the best available evidence (Level 2a) demonstrated that DLT and DDLT have comparable outcomes. As indications for liver transplantation expand, future high-quality research is encouraged to increase the DLT numbers in clinical practice, serving the growing waiting list candidates, with the caveat of uncertain de novo disease transmission risks.
Graphical Abstract
Introduction
Domino liver transplantation (DLT) was first reported in 1995 in Portugal.1 This liver transplantation (LT) technique entails the use of livers from patients undergoing transplantation due to a hereditary and/or metabolic disorder, which is implanted in another recipient.2 As such, the DLT recipient becomes a donor for another patient. DLT has emerged as a promising technique of increasing the donor pool by using otherwise discarded organs. These livers are normal in morphology and function, except for the synthesis of specific proteins or enzymes. Livers from transplant candidates with familial amyloidotic polyneuropathy (FAP) and maple syrup urine disease (MSUD) are among those most frequently used as domino grafts.2 Given the rarity of such diseases, an international registry (Domino Liver Transplant Registry) was established in 1999, which was comprehensive of the already existing FAP World Transplant Registry.3 Until December 2019, 2294 transplants for FAP were registered, of which 1288 were DLT. Almost half of DLT were performed in Portugal (45.8%), followed by France (15.1%), Sweden (6.4%), the United States (6.3%), Spain (5.2%), and Brazil (4.4%).3 While shorter waiting list time and better graft function2 are often discussed as benefits of DLT compared to deceased donor liver transplantation (DDLT), DLT is technically more challenging.2 The domino donor hepatectomy is performed carefully to provide an optimal graft for the implantation in the other recipient2 (Fig. 1). To procure a transplantable domino graft, the liver inflow and outflow vessels need to be preserved differently from a standard recipient hepatectomy.2, 4 In fact, the graft can be harvested with or without retrohepatic vena cava segment, and, after back-table reconstruction, the domino liver can be implanted in the second recipient.2 An inherent advantage is that the domino donor operation can be planned and performed in parallel with the hepatectomy of the second recipient, enabling effective communication of the two surgical teams.
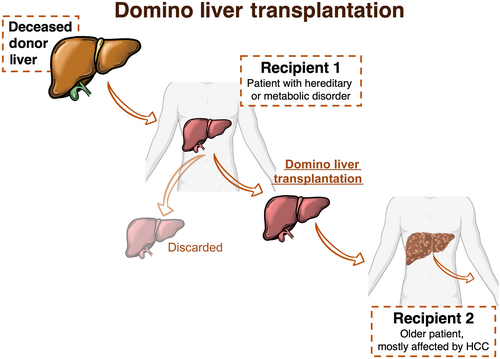
Although several single-center studies have compared the outcomes of DLT and DDLT, most were retrospective, demonstrating adequate but somewhat conflicting results.5-9 A French study showed a shorter waitlist time of 195 ± 156 days compared to 326 ± 250 days for DLT and DDLT, respectively6; however, this was not confirmed in a larger study using data from the United States.8 In addition, severe posttransplant complications were noted by a group from Portugal,7 which included piggyback syndrome, vascular complications, hemorrhage requiring reoperation, and biliary complications.7 These were not in line with earlier studies5, 6 and a most recent one from Sweden.8 Moreover, the same Portuguese study reported the incidence of de novo FAP of 11.4% after a median follow-up of 45 months.7
Posttransplant outcomes after DLT and DDLT have never been evaluated by a meta-analysis. The aim of this study was therefore to conduct a comprehensive systematic literature review and meta-analysis of available studies comparing clinical outcomes after domino and standard deceased donor LT.
Material and methods
Study design
The eligibility criteria, methodology, and investigated outcome parameters of this study were described first, in a protocol, registered at the International Prospective Register of Systematic Reviews (registration number PROSPERO: CRD42023402497). The methodology respected the standards of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.10
Eligibility criteria and participants
Included publications were studies evaluating the outcomes after DLT compared to DDLT. Non-comparative studies were excluded. Given the rarity of DLT, we considered any study describing more than five cases, provided that those were evaluated compared to standard DDLT. Studies with transplantation of other organs or combined transplantations were excluded. Living donors and pediatric cases were excluded.
Intervention and comparison of interest
DLT was considered as the intervention of interest, which was compared to DDLT.
Literature search strategy, study selection, and data extraction and management
A comprehensive search strategy was undertaken in MEDLINE, Embase, and Web of Science. The search algorithm included the following terms: “domino” AND “transplant” OR “transplantation.” The detailed search strategy, study selection, data extraction and management, and study selection are presented in the Supporting Information.
Risk of bias assessment
The Risk of Bias in Non-randomized Studies—of Interventions (ROBINS-I) tool11 was used to assess the risk of bias on the included studies. Details are illustrated in the Supporting Information.
Summary measures, synthesis, and statistical analysis
For dichotomous outcomes, odds ratios (OR) were determined as the summary measure. For the adverse dichotomous outcome variables (rejection, vascular, renal, biliary, or hemorrhagic complications), the OR of less than 1 would favor DLT over DDLT. For non-adverse dichotomous outcome variables (3-year, 5-year, or 10-year survival), the OR of more than 1 would favor DLT over DDLT. For time-to-event outcome parameter (overall survival), the natural logarithm of hazard ratios (HRs) was computed. Then, the natural logarithms of upper and lower confidence limits given for HRs were computed in order to obtain standard errors from confidence intervals (CIs). Finally, the generic inverse variance method was utilized to construct HR meta-analytical models on the natural logarithm scale. This was conducted to resolve uncertainties associated with varying follow-up periods among the included studies. For continuous parameters, a mean difference (MD) was calculated. When mean values were not available for continuous outcomes, data on median and interquartile range (IQR) were extracted and subsequently converted to mean and standard deviation (SD) using the established equation described by Hozo et al.12
One reviewer entered the extracted data into the Review Manager 5.4 software for data synthesis.13 This data set was subsequently checked by two independent reviewers, and statistical analysis was performed, using random-effects modeling as recommended by Kalkum et al.14 The results were reported in a forest plot with 95% CIs for each outcome parameter. A subgroup analysis of studies published between two different eras was performed to estimate trends and differences in outcomes among different epochs.
Heterogeneity among the studies was assessed using the Cochran Q test (χ2). Data inconsistency was quantified by calculating I2 and interpreted as follows: 0–25%: might not be important; 26–75%: may represent moderate heterogeneity; >75–100% may represent considerable heterogeneity. Sensitivity analyses were conducted to explore potential sources of heterogeneity and assess the robustness of the results. Finally, the effect of each study was evaluated based on the overall effect size and the study heterogeneity. For this purpose, the analysis was repeated following the exclusion of one study at a time (leave-one-out sensitivity analysis).
Results
Literature search and data collection
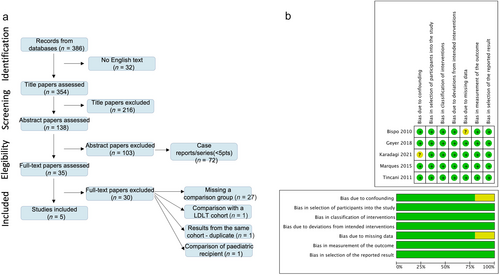
The literature search resulted in 386 articles; 32 were not in English and were excluded. Of the remaining 354 articles, 216 were excluded based on the title. After assessing the abstract of 128 articles, 103 were excluded, and 35 were reviewed for full text. Ultimately, five articles were included as retrospective comparative studies, published between 2011 and 2021 (Fig. 2). Overall, there were 945 patients who received either a DLT (n = 409) or a DDLT (n = 536). Four studies5-7, 9 were conducted in Europe using retrospective databases, whereas one study8 was performed in the United States, utilizing the United Network for Organ Sharing dataset (Table 1).
 , Yes (low risk);
, Yes (low risk);  , Unclear;
, Unclear;  , No (high risk).
, No (high risk).| First Author [ref] | Year | Center | N of centers | Country | Period of the study | DLT Group (n) | DDLT group (n) | Index disease of the domino |
|---|---|---|---|---|---|---|---|---|
| Bispo5 | 2010 | Curry Cabral Hospital, Lisbon | Single | Portugal | Jan 2005–Jun 2008 | 77 | 91 | FAP |
| Tincani6 | 2011 | Paul Brousse, Paris | Single | France | Mar 1993–Jan 2007 | 61 | 61 | FAP |
| Marques7 | 2015 | Curry Cabral Hospital, Lisbon | Single | Portugal | July 2001–Feb 2014 | 114 | 140 | FAP |
| Geyer8 | 2018 | Nationwide Children's Hospital, Columbus | UNOS | USA | Feb 2002–Dec 2016 | 123† | 123† | NR |
| Karadagi9 | 2021 | Stockholm | Single | Sweden | 2007–2017 | 34 | 115 | FAP |
- † This cohort is propensity matched.
- DDLT, deceased donor liver transplantation; DLT, domino liver transplantation; FAP, familial amyloid polyneuropathy; NR, not reported; UNOS, United Network for Organ Sharing.
The baseline characteristics of the study population and the tumor are summarized in (Table S1). Three studies5, 7, 9 reported donor age, which was significantly lower in DLT (40.1 ± 8.6 vs 56.6 ± 6.3, P = 0.003). Four5-7, 9 studies illustrated the domino graft index hereditary disease, which was FAP in all cases, for a total of 286 patients. Recipients were mostly male with 79.4% (325/409) and 76.8% (412/536) in DLT and DDLT groups, respectively (P = 0.25). All included studies reported the recipient age, which was significantly higher in the DLT group (56.9 ± 2.0 vs 54.1 ± 3.8, P = 0.04). Four studies5, 7-9 reported the recipient lab MELD, which was comparable between the two groups (14.4 ± 2.2 vs 14.5 ± 2.6, P = 0.97). Regarding the graft preservation time, four studies5-7, 9 reported the CIT, which was comparable between both groups (425.5 ± 57.4 min vs 498.2 ± 18.2 min, P = 0.13). Four studies5-8 presented the indication for transplantation. Hepatocellular carcinoma (HCC) was reported in four studies,5-8 and it was present in 213/375 (56.8%) and 231/421 (54.9%) recipients of the DLT and DDLT groups, respectively, without any significant difference (P = 0.36).
Notably, the authors of one study7 focused solely on HCC candidates. In this study, the authors reported tumor characteristics, which are summarized in Table S1. Four studies6-9 reported waiting list times, which were comparable between the two study groups, with 224.1 ± 181.7 days for DLT versus 252.2 ± 183.0 days for DDLT (P = 0.37).
Risk of bias assessment
Figure 2 presents the risk of bias assessment of the included studies. While the risk of bias due to confounding was low in four and unclear in one study, the risk of bias in selection of participants was low in all studies. The risk of bias due to missing data was low in four studies and unclear in one study. The risk of bias in measurement of outcomes and classification of interventions was low in all studies. The risk of bias due to deviations from intended intervention, or selection of the reported result was low in all studies.
Outcomes after liver transplantation
Outcomes are summarized in Table 2 and Figures 3 and 4.
| First author, year [ref] | Operative time (min) mean (SD) | Vascular events, n (%) | Renal events, n (%) | Graft rejection, n (%) | Severe hemorrhage, n (%) | ICU stay (days), mean (SD) | LOS (days), mean (SD) | Biliary complications, n (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DLT | DDLT | DLT | DDLT | DLT | DDLT | DLT | DDLT | DLT | DDLT | DLT | DDLT | DLT | DDLT | DLT | DDLT | |
| Bispo5 | 357(59) | 368 (66) | NR | NR | 6 (7.8) | 13 (14.3) | NR | 3(3.2) | 7(9.1) | 16 (17.6) | 6.0 (8.8) | 6.2 (7.3) | NR | NR | NR | NR |
| Tincani6 | 424 (113) | 482 (109) | 2 (3.2) | 3(4.9) | 12 (19.7) | 13 (21.3) | 13 (21.3) | 14 (22.9) | 3 (4.9) | 2 (5.1) | 12.2 (7.5) | 10.4 (6.4) | 34.7 (13.9) | 30.9 (11.8) | 3 (4.9) | 2 (3.2%) |
| Marques7 | 347.3 (79.4) | 335.7 (72.9) | 16 (14) | 10 (6.8) | NR | NR | 3 (2.6) | 7 (4.8) | 35 (30.7) | 28 (19.2) | 4 (3) | 3 (3) | 20 (17) | 16 (11) | 20 (17.5) | 18 (12.3) |
| Geyer8 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Karadagi†9 | 519 [404–579] | 430[372–519] | NR | NR | NR | NR | NR | NR | NR | NR | 1 [1–2] | 1 [1–2] | 17 [13–23] | 18 [14–27] | NR | NR |
- Values are reported as mean ± standard deviation or median (interquartile range), as reported in each study accordingly.
- † For Karadagi et al.,9 the operative time, the ICU stay, and LOS are reported as median [IQR].
- DDLT, deceased donor liver transplantation; DLT, domino liver transplantation; ICU, intensive care unit; LOS, length of hospital stays; NR, not reported.

Operative time
Four studies5-7, 9 reported operative times with a total of 699 patients (DLT = 286, DDLT = 413) with considerable heterogeneity (I2: 94%, P < 0.00001). The mean operative time in the DLT and DDLT groups were 408.3 ± 63.1 min versus 405.5 ± 57.4 min, respectively. There was no significant difference in those timings between DLT and DDLT (MD: 4.93, 95% CI: −40.53–50.40, P = 0.83).
Severe postoperative hemorrhage
Three studies5-7 (550 patients) were included in the analysis of severe postoperative hemorrhage. The severe postoperative hemorrhage rate in the DLT group was 17.8%, and it was 15.4% in the DDLT group. No significant difference was found in severe postoperative hemorrhage between two groups (OR: 1.09, 95% CI: 0.40–3.00, P = 0.86). Moderate heterogeneity existed among the included studies (I2: 67%, P = 0.05) (Fig. 3). Notably, there was no homogeneous definition of severe postoperative hemorrhage. One study5 reported hemorrhage needing “reintervention,” whereas another study6 reported hemorrhage needing reoperation, and the third study7 reported as “hemorrhagic complications,” without any further information.
Length of intensive care unit (ICU) stay
Four studies5-7, 9 reported the intensive care unit (ICU) stay with moderate heterogeneity (I2: 63%, P = 0.04). The mean length of ICU stay in the DLT and DDLT groups were 5.9 ± 4.0 days versus 5.2 ± 3.5 days, respectively. There were no differences in ICU stay between DLT and DDLT (MD: 0.50, 95% CI: −0.28–1.28, P = 0.21) (Fig. 3).
Length of hospital stay (LOS)
Three studies6, 7, 9 reported the length of hospital stay (LOS) with a total of 531 patients (DLT = 209, DDLT = 322) with considerable heterogeneity (I2: 85%, P = 0.001). The mean LOS in the DLT and DDLT groups were 24.1 ± 7.6 days versus 22.0 ± 6.4 days, respectively. There was no significant difference in LOS between DLT and DDLT (MD: 1.68, 95% CI: −2.83–8.19, P = 0.46) (Fig. 3).
Renal events
Renal events, that is, renal failure requiring dialysis, were reported by two studies5, 6 (290 patients). Renal events were present in 13% of DLT and 19% of DDLT. There was no significant difference in the renal event rate between the two groups (OR: 0.62, 95% CI: −0.28–1.39, P = 0.24). Moderate between-study heterogeneity was detected (I2: 33%, P = 0.22) (Fig. 3).
Vascular events
Two studies6, 7 reported vascular events for a total of 382 patients (DLT = 175, DDLT = 207). Vascular events occurred in 10.2% (18/175) and 6.2% (13/207) in DLT and DDLT, respectively, without significant difference in vascular event rates between the two groups (OR: 1.60, 95% CI: −0.55–4.62, P = 0.39). Moderate between-study heterogeneity was detected (I2: 30%, P = 0.23) (Fig. 3). Of note, only one study6 differentiated between arterial, portal, and outflow vascular complications.
Overall biliary complications
Two studies6, 7 reported biliary events for a total of 382 patients (DLT = 175, DDLT = 207), which were higher in the DLT group (n = 23/175, 13.1%) compared to the DDLT cohort (n = 20/207, 9.6%). However, this did not reach statistical significance (OR: 1.51, 95% CI: 0.79–2.89, P = 0.21); the between-study heterogeneity was low (I2: 0%, P = 0.99). Notably, the nature of biliary complications reported by the included studies remained unspecified (Fig. 3).
Graft rejection
Only two studies6, 7 (382 patients) reported rates of acute cellular rejection (ACR). One study6 reported ACR incidence, whereas another study7 reported the incidence of immune-related complications, which were considered as ACR for our analysis. ACR occurred in 9.1% (16/175) and 10.1% (21/207) in DLT and DDLT, respectively. There was no significant difference in ACR rates between the two groups (OR: 0.78, 95% CI: 0.38–1.62, P = 0.51). Low between-study heterogeneity was detected (I2: 0%, P = 0.52) (Fig. 3).
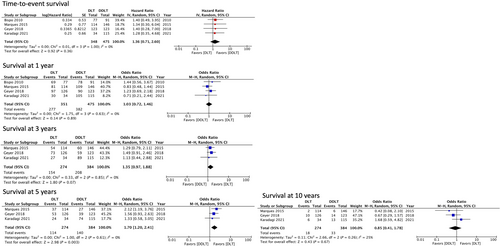
Recipient overall survival (time-to-event)
Four studies5, 7-9 (826 patients) were included in the analysis of overall survival. The time-to-event analysis demonstrated that there was no significant difference in overall survival between the DLT and DDLT groups (HR 1.36, 95% CI: 0.71–2.60, P = 0.36). Low heterogeneity was detected among the included studies (I2: 0%, P = 1.00).
Recipient survival at 1, 3, 5 and 10 years
Survival outcomes are summarized in Figure 4. Four studies5, 7-9 (826 patients) were included in the analysis of 1-year survival. The rates of 1-year survival in the DLT and DDLT groups were 78.9% and 80.4%, respectively, without statistically significant differences (OR: 1.03; 95% CI: 0.72–1.46, P = 0.89). Low between-study heterogeneity was detected (I2: 0%, P = 0.63).
Three studies5-7 (658 patients) were included in the analysis of 3-year, 5-year, and 10-year survival. The rates of 3-year, 5-year, and 10-year survival were rather low in both groups with 56.2%, 41.6%, and 6.5% for DLT, versus 54.1%, 36.4%, and 8.5% for DDLT, respectively. There was no statistically significant difference in the 3-year survival rates (OR: 1.35; 95% CI: 0.97–1.88, P = 0.07); however, a survival advantage toward DLT was showed at 5 years (OR: 1.70; 95% CI: 1.20–2.40, P = 0.003), which was not paralleled by the survival after 10 years (OR: 0.85; 95% CI: 0.41–1.78, P = 0.67). Low between-study heterogeneity was detected at 3 years (I2: 0%, P = 0.85), 5 years (I2: 0%, P = 0.61), and 10 years (I2: 25%, P = 0.26).
Notably, none of the studies included in this meta-analysis reported the graft survival; therefore, solely the recipient survival was analyzed.
Subgroup analysis of studies published between 2010–2016 and 2016–2021
This subgroup analysis was performed when more than three studies could be included, and it is presented in the Supporting Information.
De novo development of the index disease in the recipient
Only one study7 reported this outcome; therefore, a formal analysis was not performed. The authors illustrated that, after a median follow-up of 45 months (1–136 months), 13/114 patients (11.4%) developed symptoms of FAP disease. Median elapsed time until the appearance of clinical features was reported to be 75 months (60–121 months). The diagnosis of acquired FAP was made after repeated neurological assessment and electroneurography. Labial salivary gland or sural nerve biopsies were performed in five and two patients, respectively. One patient underwent retransplantation with a deceased donor, 6.5 years after the first transplant. The only variable independently associated with de novo amyloidosis was an elapsed time of more than 60 months post-LT.7
Sensitivity analysis
This was performed only when ≥3 studies were available for the outcome. The direction of the described pooled effects size remained unchanged during the sensitivity analysis for most outcomes, in particular survival rates (Table S2). An individual removal of Karadagi9 turned the results for ICU stay (P = 0.005) to significant in favor of DDLT. In the analysis of length of ICU stay, removal of study of Marques et al.7 decreased I2 from 63% to 0% while maintaining the insignificant difference between two groups. In the analysis of LOS, removal of study of Karadagi et al.9 not only reduced the decreased I2 from 85% to 0%; it made the results in favor of DDLT (P = 0.007). For severe hemorrhage, an individual removal of Bispo5 (P = 0.03) turned the results significant in favor of DDLT. An individual removal of Marques et al.7 (P = 0.07) turned the results nonsignificant for survival at 5 years.
Discussion
This is the first meta-analysis comparing short-term and long-term outcomes of DLT and DDLT based on five published, retrospective cohort studies. The following main results were found. Firstly, both DLT and DDLT have the same waitlist timings and early in hospital outcomes, including operative time, and length of ICU and hospital stay. Secondly, similar rates of relevant posttransplant complications, including hemorrhage, acute rejection, and biliary complications, were found. Finally, survival at 1 and 3 years appeared to be comparable between the two groups. The survival advantage seen after DLT at 5 years was not confirmed at a follow-up of 10 years.
LT represents the only definitive treatment for hereditary and metabolic liver disorders. The use of these livers for DLT was developed to expand the donor pool throughout the years, especially in countries like Portugal, Sweden, and Japan, where FAP is endemic,15, 16 utilizing these grafts have for elderly recipients with hepatic tumors. Looking at the Domino Liver Transplant Registry, by December 2019, HCC candidates were the most common DLT recipients (540/2294, 23.5%), followed by alcoholic cirrhosis (239/2294, 10.4%) and viral hepatitis (216/2294, 9.4%).3 Such registry data were supported by our study, where we have demonstrated that HCC was diagnosed in more than half of the recipient (55.7%), pooling the results of four included studies.5-8 Of note, one study7 included solely domino recipients with HCC.
A Swedish study has demonstrated that the incidence of hereditary amyloidosis per 100 000 persons/year increased from 1.50 in 2006–2009 to 4.92 cases in 2016–2018, possibly due to previous underdiagnosis. However, authors showed a lower mortality during later years, which was likely based on the introduction of disease-modifying drugs.17 The agent patisiran has been demonstrated to improve the clinical manifestation of FAP.18 In some reports, this novel therapeutic drug has been successfully used in de novo FAP among DLT recipients, with good results.19, 20 This trajectory could have interesting implications in the transplantation setting. DLT could be a potential source of more grafts when combining it with this novel drug, thus reducing waitlist time and enable transplantation of more patients.
Although there was a trend toward shorter waitlist time in the DLT group in our study, this did not reach statistical significance. This could have been caused by the limited number of studies included in this data synthesis. However, Karagadi et al. showed that, despite DLT did not affect the waitlist time compared to DDLT, it enabled transplantation of more patients.9 Notably, the reports in our study focused only on FAP; therefore, it was not possible to explore the impact of DLT on waiting list times when considering other indications, such as other metabolic disorders. At present, while there is a constant shortage of transplantable organs, the number of indications for LT is expanding, especially for secondary liver tumors and metastases.21 In the future, DLT could be a compelling option to expand donor pool and enable transplantation of patients even with biologically unfavorable liver tumors or metastatic diseases, who might have limited access to organs, especially in countries with longer waitlist times and where the number of deceased donors is limited.22-24 In our study, we were able to demonstrate similar long-term recipient survival rates between DLT and DDLT. Although a formal comparison has not been reported yet, overall outcomes after DLT appear comparable to recently presented benchmark values for the best possible outcomes with utilization of donation after brain death whole liver grafts.25
Despite such promising results and the normal function of domino grafts, DLT may also raise some ethical concerns. DLT recipients have to be aware of the risk of de novo development of the index disease. In our study, we were not able to draw robust conclusions because only one study7 reported the rates of de novo development of the index disease; therefore, a formal analysis was not possible. In their study, Marques et al. reported that 13/114 patients (11.4%) developed symptoms of FAP. However, only one patient underwent retransplantation 6.5 years after the first transplant.7 Another study26 found that four out of 17 domino recipients developed FAP symptoms (23.5%) within a mean follow-up of 5.2 years (62.6 ± 2.9 months). A few other studies reported the occurrence of de novo amyloidosis in six of 84 domino recipients (7.1%), 3–11 years after DLT.27, 28 Of additional interest is the study by Asonuma et al., where authors found that three of 39 domino recipients (7.7%) developed signs of de novo amyloid polyneuropathy about 6 years after transplantation.16 The largest series reporting the onset of the index disease after DLT was presented by the Hospital Curry Cabral, where 55 out of 335 recipients (16.4%) developed symptoms of FAP disease at a median time of 8.7 years (IQR: 3.6–13.1 years; 105 months [IQR: 44–158 months]).2 These studies underline that de novo development of FAP may occur earlier than previously expected; however, it appears not to impact long-term DLT recipient survival rates.2, 7 Given the new therapeutic drugs used in DLT recipients with promising outcomes so far,19, 20 the development of de novo FAP might become better controlled without major risk for the domino recipients in the future. The literature is however lacking large reports regarding the de novo development of metabolic disease. The two largest series reported only short-term outcomes of 14 domino recipients29 and 21 patients underwent LT for MSUD receiving living donor liver grafts.4 Based on these findings, the proper recipient selection for the domino graft appears of major importance. Given the limited reports and the lack of high-quality data, it is not possible to draw robust conclusions regarding de novo development of the index disease and long-term outcomes for DLT when considering metabolic diseases.
Additionally, DLT poses some technical challenges given its surgical complexity. The domino graft has to be procured by experienced surgeons to provide optimally preserved inflow and outflow vessels for the implantation in the domino recipient, without jeopardizing the domino donor, who receives a whole or living donor graft as well (Fig. 1). Based on an overall increasing number of DLT procedures worldwide since 1995, several techniques have been developed achieving a safe transplant surgery for both the domino donor and the recipient.2 Such techniques have been further advanced with the use of living donor liver grafts as the new liver of the domino donor, without increasing the number of posttransplant complications.30
The piggyback technique for domino graft procurement and implantation was first developed in the Hospital Curry Cabral, avoiding utilization of veno-venous bypass.2 Other techniques involve the outflow reconstruction with an autologous portal vein31 or the use of cadaveric vein grafts (IVC, auricles, Y-shaped IVC-iliac bifurcation) as interposition between the hepatic vein stump of the domino liver and the recipient hepatic vein or arterial/aortic segments.32 As a general concept, domino grafts are high-quality organs comparable to living donor liver grafts, including stable hemodynamics during procurement, short ischemic time, and a planned procedure. Some authors have also reported domino graft splitting with excellent results in both domino donor and recipient.33, 34 Recently, a 180° rotated, free iliaco-caval venous graft was used to reconstruct the domino graft outflow, with an also procured common left iliac vein, anastomosed to the right hepatic vein of the domino graft. The common right iliac vein was then anastomosed to the common stump of the domino graft middle and left hepatic vein; ultimately, the cadaveric IVC cuff was connected to the recipients middle and left hepatic veins.35 Some studies have explored combined domino heart–liver transplantation for FAP with good outcomes.36, 37 However, give the small number of reports, we were not able to analyze and draw robust conclusions in the current study.
Given the aforementioned concerns, a close monitoring for early detection of de novo FAP in domino recipients should be considered. A periodic cardiac workup and neurological assessment are recommended, especially for recipients from non-Val30Met grafts, in particular if a cardiac involvement was documented in the donor and if the FAP liver is placed into an old male recipient.38, 39
This meta-analysis has several limitations, which have to be acknowledged when interpreting the results. First, only five retrospective studies were identified. This has possibly led to an inherent selection bias, thus limiting the robustness of our results. Second, the outcomes were not homogeneously reported by all studies, especially regarding postoperative complications. For instance, we were not able to assess the occurrence of early allograft dysfunction/primary non-function as only two studies5, 6 reported these outcomes without homogeneous criteria. In addition, the definition of some of the analyzed postoperative complications such as hemorrhagic, biliary, vascular, and rejection complications was not reported homogeneously, which might limit the robustness of these results. Third, it was possible to estimate HRs when performing survival synthesis, but some survival outcomes had to be extrapolated by the survival curves and were used as dichotomous variable. In particular, one study8 reported survival curves after DLT exclusively before propensity matching. Fourth, the period of the included study was rather long (1993–2017), which could cause selection bias due to management changes throughout that time period. It was therefore not possible to estimate the effects of the learning curve, as more experience was gathered for DLT throughout the years in however a few specialized centers with the required patient's number. Furthermore, all included studies reported DLT for FAP; therefore, it was not possible to compare the outcomes for other metabolic DLT indications. In addition, it was not feasible to conduct outcome synthesis of the de novo development of the index disease in domino recipients, because only one study7 reported this outcome adequately.
Despite several limitations, this meta-analysis based on five retrospective cohort studies provides the currently highest evidence and demonstrates similar clinical outcomes after DLT compared to standard DDLT. Therefore, DLT could be a potential source of additional grafts to expand the donor pool. This appears to be of greater importance as novel indications for transplantation are emerging. However, the real-life risks of de novo development on the index disease could not be assessed in the present study given the lack of data; therefore, we believe that a proper recipient selection is key to success. The findings of our study support any further attempt to evaluate regional opportunities to increase the number of DLT in the future. Nevertheless, the risk of index disease transmission in a proportion of recipients is possible, and it needs to be considered when counseling patients for DLT.
Acknowledgments
None.
Ethical approval
Considering the design of our study, ethical approval was not required. This article does not contain original data of human participants.
Informed consent
No patient data were collected for our study; thus, informed consent was not required.