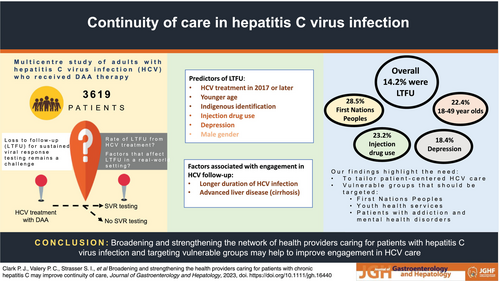
Broadening and strengthening the health providers caring for patients with chronic hepatitis C may improve continuity of care
Abstract
Background
Direct-acting antiviral (DAA) therapies for hepatitis C virus infection (HCV) lead to excellent rates of sustained virological response (SVR). However, loss to follow-up (LTFU) for SVR testing remains a challenge. We examine factors associated with LTFU in a real-world setting.
Methods
Adults who received DAA therapy for HCV in one of 26 centers across Australia during 2016–2021 were followed up for 2 years. Data sources included the patient medical records and the national Pharmaceutical and Medicare Benefits Schemes. Linkage to Medicare provided utilization data of other health-care providers and re-treatment with DAAs. LTFU was defined as no clinic attendance for SVR testing by at least 52 weeks after DAA treatment commencement. Multivariable logistic regression assessed factors associated with LTFU.
Results
In 3619 patients included in the study (mean age 52.0 years; SD = 10.5), 33.6% had cirrhosis (69.4% Child–Pugh class B/C), and 19.3% had HCV treatment prior to the DAA era. Five hundred and fifteen patients (14.2%) were LTFU. HCV treatment initiation in 2017 or later (adj-OR = 2.82, 95% confidence interval [CI] 2.25–3.54), younger age (adj-OR = 2.63, 95% CI 1.80–3.84), Indigenous identification (adj-OR = 1.99, 95% CI 1.23–3.21), current injection drug use or opioid replacement therapy (adj-OR = 1.66, 95% CI 1.25–2.20), depression treatment (adj-OR = 1.49, 95% CI 1.17–1.90), and male gender (adj-OR = 1.31, 95% CI 1.04–1.66) were associated with LTFU.
Conclusions
These findings stress the importance of strengthening the network of providers caring for patients with HCV. In particular, services targeting vulnerable groups of patients such as First Nations Peoples, youth health, and those with addiction and mental health disorders should be equipped to treat HCV.
Graphical Abstract
Introduction
Direct-acting antiviral (DAA) therapies for hepatitis C virus (HCV) infection are associated with very high rates (>95%) of sustained virological response (SVR) at 12 weeks.1-3 Nevertheless, non-attendance for SVR testing remains a challenging aspect in accurately documenting SVR and ensuring continuity of care.4-7 A concern in marginalized populations is treatment adherence to maximize chance of cure while also engaging to ensure patients can be treated again, should treatment failure occur. Other patients with greater risk for advanced liver disease or at risk of progression may need regular review and/or ongoing surveillance for liver cancer. While the rate of loss to follow-up (LTFU) has been reported to be small in randomized clinical trials,8, 9 for various reasons, trial patients may differ from real-world settings, and data are conflicting with LTFU rates varying with patient characteristics (e.g. people who inject drugs, gender, genotype, prior HCV treatment).4, 5, 7, 10
The rapid uptake of DAA therapy in Australia has led to a 30% reduction in liver-related deaths due to HCV since 2015 and a plateauing of HCV-related hepatocellular carcinoma (HCC) cases.11 Having data on the rate of LTFU and the factors that may affect engagement with treatment and follow-up are crucial to inform further improvement of HCV service delivery and treatment. Moreover, continued engagement after HCV therapy is vital to monitor for progressive liver disease to help manage comorbid risks and follow-up for potential HCV reinfection. Using data from a large multicenter prospective study of people with HCV infection who received DAA therapy in Australia, we report the rate of LTFU from HCV treatment and examine factors that affect LTFU in a real-world setting.
Methods
Details of the OPERA-C study have been previously described.12 Briefly, the OPERA-C is a multicenter prospective study of adults with HCV who received DAA therapy during February 2016 to July 2021 in one of 26 centers across Australia (six states and one territory).
Data collection and measures
At study enrollment, sociodemographic and clinical characteristics were captured from the patient medical records where participants were recruited. Patient data were reviewed at 6-monthly intervals for 2 years to assess treatment and liver-related outcomes. Probabilistic data linkage was undertaken using the federal Medicare Pharmaceutical Benefits Scheme (PBS) and Medicare Benefits Schedule (MBS) records.
Area-based measures for classification of remoteness of residence13 and socioeconomic status14 were based on the patients' residential postcode. Clinical data included HCV diagnosis and treatment details, concurrent injection drug use (IDU) and opioid replacement therapy (ORT), and selected risk factors for liver disease.
Liver fibrosis was assessed using transient elastography and Fibrosis-4 (FIB-4) scores.15 Thresholds using liver stiffness measurement (LSM)16 were LSM < 8 kPa was considered as no or minimal liver fibrosis, between 8–12.5 kPa moderate or advanced fibrosis, and >12.5 kPa was considered cirrhosis. FIB-4 test >3.25 was used to predict advanced liver fibrosis.15 Cirrhosis was classified using the Child–Pugh and MELD scores.
Australia's universal health-care system provides citizens and eligible residents with subsidized prescription medicines through the PBS and consultations with doctors and allied health services for eligible patients. Patient data were linked to the PBS and MBS records to obtain complete drug dispensing and MBS histories (which includes consultation history and provider type as well as pathology testing) from May 2015 to September 2021. Data were used to identify subsidized use of medications including HCV treatment. Medication dispensing histories and service use 12-month prior to and during DAA therapy were included in the analysis. Comorbid medical conditions were derived using the RxRisk-V17 model as previously described.18
LFTU was defined as patients who had not attended clinic for HCV PCR testing by at least 52 weeks after DAA treatment and had not been reported as deceased or discharged from clinic. The choice of 52 weeks posttreatment for LTFU allowed better capture of SVR data in a real-world setting where some patients may present late for SVR testing.
Statistical analysis
Analyses were conducted using Stata/SE (Version 15; Stata Corporation, College Station, TX). Group comparisons (e.g. according to LTFU) were made using Student's t-test, the Mann–Whitney test, the chi-squared test, or Fisher's exact test, as appropriate. Rate of LTFU was calculated as the proportion of patients who were LTFU and 95% confidence intervals (CI).
Logistic regression models examined factors associated with LTFU. We have excluded factors where prevalence of exposure was missing for >15% of the patients and combined IDU and ORT. Presence of cirrhosis at enrollment was considered as a marker for liver disease severity as data were more complete when compared to LSM for which data were missing for 24.9% of patients. Unadjusted odds ratios (ORs) and its corresponding 95% CI were estimated. Stepwise selection (P-value for addition/removal <0.10) was used to create a multivariable logistic regression model to identify factors associated with LTFU (also referred to as predictors of LTFU). The final multivariable model was determined based on the results of the bivariable analysis but also taking into account our understanding of the relationships and dependencies among variables, their clinical relevance, and our previous analysis.18 The interaction term IDU and/or ORT and psychotic illness was statistically significant (P < 0.001) and, therefore, included in the final model. The calibration of the final model was performed using Hosmer–Lemeshow test and discrimination power using receiver operating characteristic (ROC) curve. Statistical significance was set at alpha = 0.05, and all P values were two-sided.
Results
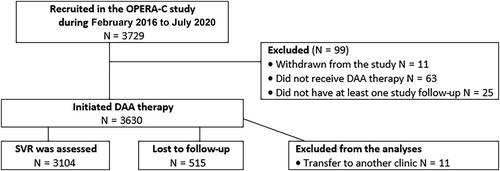
OPERA-C study recruited 3729 patients, and after exclusions (n = 110 patients; see Fig. 1), 3619 were included in the analysis. Most patients (86.5%) were recruited from gastroenterology/hepatology clinics at tertiary centers. At recruitment, patients were 52.0 years (SD = 10.5), 33.6% had cirrhosis (69.4% Child–Pugh class B/C), and 19.3% had HCV treatment prior to DAA era. Over half of the cohort had genotype 1 (53.7%) and 37.6% genotype 3.
Differences in sociodemographic and clinical factors according to LTFU
The overall rate of LTFU was 14.2% (95% CI 13.1–15.4). Compared to patients not LTFU, patients LTFU were significantly younger (46.7 years [SD = 10.7] vs 52.9 years [SD = 10.2]; P < 0.001), a higher proportion were Australian born (88.1% vs 80.5%; P < 0.001), First Nations Peoples (6.5% vs 2.7%; P < 0.001), and lived in the most disadvantaged areas (55.6% vs 50.4%; P = 0.027) (Table 1). A greater number of patients LTFU were actively injecting drugs (14.2% vs 5.8%; P < 0.001) and were prescribed ORT (22.9% vs 13.9%; P < 0.001). Patients LTFU had less severe liver disease as assessed by median APRI (P = 0.035), FIB-4 (P < 0.001), and LSM (P < 0.001), and fewer were cirrhotic (22.8% vs 35.3%; P < 0.001). In those LTFU, cirrhosis was less likely to be advanced, with MELD significantly lower (P = 0.035). There was no difference in Child–Pugh class (P = 0.22) or presence of portal hypertension (P = 0.43) according to LTFU status.
| SVR was assessed | LTFU | |||
|---|---|---|---|---|
| N = 3104 | N = 515 | P-value | ||
| Age (mean, SD) | 52.9 (10.2) | 46.7 (10.7) | <0.001 | |
| Age group | 18–49 years | 1014 (32.7%) | 293 (56.9%) | <0.001 |
| 50–59 years | 1273 (41.0%) | 170 (33.0%) | ||
| 60 + years | 817 (26.3%) | 52 (10.1%) | ||
| Gender | Female | 1058 (34.1%) | 171 (33.2%) | 0.70 |
| Male | 2046 (65.9%) | 344 (66.8%) | ||
| Country of birth‡ | Overseas | 592 (19.5%) | 60 (11.9%) | <0.001 |
| Australia | 2442 (80.5%) | 443 (88.1%) | ||
| Identified as First Nations Peoples† | 83 (2.7%) | 33 (6.5%) | <0.001 | |
| Socioeconomic status† | Q1 most affluent/Q2/Q3 | 1539 (49.6%) | 228 (44.4%) | 0.027 |
| Q4/Q5 most disadvantaged | 1562 (50.4%) | 286 (55.6%) | ||
| Remoteness of residence† | Major city | 2322 (74.8%) | 385 (74.9%) | 0.97 |
| Regional/remote | 781 (25.2%) | 129 (25.1%) | ||
| Hepatitis B surface antigen¶ | 43 (1.7%) | 13 (3.1%) | 0.079 | |
| Diabetes† | 350 (11.3%) | 41 (8.1%) | 0.028 | |
| Current injection drug use§ | 164 (5.8%) | 64 (14.2%) | <0.001 | |
| Prescribed opioid replacement therapy‡ | 409 (13.9%) | 112 (22.9%) | <0.001 | |
| Cirrhosis at enrollment† | 1089 (35.3%) | 115 (22.8%) | <0.001 | |
| Child–Pugh class A | 273 (30.0%) | 35 (36.1%) | 0.22 | |
| B/C | 637 (70.0%) | 62 (63.9%) | ||
| MELD (median, IQR) | 7.90 (7.50–9.91) | 7.50 (6.43–8.47) | 0.035 | |
| Current cirrhosis decompensation | 76 (7.0%) | 5 (4.3%) | 0.43 | |
| Liver fibrosis assessment (median, IQR) | ||||
| APRI score§ | 0.66 (0.37–1.41) | 0.62 (0.34–1.19) | 0.035 | |
| FIB-4 score§ | 1.71 (1.07–3.11) | 1.27 (0.84–2.11) | <0.001 | |
| Liver stiffness (kPa)†† | 7.80 (5.60–14.30) | 6.80 (5.00–10.30) | <0.001 | |
| Data source: PBS‡ | ||||
| Rx-Risk categories | Depression | 792 (26.6%) | 179 (36.6%) | <0.001 |
| Gastric acid disorder | 772 (25.9%) | 102 (20.9%) | 0.017 | |
| Anxiety | 587 (19.7%) | 139 (28.4%) | <0.001 | |
| Psychotic illness | 378 (12.7%) | 85 (17.4%) | 0.006 | |
| Hypertension | 356 (12.0%) | 36 (7.4%) | 0.003 | |
| Data source: MBS‡ | ||||
| Mental health services | 684 (22.6%) | 166 (33.4%) | <0.001 | |
| Number of visits (mean, SD) | 1.15 (4.03) | 1.48 (3.98) | 0.088 | |
| General practitioner or specialist (excluding psychiatrist) | 2911 (96.0%) | 493 (99.2%) | <0.001 | |
| Number of visits (mean, SD) | 11.74 (11.66) | 12.93 (11.39) | 0.032 | |
| HCV assessment and treatment | ||||
| Duration of HCV infection (years) (mean, SD)‡‡ | 23.6 (11.6) | 18.0 (11.8) | <0.001 | |
| HCV treatment prior to DAA era | 645 (20.8%) | 54 (10.5%) | <0.001 | |
| Year of HCV treatment initiation | 2016 or earlier | 1903 (61.3%) | 159 (30.9%) | <0.001 |
| 2017 or later | 1201 (38.7%) | 356 (69.1%) | ||
| Had a second DAA treatment§§ | 184 (5.9%) | 42 (8.2%) | 0.05 | |
- † Missing data for <1% of patients.
- ‡ Missing data for 1–5% of patients.
- § Missing data for 6–15% of patients.
- ¶ Missing data for 16–25% of patients.
- †† Missing data for 26–43% of patients.
- ‡‡ Data missing for 449 patients.
- §§ Data on second treatment was not available for 26 patients who were LTFU and PBS data was not available.
- IQR, interquartile range; SD, standard deviation.
Fewer patients LTFU were dispensed medications for other medical conditions, namely, diabetes (8.1% vs 11.3% in patients not LTFU; P = 0.028), hypertension (7.4% vs 12.0%; P = 0.003), or gastric acid disorders (20.9% vs 25.9%; P = 0.017). Medicines for anxiety and depression were more commonly dispensed to patients LTFU (28.4% vs 19.7%; P < 0.001 and 36.6% vs 26.6; P < 0.001, respectively). A greater proportion of patients LTFU used publicly funded mental health consultations (33.4% vs 22.6%; P < 0.001) and had at least one visit to general practitioner (GP) or specialist (P < 0.001) in the 12 months prior to and during DAA therapy.
Patients LTFU had a shorter duration of infection (18.0 years vs 23.6 years, P < 0.001), and fewer had prior pre-DAA era treatment (10.5% vs 20.8%; P < 0.001). Among patient who had prior pre-DAA era treatment, SVR status after prior HCV treatment was unknown for 24% of patients LTFU compared to 10.4% of patients not LTFU (P < 0.001).
Regarding second DAA treatment, 40 patients LTFU (8.2%) had a second DAA treatment compared to 184 patients not LTFU (5.9%; P = 0.05). Data on second treatment were not available for 26 patients who were LTFU, and PBS data were not available. The average time between the start of the first and second DAA treatment was 684.7 days (SD = 392.6) for patients who were assessed for SVR and 737.8 days (SD = 406.6; P = 0.43) for patients who were LTFU.
Rate of LTFU according to sociodemographic and clinical factors
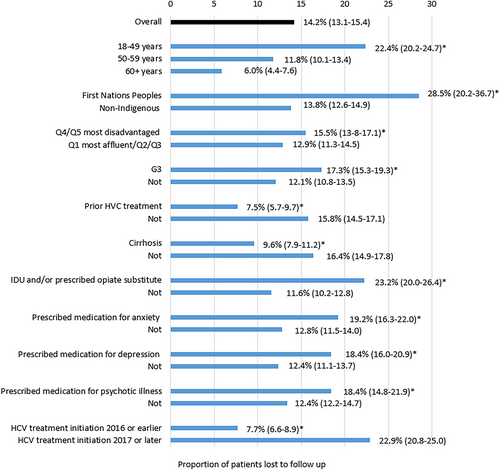
The rate of LTFU was highest among First Nations Peoples (28.5%, 95% CI 20.2–36.7) and patients with a history of IDU and/or ORT (23.2%, 95% CI 20.0–26.4), anxiety (19.2%, 95% CI 16.3–22.0), and depression (18.4%, 95% CI 16.0–20.9) (Fig. 2). Compared to patients aged ≥60 years who had an LTFR of 6.0% (95% CI 4.4–7.6), the rate of LTFU was over threefold higher for patients 18–49 years (22.4%, 95% CI 20–2-24.7) and nearly double for 50–59 years (11.8%, 95% CI 10.1–13.4).
Factors associated with LTFU
In multivariable analysis, HCV treatment initiation in 2017 or later (vs ≤2016, adj-OR = 2.82, 95% CI 2.25–3.54), younger age (18–49 years vs 60+ years adj-OR = 2.63, 95% CI 1.80–3.84), and Indigenous identification (adj-OR = 1.99, 95% CI 1.23–3.21) were the strongest predictors of LTFU (Table 2). Current IDU and/or ORT (adj-OR = 1.66, 95% CI 1.25–2.20), prescription of medication for depression (adj-OR = 1.49, 95% CI 1.17–1.90), and male gender (adj-OR = 1.31, 95% CI 1.04–1.66) were also associated with LTFU. Absence of cirrhosis at enrollment was significantly associated with LTFU; patients were 29% less likely to be LFTU if they had cirrhosis (adj-OR = 0.71 95% CI 0.55–0.93). Longer duration of HCV infection was inversely associated with LFTU (adj-OR = 0.98, 95% CI 0.97–0.99).
| Unadjusted OR (95% CI) | Adjusted OR (95% CI) | ||
|---|---|---|---|
| Socioeconomic and clinical characteristics | |||
| Age group | 18–49 years | 4.45 (3.33–6.18) | 2.63 (1.80–3.84) |
| 50–59 years | 2.10 (1.52–2.90) | 1.89 (1.31–2.73) | |
| 60+ years | Reference | Reference | |
| Gender | Female | Reference | Reference |
| Male | 1.04 (0.85–1.27) | 1.31 (1.04–1.66) | |
| Country of birth | Overseas | 0.56 (0.42–0.74) | n/s |
| Australia | Reference | ||
| Indigenous status | Non-Indigenous | Reference | Reference |
| First Nations Peoples | 2.49 (1.65–3.77) | 1.99 (1.23–3.21) | |
| Socioeconomic status | Q1 most affluent/Q2/Q3 | Reference | n/s |
| Q4/Q5 most disadvantaged | 1.24 (1.02–1.49) | ||
| Remoteness of residence | Major city | Reference | n/s |
| Regional/remote | 1.00 (0.80–1.24) | ||
| Diabetes | 0.69 (0.49–0.96) | n/s | |
| Current IDU and/or ORT | 2.32 (1.86–2.88) | 1.66 (1.25–2.20) | |
| Cirrhosis at enrollment | 0.54 (0.43–0.67) | 0.71 (0.55–0.93) | |
| FIB-4 groups | FIB-4 < =3.25 | Reference | n/s |
| FIB-4 > 3.25 | 0.62 (0.48–0.81) | ||
| No FIB-4 data | 0.64 (0.47–0.87) | ||
| Rx-Risk categories | Depression | 1.59 (1.30–1.95) | 1.49 (1.17–1.90) |
| Gastric acid disorder | 0.75 (0.60–0.95) | n/s | |
| Anxiety | 1.62 (1.30–2.01) | 1.17 (0.90–1.53) | |
| Hypertension | 0.59 (0.41–0.84) | n/s | |
| Psychotic illness | 1.45 (1.12–1.87) | 0.86 (0.58–1.28) | |
| Factors related to HCV assessment and treatment | |||
| Duration of HCV infection | 0.96 (0.95–0.97) | 0.98 (0.97–0.99) | |
| HCV treatment prior to DAA era | 0.45 (0.33–0.60) | n/s | |
| Year of HCV treatment initiation | 2016 or earlier | Reference | Reference |
| 2017 or later | 2.43 (1.90–3.12) | 2.82 (2.25–3.54) | |
| Type of service | Secondary center (vs Tertiary hospital) | 0.93 (0.71–1.23) | 0.74 (0.54–1.02) |
- Results in bold indicate statistical significance (P < 0.05).
- IDU, injection drug use; n/s, not selected as a predictor; ORP, opioid replacement therapy.
With the inclusion of LSM in the multivariable model instead of cirrhosis, the results were similar to the main analysis. In multivariable analysis, patients with >12.5 kPa appear to be less likely to be LTFU (adj-OR = 0.73, 95% CI 0.52–1.01), but chance could not be ruled out (P = 0.059; see Table S1 for the results of sensitivity analyses).
Overrepresentation of vulnerable groups in the later HCV treatment cohort
Compared to people who initiated HCV treatment in 2016 or earlier, the later HCV treatment cohort was significantly younger, and a higher proportion of them were First Nations Peoples, of lower socioeconomic status, current IDU, and/or on ORT, and were prescribed medication for anxiety and psychotic illness. In contrast, fewer people in the later HCV treatment cohort had cirrhosis, had HCC, or were prescribed medication for gastric disorders. They also had lower scores for liver fibrosis (Table S2).
Discussion
In this real-world experience of over 3600 Australians with HCV infection treated with DAAs over a 4.5-year period, we assessed factors associated with being lost to follow-up. Younger age and identifying as First Nations Peoples more than doubled a person's risk of being lost to follow-up after HCV treatment. People with mental health comorbidity were more likely to be lost to follow-up from HCV treatment services. Interestingly, these people did continue to utilize mental health services, providing an important opportunity for continuity of care. Report of current IDU and receipt of opioid replacement therapy at the time of DAA initiation were also important determinants of LTFU. As might be anticipated, younger people and those with a shorter duration of infection and less severe liver disease were more likely to be LTFU. Taken together, these data point to a number of vulnerable groups who might be prioritized for more intense efforts to follow-up to reduce the risk of being lost to care. Furthermore, the data reflect the diverse nature of the HCV-affected community and that developing equivalent diversity in the health services treating HCV and following up post-treatment are critical. Key opportunities for expanding treatment and follow-up are in First Nations primary health care, addiction treatment, and mental health services.19
Unlike many patient HCV registries, patients in the OPERA-C cohort were well characterized for liver disease and cirrhosis. In this cohort, people with HCV cirrhosis were three times less likely to be LTFU, confirming previous findings of the REACH-C study.20 The Australian experience differs from previously published data showing either no difference in LTFU rates or an increased risk of LTFU from HCV treatment among patients with advanced liver disease. A German study including 7747 patients reported no association between LTFU from HCV treatment and presence of cirrhosis.7 In the OPERA-C study, participants were mostly recruited from gastroenterology/hepatology clinics located in tertiary hospitals, while half the centers included in the German study were clinics specialized in ORT treatment. In Germany and in Australia, ORT and cirrhosis treatment rarely occur in the same site, leading to a disjunction in care. Therefore, in Australia, as patients with cirrhosis received HCV treatment in the same clinic as their cirrhosis treatment, they were less likely to be LFTU for HCV treatment. Moreover, novel data from the OPERA-C study showed that patients with less liver fibrosis (higher LSM) appeared to be more likely to be LTFU.
Importantly, patients with a longer history of infection or prior treatment were nearly three times more likely to remain engaged in follow-up than patients whose first treatment was with DAAs. The study population who initiated HCV treatment in 2017 or later, which included younger patients and more vulnerable groups, were 2.82 more likely to be LTFU compared to those treated early, when DAA medication for HCV became funded through Australia's universal health-care system.
The rate of LTFU reported here were in line with previous reports from comparable countries such as Canada5 (13%), Italy4 (10.5%), and Germany7, 21 (2.2–13%), as well as data from Australia (6.8–15%).1, 20 These studies also reported that rates of LTFU from HCV treatment varied according to patient group. The high rate of LTFU from HCV treatment among younger patients included in the OPERA-C study was consistent with that of another Australian20 and an Italian study.4 In contrast, a Canadian5 study reported no association with age at treatment initiation and LTFU. While male sex was associated with LTFU in some studies in Europe,4, 7 this has not been shown previously in our or prior Australian data.20 Many studies have reported differences in health-seeking behavior according to gender, with adult men consistently consulting less than adult women in primary care, after adjusting for consultations for reproductive reasons.22, 23 The lower health-seeking behavior in men may partly explain our findings of poor engagement in HCV follow-up compared to women.
First Nations Peoples in our study were twice as likely to be LTFU from HCV treatment compared to non-Indigenous Australians. HCV prevalence and incidence is higher for First Nations Peoples,24 and liver disease is a major contributor to the mortality gap between First Nations Peoples and non-Indigenous Australian adults.25 In 2015, the rate of newly acquired HCV was 13-fold higher among First Nations Peoples compared to non-Indigenous Australians.24 Understanding the sociodemographic and clinical characteristics of First Nations Peoples with HCV who initiate HCV treatment is key to inform potential interventions which may improve delivery of HCV care for this patient group. In the OPERA-C study, prior analysis focused on First Nations patients identified younger age, less advanced liver disease (as measured by FIB4), and a recency of treatment as factors associated with higher LTFU.18
The characteristics of the HCV population who initiated treatment early was significantly different from those treated after 2016. The “early HCV treatment cohort” were older and had more advanced liver disease (cirrhosis, HCC) and possibly patients engaged in care for other conditions. In contrast, the “later HCV treatment cohort” comprised younger patients and more vulnerable groups, namely, First Nations Peoples, patients of lower socioeconomic status, IDU, and/or ORT and patients on medication for depression and psychotic illness. This might suggest that patients with less liver disease defined care needs may predominate. In the REACH-C study,20 year of initiation of DAA treatment was also the strongest predictor of LTFU; patients who initiated HCV treatment in 2019 were 4.5 times (95% CI 3.57–5.64) more likely to be LTFU compared to those initiating treatment in 2016. Our study did not directly assess models of HCV treatment, but does point to opportunities to optimize engagement for vulnerable groups.
Our study found higher LTFU in patients on ORT. Other studies have shown patients who received ORT in a different clinic than their HCV treatment were over three times more likely to be LTFU from HCV treatment (adj-OR = 3.35 95% CI 2.35–4.78).7 In a Canadian study,5 participants receiving ORT in the same clinic as HCV treatment were less likely to be LTFU (adj-OR = 0.09 95% CI 0.02–0.46).5 In our study, patients who were current IDU or were on ORT were 1.66 times more likely to be LTFU for HCV treatment. In the Australian clinical environment, these data highlight the need to expand treatment into ORT centers and practitioners.19
HCV infection often occurs concurrently with mental health disorders. Prior to DAA era, mental health comorbidity often precluded interferon-based treatment due to the risk of neuropsychiatric side effects and worsening of existing mental health symptoms.26 With the uptake of DAA therapy for HCV, mental health illness is no longer a barrier to treatment,27 but appears to be an ongoing barrier to continuing care after treatment.
Strengths of this study are the prospective and multicenter design, linked data from national databases (MBS/PBS), the use of validated methods to assess fibrosis, cirrhosis, and liver disease severity. The use of medications as a surrogate to identify people with comorbidities may underestimate comorbidity burden as non-pharmacological approaches are often used (e.g. diet-controlled diabetes mellitus). Notwithstanding these limitations, the RxRisk-V has been validated and used in Australian and international cohorts.18, 28, 29 While patients without follow-up data for >12 months were LTFU from the treating center, it is possible other clinical follow-up occurred elsewhere, and such data were not captured. Lastly, there may be other factors that may explain LTFU that were not examined in this study such as history of incarceration or the use of non-MBS-related services.
Sociodemographic and clinical factors were associated with LTFU. Broadening and strengthening the network of health providers caring for patients with HCV may help to address this. Targeting vulnerable groups such as First Nations Peoples, youth health, and those with addiction and mental health disorders should be enabled to care for patient assessment, treatment, and follow-up as well as screen for advanced liver disease. For patients who remain at risk of reinfection or of progressive liver disease, engagement is critical. Expanding the role of GPs and other health providers in relation to HCV care will require sufficient support from specialists to enable this. It will require formal education and training programs focused on HCV screening, assessment, treatment, follow up, and the importance of continuity of care. Our findings emphasize the need to tailor patient-centered HCV care and may inform strategies to improve continuity after HCV treatment. Provision of telehealth, outreach nursing from the tertiary clinic, access to point-of-care RNA testing, and more flexible clinic locations and appointment availability could also encourage priority populations to engage with HCV care.
Acknowledgments
Project governance was overseen by the Project Steering Committee, via the GESA Liver Clinical Research Network. QIMR Berghofer was the administering institution. We thank the patients for participating in the study. We also thank David Roche (GESA) and Karen Martin (QIMR Berghofer) for their support coordinating the study, Therese Lawton (QIMR Berghofer) for data management, and the research nurses from each study site for recruitment and data collection.
Informed consent
Informed consent was obtained from all subjects who participated in the study. The Human Ethics Committees of the Royal Brisbane and Women's Hospital (HREC/15/QRBW/183), participating hospitals, and QIMR Berghofer Medical Research Institute (P2126) approved this study. All procedures followed were in accordance with the ethical standards of the abovementioned committees and with the Helsinki Declaration of 1975, as revised in 2008.
Open Research
Data availability statement
The data that support the findings of this study contain potentially sensitive and/or identifying information that could compromise the privacy of the participants. Therefore, data are not publicly available. Data may, however, be available from the authors upon reasonable request with approval from relevant ethics committees.